Abstract
To identify pharmacokinetic (PK) drug-drug interactions between tipranavir-ritonavir (TPV/r) and rosuvastatin and atorvastatin, we conducted two prospective, open-label, single-arm, two-period studies. The geometric mean (GM) ratio was 1.37 (90% confidence interval [CI], 1.15 to 1.62) for the area under the concentration-time curve (AUC) for rosuvastatin and 2.23 (90% CI, 1.83 to 2.72) for the maximum concentration of drug in serum (Cmax) for rosuvastatin with TPV/r at steady state versus alone. The GM ratio was 9.36 (90% CI, 8.02 to 10.94) for the AUC of atorvastatin and 8.61 (90% CI, 7.25 to 10.21) for the Cmax of atorvastatin with TPV/r at steady state versus alone. Tipranavir PK parameters were not affected by single-dose rosuvastatin or atorvastatin. Mild gastrointestinal intolerance, headache, and mild reversible liver enzyme elevations (grade 1 and 2) were the most commonly reported adverse drug reactions. Based on these interactions, we recommend low initial doses of rosuvastatin (5 mg) and atorvastatin (10 mg), with careful clinical monitoring of rosuvastatin- or atorvastatin-related adverse events when combined with TPV/r.
Tipranavir coadministered with low-dose ritonavir (TPV/r) is an effective treatment option in treatment-experienced human immunodeficiency virus (HIV)-infected patients with resistance to more than one protease inhibitor (PI) (9). TPV/r is associated with adverse effects (AEs) that include increased triglycerides and cholesterol. NIAID Division of AIDS (DAIDS) grade 3 to 4 cholesterol elevation (>400 mg/dl) and grade 3 to 4 triglyceride elevation (>750 mg/dl) were higher in the TPV/r-treated patients than in the comparator-boosted PI-treated patients in phase III studies (9). Grade 3 to 4 cholesterol elevation was 4.3 versus 0.6/100 patient exposure years, and triglyceride elevation was 27.8 versus 21.6/100 patient exposure years in the TPV/r versus comparator-boosted PI-treated patients. The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study (N. Friis-Moller, P. Reiss, W. El-Sadr, A. D'Arminio Monforte, R. Thiébaut, R. De Wit, S. Weber, E. Fontas, M. Law, A. Phillips, and the DAD Study Group, presented at the 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO, 5 to 8 February 2006) found a 16% increase in the relative risk of myocardial infarction in PI-treated patients. This association is possibly explained by dyslipidemia. Since the HIV-infected patient population is getting older, it is critical to control hyperlipidemia in PI-treated patients in order to reduce the risk of long-term cardiovascular complications. Potent 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors (e.g., atorvastatin [Lipitor; Pfizer, Inc., New York, NY] and rosuvastatin [Crestor; AstraZeneca Pharmaceuticals, Wilmington, DE]) are recommended for the treatment of hypercholesterolemia (1); however, their use in HIV-infected patients may be limited by clinically significant drug-drug interactions with PIs (5). Atorvastatin is metabolized extensively by cytochrome P450 3A4 (CYP3A4) to metabolites that have in vitro inhibitory activity for HMG-CoA reductase similar to that of atorvastatin. Approximately 70% of the circulating inhibitory activity for HMG-CoA reductase has been attributed to these active metabolites (15). Since TPV/r has a net inhibitory effect on CYP3A4 (M. Vourvahis, J. Dumond, K. Patterson, N. Rezk, N. White, S. Jennings, H. Tien, J. Sabo, T. MacGregor, and A. Kashuba, presented at the 14th Congress on Retroviruses and Opportunistic Infections, Los Angeles, CA, 25 to 28 February 2007), it has the potential to significantly increase circulating atorvastatin concentrations.
However, rosuvastatin is unlikely to interact with TPV/r since it is not a CYP3A4 substrate and it is not extensively metabolized (Crestor package insert; AstraZeneca Pharmaceuticals, Wilmington, DE). In order to provide guidance for clinical use, pharmacokinetic (PK) studies in healthy volunteers evaluated the drug-drug interactions between steady-state TPV/r and single-dose rosuvastatin (“rosuvastatin study”) and atorvastatin (“atorvastatin study”).
MATERIALS AND METHODS
Subjects.
Each study protocol was approved by the local institutional review board, and written informed consent was obtained from all volunteers before enrollment in these studies.
Rosuvastatin study.
HIV-negative healthy men and nonpregnant women volunteers 18 to 65 years old with body mass indexes of 18 to 30 kg/m2 were enrolled in this study. Exclusion criteria included the use of any medications, herbal therapies, or grapefruit juice within 14 days before study entry or alcohol intake within 48 h before PK sampling days, active hepatitis B or C infection, and significant laboratory abnormalities. All women were required to have a negative pregnancy test result at study entry and were instructed to use a barrier contraceptive method during the study period.
Atorvastatin study.
Healthy men and women volunteers 18 to 60 years old, with body mass indexes of 18 to 29 kg/m2, were eligible for this study. Subjects were considered to be healthy based on their medical histories, physical examination, electrocardiogram, urinalysis, routine tests of biochemistry and hematology, hepatitis B and C status, and HIV status. Throughout the study period, subjects were instructed to abstain from alcohol, grapefruits/grapefruit juice (starting 10 days prior to the first study day), Seville oranges, and over-the-counter herbal medications (garlic supplements, St. John's wort, milk thistle) starting 5 days prior to the first study day. Methylxanthine-containing foods or drinks were not allowed within 72 h prior to and during PK sampling days.
Study design and procedures. (i) Rosuvastatin study.
This was a prospective, open-label, single-arm, inpatient, steady-state PK study. Subjects received a single 10-mg dose of rosuvastatin on day 1, followed by tipranavir 500 mg-ritonavir 200 mg twice daily for 11 days (days 3 to 13), with a single 10-mg dose of rosuvastatin given on day 12. All study drug doses were given under direct observation by the nursing staff of the Johns Hopkins University General Clinical Research Center. All subjects received 100% of study medications. Intensive PK sampling was performed on days 1 to 3 (before the addition of TPV/r) and days 12 to 14 (with TPV/r at steady state) for rosuvastatin and days 12 and 13 for tipranavir and ritonavir. A standard 670-kcal (33% fat) breakfast was served to subjects within 30 min before morning medication administration. No caffeine or food was allowed until 5 h postdose. No grapefruit juice was allowed on PK sampling days. Blood samples were collected at the following time points: 0 h predose and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h postdose for tipranavir and ritonavir and 0 h predose and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, and 48 h postdose for rosuvastatin. A pharmacokinetic comparison of single-dose rosuvastatin with single-dose rosuvastatin coadministered with TPV/r 500 mg/200 mg twice daily at steady state was undertaken. The short-term safety and steady-state PKs of tipranavir and ritonavir coadministered with single-dose rosuvastatin (10 mg) were also evaluated.
(ii) Atorvastatin study.
The single-dose PKs of atorvastatin, orthohydroxy atorvastatin, and parahydroxy atorvastatin were assessed on day 1 after ingestion of 40 mg of atorvastatin alone. From days 14 to 21, subjects received TPV/r 500 mg/200 mg twice daily. TPV/r dosages from the evening of day 13 to the morning of day 18 were taken at home. All dosages before and after this period were taken as directly observed therapy. Subjects were asked to log their adherence to study medications. All subjects had 100% adherence with study medication. The steady-state PKs of tipranavir were assessed on day 19 after ingestion of TPV/r alone and on day 20 after administration of TPV/r 500 mg/200 mg twice daily plus a single dose of atorvastatin 10 mg, as an increase in atorvastatin concentrations was expected in combination with steady-state TPV/r at 500 mg/200 mg twice daily. Blood samples for analysis of atorvastatin and hydroxy metabolites were collected on days 1 and 20 in EDTA-containing tubes by an indwelling catheter or the venipuncture of a forearm vein just before and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, and 48 h after ingestion of atorvastatin (14 samples).
Blood samples for analysis of tipranavir concentrations were collected in heparinized tubes on days 19 and 20 by an indwelling catheter or the venipuncture of a forearm vein just before and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after ingestion of TPV/r (12 samples). Ritonavir plasma concentrations were not measured in this study.
On PK sampling days, atorvastatin and TPV/r were administered orally at 8 a.m. with 240 ml of water after an overnight fast. A light snack (i.e., 240 ml of low-fat milk and dry bread) was allowed at no less than 1 h prior to dosing or 2 h after dosing to minimize nausea and vomiting if necessary. Subjects were kept in an upright position for the first 2 h after drug administration. A standardized lunch and dinner were served at 4 and 10 h after the morning dose, respectively (500 to 682 kcal; 23% to 25% from fat). The total daily fluid intake was restricted to a maximum of 3 liters.
Subject safety was monitored by an assessment of all AEs at each visit, in addition to a laboratory assessment of safety parameters.
Bioanalysis. (i) Rosuvastatin assay.
A bioanalytic method has been developed and validated by PPD (Richmond, VA) for the analysis of rosuvastatin in human plasma containing dipotassium EDTA. A 200-μl-sample aliquot was fortified with 200 μl of internal standard (IS) working solution. Analytes were isolated by protein precipitation with 400 μl of acetonitrile. Sample extraction steps were controlled and automated using a Quadra 96 model 320 (Tomtec, Hamden, CT). The supernatant was transferred to another plate and evaporated to dryness under a nitrogen stream at 45°C to 50°C. The remaining residue was reconstituted with 150 μl of 20:80:0.1 acetonitrile-water-formic acid (vol/vol/vol). The final extract was analyzed via high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS-MS) detection.
Eight calibration standards were analyzed in duplicate over the nominal concentration range of 0.100 to 100 ng/ml. For this validation, the lower limit of quantitation was nominally 0.100 ng/ml for rosuvastatin.
(ii) Atorvastatin assay.
Plasma concentrations of atorvastatin and its hydroxy metabolites were measured by validated liquid chromatography (LC)-MS-MS at MDS Pharma Services (Saint-Laurent, Quebec, Canada). The respective deuterated analogs of the analytes were used as ISs. Briefly, the analytes were extracted from 200 μl of EDTA plasma by solid-phase extraction using C8 cartridges. After isolation and evaporation to dryness, the analytes were reconstituted and 200 μl was injected for LC-MS-MS analysis. The analytes were separated on a Luna C8 column (Phenomenex, Torrance, CA) and quantified by MS under multiple-reaction monitoring mode. The lower limits of quantitation were 0.225 ng/ml for atorvastatin, 0.250 ng/ml for parahydroxy atorvastatin, and 0.175 ng/ml for orthohydroxy atorvastatin using a 200-μl sample.
(iii) Tipranavir assay.
A validated HPLC-MS-MS method was used to measure plasma drug concentrations of tipranavir and ritonavir (16). In general terms, ritonavir, tipranavir, and an IS are extracted from EDTA human plasma by a two-step liquid-liquid extraction using an ethyl acetate-hexane mixture followed by a hexane wash. The analytes are separated and detected by an LC-MS-MS system utilizing a 2.0- by 30-mm Synergi Polar RP column (Phenomenex) with a formic acid-acetic acid-acetonitrile mobile phase. For the atorvastatin study, tipranavir and an IS (PNU-109011) were extracted from 50 μl of heparinized plasma by liquid-liquid extraction using 600 μl of a mixture of ethyl acetate-hexane (9/1 [vol/vol]) after the addition of 100 μl of borate buffer (pH 9).
High and low calibration ranges were used to predict unknown concentrations. The high calibration curve ranged from 20,000 ng/ml to 1,000 ng/ml. The low calibration curve ranged from 2,000 ng/ml to 25.0 ng/ml.
Data analysis and statistical methods. (i) PK analysis.
For both studies, noncompartmental methods were used for PK analysis (WinNonlin versions 5.01 and 4.0; Pharsight Corporation, Mountain View, CA). The highest observed plasma concentration was defined as the maximum concentration of drug in serum (Cmax), with the corresponding sampling time to Cmax (Tmax). The elimination rate constant (λz) was determined by least squares linear regression analysis (log C versus t) of the last data points (n ≥ 3). The half-life (t1/2) was calculated by the equation t1/2 = ln2/λz. After single-dose administration, the area under the concentration-time curve from 0 h to infinity (AUC0-∞) was calculated using the linear-log trapezoidal rule (linear up/log down) from zero to the last measured concentration, with extrapolation to infinity by dividing the last measured concentration by λz (Clast/λz). The AUC0-12 was estimated using the linear-log trapezoidal rule (linear up/log down). The concentration at 12 h postdose was defined as C12. The CL/F, where F represents the oral bioavailability, was calculated as dose/AUC, and the volume of distribution (V) was calculated as (CL/F)/λz.
(ii) Statistical analysis.
Statistical analysis was performed with SAS (version 8.01; SAS Institute, Inc., Cary, NC) and Stata (version 8; Stata Corp., College Park, TX). Statistical comparisons were performed after logarithmic transformation. Summary statistics for each of the PK parameters were tabulated by regimen and study day. Geometric mean (GM) ratios and corresponding 90% confidence intervals (CI) of PK parameters were calculated. The lack of a clinically relevant interaction was declared if the 90% CI of the GM ratio was completely contained in the acceptance range of 0.80 to 1.25.
Prior to statistical analysis of the effect of TPV/r on atorvastatin (day 1 versus day 20), the atorvastatin AUC0-∞ and Cmax were multiplied by 4 to adjust for the difference in atorvastatin dose between day 1 (40 mg) and day 20 (10 mg). No dose adjustments were made for the analysis of the hydroxy metabolite concentrations.
In addition to the analysis of atorvastatin and its hydroxy metabolites individually, the effect on total HMG-CoA reductase inhibitory activity was assessed. The total HMG-CoA reductase inhibitory activity was calculated as the sum of the time-specific molar concentrations of atorvastatin (normalized for a 40-mg dose), orthohydroxy atorvastatin, and parahydroxy atorvastatin.
Nonparametric tests were used for comparisons between PK parameters (Mann-Whitney U test or Wilcoxon signed-rank test as appropriate). Spearman's correlation was used to test for associations between continuous variables. A P value of <0.05 was considered statistically significant for all comparisons. The within-subject variability in the steady-state tipranavir AUC0-12 was calculated as the percent difference between the individual tipranavir AUC0-12 values on day 19 and day 20.
For sample size calculations in both studies, it was assumed that the coefficient of variation (CV) would range from 20% to 40% for the AUCs of rosuvastatin and atorvastatin. Therefore, a sample size of 20 would provide >90% power to detect a ≥25% change in the AUCs of rosuvastatin and atorvastatin.
RESULTS
Rosuvastatin study. (i) Subjects.
Of the 29 subjects (5 women, 24 men), 16 evaluable subjects completed the study. The population was mostly African-American (76%), with a median age of 42 (range, 18 to 64) years, a median weight of 78 (range, 51 to 96) kg, and a median height of 175 (range, 162 to 196) cm. No Asian-Americans participated in the study.
(ii) Interaction between TPV/r and rosuvastatin.
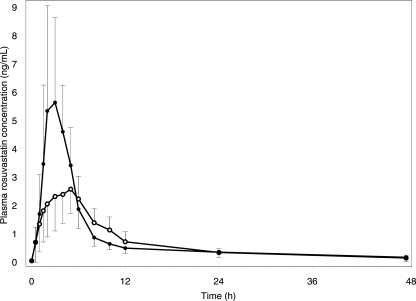
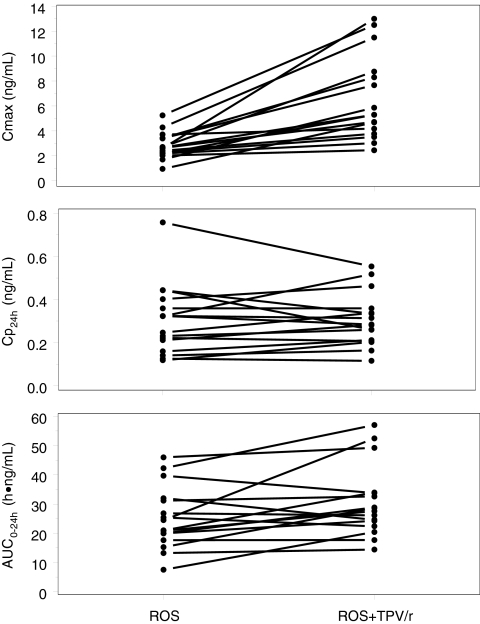
With TPV/r coadministration, the GM AUC for rosuvastatin was 38.6 ng·h/ml, a 37% increase compared with that of rosuvastatin alone (P = 0.0006) (Fig. 1 and 2). The GM Cmax for rosuvastatin was 5.78 ng/ml with TPV/r coadministration, a 123% increase compared with that of rosuvastatin alone (P < 0.001) (Table 1). Rosuvastatin clearance (CL) also was decreased by 27% with TPV/r coadministration; this resulted in a significant increase in plasma t1/2 (20.6 h versus 9.01 h; P < 0.001). Tipranavir and ritonavir PK parameters were not affected by single-dose rosuvastatin (see Table 3).
FIG. 1.
Plasma rosuvastatin concentration-time profile in the absence (open circles) and presence (filled circles) of steady-state TPV/r.
FIG. 2.
Effect of steady-state TPV/r on single-dose rosuvastatin (ROS) Cmax, plasma drug concentration at 24 h (Cp24h), and AUC0-24.
TABLE 1.
PK parameters of rosuvastatin alone or rosuvastatin plus TPV/ra
| Parameter | Rosuvastatin GM (% CV) | Rosuvastatin + TPV/r GM (% CV) | GM ratio (90% CI) | P value |
|---|---|---|---|---|
| Cmax (ng/ml) | 2.59 (41) | 5.78 (55) | 2.23 (1.83-2.72) | <0.001 |
| Tmax (h) | 5 (1.48-6.02) | 3 (2-4) | NA | <0.001 |
| C24 (ng/ml) | 0.186 (46) | 0.163 (21) | 0.88 (0.73-1.06) | 0.26 |
| AUC0-∞ (ng·h/ml) | 28.2 (59) | 38.6 (38) | 1.37 (1.15-1.62) | 0.006 |
| t1/2 (h) | 9.01 (69) | 20.6 (48) | 2.29 (1.63-3.21) | <0.001 |
| CL/F (liters/h) | 355 (59) | 259 (38) | 0.73 (0.62-0.87) | 0.006 |
Tmax values presented as median (range).
TABLE 3.
Effect of rosuvastatin (n = 16) and atorvastatin (n = 22) on the PK of TPV/ra
| TPV PK parameter | GM of TPV/r alone (% CV) | GM of TPV/r + rosuvastatin (% CV) | Rosuvastatin GM ratio (90% CI)b | GM of TPV/r alone (% CV) | GM of TPV/r + rosuvastatin (% CV) | Atorvastatin GM ratio (90% CI)b |
|---|---|---|---|---|---|---|
| Cmax (μM) | 72.5 (22) | 78.5 (24) | 1.08 (1.00-1.17) | 122 (52) | 118 (44) | 0.96 (0.86-1.07) |
| Tmax (h) | 3.5 (2.0-5.0) | 3.0 (1.6-5.0) | NA | 3.0 (1.5-5.0) | 2.5 (1.5-5.0) | NA |
| C12 (μM) | 15.4 (59) | 15.2 (40) | 0.99 (0.88-1.11) | 30.1 (82) | 31.5 (77) | 1.04 (0.89-1.22) |
| AUC0-12 (μM·h) | 477 (30) | 504 (27) | 1.06 (0.97-1.15) | 769 (56) | 827 (43) | 1.08 (1.00-1.15) |
| t1/2 (h) | 3.94 (33) | 3.77 (23) | NA | 5.54 (60) | 5.07 (50) | NA |
| CLss (liters/h) | 1.74 (38) | 1.65 (38) | NA | 1.08 (57) | 1.00 (42) | NA |
| V/F (liters) | 9.9 (38) | 9.0 (22) | NA | 8.6 (81) | 7.3 (41) | NA |
Tmax values presented as median (range). CLss, steady-state plasma clearance.
Calculated as the ratio of TPV/r plus the statin to TPV/r alone.
(iii) Safety.
The most common AEs associated with study drug administration were diarrhea (10.3%), nausea (13.8%), abdominal cramps (10.3%), flatulence (10.3%), headache (13.8%), and grade 1 liver enzyme elevations (34.5%) not resulting in study drug discontinuation. With the exception of one grade 2 nausea, one grade 2 rash, and eight grade 2 to 3 liver enzyme elevations, all AEs were of mild intensity (grade 1). Of 29 subjects enrolled, 13 subjects did not complete the study. Eight subjects discontinued because of grade 2 to 3 liver enzyme elevations, which were reversible upon study drug discontinuation. None of the subjects who had an increase in liver enzyme levels developed signs or symptoms of clinical hepatitis. One subject withdrew because of a grade 3 hypersensitivity reaction (mild shortness of breath, diffuse rash, and liver enzyme elevations). Three subjects withdrew consent, and one subject was administratively discharged due to a positive drug screen. The frequency and severity of these AEs are consistent with those observed in clinical trials involving HIV-infected patients treated with TPV/r. The rate of discontinuation in this study was high due to a lower threshold for study drug discontinuation (9). The potential for increased risk of hepatotoxicity with the coadministration of TPV/r plus rosuvastatin could not be assessed since all liver enzyme elevations occurred before coadministration began (day 8). All AEs resolved with study drug discontinuation.
Atorvastatin study. (i) Subjects.
Twenty-three subjects (11 men, 12 women) were recruited into this study. The population was mostly white (95.7%), with a median age of 32 (range, 18 to 55) years, a median weight of 72 (range, 55 to 99) kg, and a median height of 168 (range, 158 to 189) cm. The atorvastatin PK results from one subject and the steady-state tipranavir PK results from another subject were excluded from all statistical analyses because of nonphysiologic results (more than fivefold increase in drug concentration at the end of the dosing interval). However, the outcome of the study was not different with or without the data from these subjects (data not shown).
(ii) Interaction between TPV/r and atorvastatin.
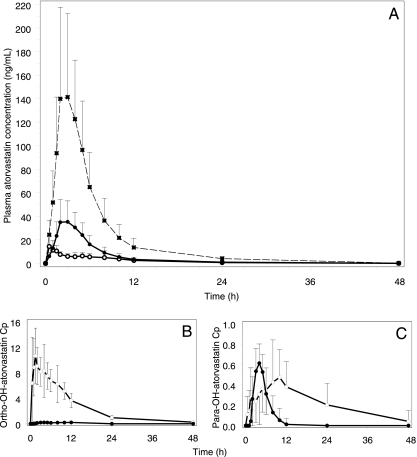
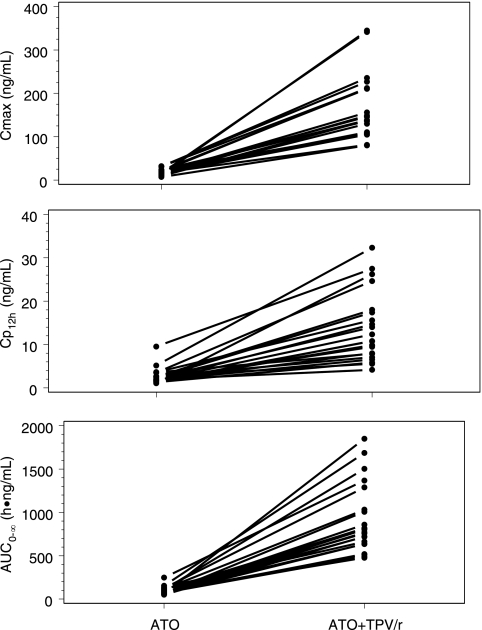
TPV/r increased the dose-adjusted atorvastatin AUC0-∞ and Cmax by approximately ninefold (Table 2; Fig. 3 and 4). As both apparent CL/F and apparent V/F were decreased almost proportionally, there was no effect on the t1/2 of atorvastatin. TPV/r inhibited the formation of orthohydroxy and parahydroxy atorvastatin and reduced the AUC0-∞ by 89% (P = 0.002) and 82% (P = 0.001), respectively. Total HMG-CoA reductase inhibitory activity increased by approximately fourfold in the presence of TPV/r. The GM ratios for the AUC0-∞ and Cmax of the total HMG-CoA reductase inhibitory activity were 3.87 (90% CI, 3.13 to 4.33; P < 0.001) and 4.97 (90% CI, 3.71 to 5.51; P < 0.001), respectively.
TABLE 2.
Single-dose PK parameters of atorvastatin, orthohydroxy atorvastatin, and parahydroxy atorvastatin after administration of atorvastatin 40 mg alone and coadministration of atorvastatin 10 mg and steady-state TPV/r 500 mg/200 mg twice daily (n = 22)a
| Analyte and PK parameter | Atorvastatin 40 mg alone (day 1) GM (% CV) | Atorvastatin 10 mg + TPV/r (day 20) GM (% CV)b
|
GM ratio (90% CI)c | P valued | |
|---|---|---|---|---|---|
| Observed | Normalized | ||||
| Atorvastatin | |||||
| AUC0-∞ (h·ng/ml) | 89.3 (38) | 209 (41) | 836 (41) | 9.36 (8.02-10.94) | <0.001 |
| Cmax (ng/ml) | 17.6 (40) | 37.8 (42) | 151 (42) | 8.61 (7.25-10.21) | <0.001 |
| Tmax (h) | 0.5 (0.5-8.0) | 3.0 (1.5-4.0) | 0.001 | ||
| CL/F (liters/h) | 448 (38) | 47.8 (41) | <0.001 | ||
| V/F (liters) | 4536 (43) | 432 (54) | <0.001 | ||
| t1/2 (h) | 7.0 (32) | 6.3 (36) | 0.30 | ||
| Orthohydroxy atorvastatin | |||||
| AUC0-∞ (h·ng/ml) | 117 (21) | 13.6 (124) | 0.11 (0.08-0.17) | 0.002 | |
| Cmax (ng/ml) | 12.4 (41) | 0.301 (35) | 0.02 (0.02-0.03) | <0.001 | |
| Tmax (h) | 1.5 (0.5-8.0) | 4.0 (1.0-12.0) | 0.006 | ||
| t1/2 (h) | 8.6 (21) | 27.7 (158) | 0.010 | ||
| Parahydroxy atorvastatin | |||||
| AUC0-∞ (h·ng/ml) | 19.5 (65) | 4.07 (35) | 0.18 (0.14-0.24) | 0.001 | |
| Cmax (ng/ml) | 0.586 (54) | 0.610 (32) | 1.04 (0.87-1.25) | 0.795 | |
| Tmax (h) | 9.0 (0.5-24.0) | 4.0 (2.0-5.0) | 0.002 | ||
| t1/2 (h) | 21.0 (99) | 3.17 (41) | 0.001 | ||
| Total HMG-CoA reductase inhibitory activity | |||||
| AUC0-∞ (h·nM) | 393 (27) | 396 (43) | 1,519 (42) | 3.87 (3.13-4.33) | <0.001 |
| Cmax (nM) | 54.8 (37) | 69.4 (42) | 272.6 (42) | 4.97 (3.71-5.51) | <0.001 |
Tmax values presented as median (range).
Dose adjusted assuming linear PK for atorvastatin (normalized value = observed value × 4).
Calculated as the ratio of atorvastatin plus TPV/r to atorvastatin alone.
P value for difference between atorvastatin alone and atorvastatin plus TPV/r determined by using the Wilcoxon matched-pairs signed-rank test.
FIG. 3.
Plasma drug concentration (Cp)-time profiles for atorvastatin (A), orthohydroxy atorvastatin (B), and parahydroxy atorvastatin (C). Shown are 40 mg atorvastatin alone (open circles) (A to C), 10 mg atorvastatin plus steady-state TPV/r (closed circles) (A to C), and an atorvastatin 10-mg profile corrected to a 40 mg dose (asterisks) (A). Values are mean ng/ml ± SD.
FIG. 4.
Effect of steady-state TPV/r on single-dose atorvastatin (ATO) Cmax, plasma drug concentration (Cp), and AUC0-∞.
Single-dose atorvastatin did not affect the steady-state PKs of tipranavir (Table 3).
(iii) Safety.
In general, treatment with TPV/r 500 mg/200 mg twice daily alone and in the presence of single-dose atorvastatin was well tolerated in this study. There were no study discontinuations due to AEs or for any other reason. One serious AE was reported in this study (a sprained ankle due to exercise), which was unrelated to the study drugs.
The most frequently reported AEs during all treatment phases were related to the gastrointestinal tract (n = 22 [95.7%]), followed by the nervous system (n = 16 [69.6%]). During treatment with TPV/r alone, 17 (73.9%) subjects reported diarrhea, 11 (47.8%) reported nausea, and 9 (39.1%) reported abdominal pain. Flatulence, loose stools, and dyspepsia occurred in six (26.1%), five (21.7%), and three (13%) subjects, respectively, during treatment with TPV/r. With the exception of one case of moderate nausea, all gastrointestinal events were of mild intensity and rarely required treatment intervention. Headache and dysgeusia were reported by eight (34.8%) and four (17.4%) subjects, respectively, during treatment with TPV/r.
With the exception of one subject with an asymptomatic DAIDS grade 3 elevation in alanine aminotransferase, there were no clinically relevant changes (grade 3 or higher) in any of the laboratory tests. The largest deviations from the median baseline were observed for triglycerides and alanine aminotransferase levels, which increased by 1.7-fold and 3.1-fold, respectively, relative to the baseline.
DISCUSSION
A PK drug interaction was predicted to be unlikely to occur between rosuvastatin and TPV/r since rosuvastatin is not a known substrate, inhibitor, or inducer of CYP3A4. Furthermore, rosuvastatin is not extensively metabolized, with approximately 10% of a radiolabeled dose recovered as N-desmethyl rosuvastatin. This major metabolite is formed principally by CYP2C9 (Crestor package insert; AstraZeneca Pharmaceuticals, Wilmington, DE). Although in vitro studies indicate that tipranavir is an inhibitor of CYP2C9, an in vivo cocktail study determined that the steady-state levels of tipranavir (500 mg) plus ritonavir 200 mg twice daily did not affect concentrations of plasma S-warfarin, a CYP2C9 substrate (M. Vourvahis, presented at the Eighth International Workshop on Clinical Pharmacology of HIV Therapy, Budapest, Hungary, 16 to 18 April 2007). When TPV/r was coadministered with rosuvastatin at steady state, the AUC0-∞ and Cmax of rosuvastatin were increased by 37% and 123%, respectively, after single-dose rosuvastatin. Similarly, concomitant administration of steady-state TPV/r significantly increased the AUC0-∞ of atorvastatin. However, the atorvastatin AUC increase was significantly higher. The differential effect of TPV/r on the PKs of rosuvastatin and atorvastatin may be due to the difference in the mechanisms of interaction.
Rosuvastatin is not a P-glycoprotein (Pgp) substrate, but it is a substrate of organic anion-transporting polypeptide 1B1 (OATP1B1) and breast cancer resistance protein (BCRP), also known as ATP-binding cassette transporter G2 (12). OATP1B1 is located at the basolateral membrane of hepatocytes and functions as an influx transporter to facilitate the entry of drugs into hepatocytes. These types of substrates include several statins (i.e., pravastatin, cerivastatin, atorvastatin, and rosuvastatin) (7, 10). BCRP is expressed in many tissue barriers throughout the body, including the epithelium of the small intestine, liver canalicular membrane ducts, placental syncytiotrophoblasts, and lobules of the breast (4, 17). In the gastrointestinal tract, BCRP is localized in the apical membrane of the intestinal epithelia, with the highest BCRP mRNA expression in the duodenum (6). Unlike OATP1B1, BCRP generally functions as an efflux transporter that facilitates hepatobiliary excretion and decreases intestinal absorption of BCRP substrates. The marked effect on the oral bioavailability of BCRP substrates was demonstrated in a BCRP 1 knockout murine model. The plasma concentrations of nitrofurantoin, a substrate of BCRP, were increased by fourfold and twofold compared with those in wild-type mice after oral and intravenous administration, respectively (20). Similarly, BCRP (ATP-binding cassette transporter G2) polymorphism (c.421AA genotype) in healthy volunteers was associated with significant increased atorvastatin and rosuvastatin AUC values of 72% and 100%, respectively (13). Cyclosporine, an OATP1B1 and BCRP inhibitor, significantly increases rosuvastatin and atorvastatin serum concentrations, by 11-fold and 8-fold, respectively (8, 24). Tipranavir, ritonavir, and lopinavir are also BCRP inhibitors in vitro (19, 27). Concentration-dependent inhibition of [3H]estradiol-17β-d-glucuronide uptake by ritonavir is also observed in OATP1B1-transfected HeLa cells and OATP-mediated CGamF (25, 28). The potential mechanism of interaction in this study may be the result of dual inhibition of OATP1B1 and BCRP transporters by ritonavir and tipranavir. Inhibition of OATP1B1 may lead to a decrease in hepatocyte uptake of rosuvastatin and atorvastatin, whereas inhibition of BCRP decreases hepatobiliary excretion and increases rosuvastatin and atorvastatin absorption.
In addition to OATP1B1 and BCRP transporter-mediated interaction, inhibition of first-pass metabolism of atorvastatin, rather than inhibition of its systemic metabolism, may explain the 8.6-fold increase in atorvastatin Cmax (an 8.6-fold increase) without an effect on the t1/2. The absolute oral bioavailability of atorvastatin is low (14%), which is likely a result of the combined effects of intestinal metabolism by CYP3A4 and transport into the gut lumen by the multidrug efflux transporter Pgp (4). Assuming the linear PKs of atorvastatin (23), coincidentally, the formation of orthohydroxy and parahydroxy atorvastatin was inhibited. The AUC0-∞ values of these metabolites were reduced by 89% and 82%, respectively, in the presence of TPV/r. Steady-state TPV/r has an inhibitory effect on CYP3A4/5 but minimal effects on Pgp (5) and may thus increase the bioavailability of atorvastatin, as suggested in the current study. Previous studies have reported an increased bioavailability of various CYP3A4 and/or Pgp substrates in the presence of ritonavir (e.g., digoxin and saquinavir) (22, 26). Similarly near-complete absorption of atorvastatin in the presence of TPV/r (F ≈ 100%) may explain the observed changes in the AUC0-∞ and Cmax, the delayed Tmax, and the proportional reduction in both the CL/F and the V/F, resulting in no change in the t1/2. On the other hand, the rosuvastatin t1/2 was increased by approximately twofold. This finding suggests that alternative metabolic pathways for rosuvastatin may be present.
These findings are consistent with those of drug interaction studies evaluating the effect of another PI, lopinavir-ritonavir (LPV/r), when coadministered with rosuvastatin at steady state. A prospective study conducted with healthy volunteers found that rosuvastatin AUC0-∞ and Cmax were increased 2.1- and 4.7-fold, respectively, when coadministered with LPV/r (14). Similar to the results of the current atorvastatin study, the t1/2 was not affected; however, the magnitude of the drug interaction observed between rosuvastatin and LPV/r was severalfold higher. This effect may be due to the higher inhibitory potency of BCRP observed with lopinavir in vitro (27). Coadministration of atazanavir-ritonavir with rosuvastatin was found to result in a 210% increase in the rosuvastatin AUC; however, no significant interaction was observed with fosamprenavir-ritonavir (2). The lack of interaction observed with fosamprenavir-ritonavir may be due to its low BCRP and OATP1B1 inhibitory potential (V. E. Theil-Demby, K. Harmon, M. Naqwe, J. Humphreys, M. B. Wire, and J. W. Polli, presented at the 2008 Annual Meeting and Exposition of the American Association of Pharmaceutical Scientists [AAPS], November 2008). Increased exposure to atorvastatin has previously been observed in combination with other HIV PIs, which are all substrates/inhibitors of CYP3A4. Coadministration with LPV/r 400 mg/100 mg twice daily increased the atorvastatin (20 mg once daily) AUC0-24 and Cmax by 5.9- and 4.7-fold, respectively, and increased the AUC0-24 and Cmax of the total HMG-CoA reductase inhibitory activity by 2.5- and 4.5-fold, respectively (R. A. Carr, A. K. Andre, and R. J. Bertz, presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000) (5). The combination of saquinavir-ritonavir 400 mg/400 mg twice daily increased the median atorvastatin (40 mg) AUC0-24 by 3.5-fold and the AUC0-24 of the total HMG-CoA reductase inhibitory activity by 1.8-fold (5). Nelfinavir, a moderately strong inhibitor of CYP3A4, increased the AUC0-24 and Cmax of the total HMG-CoA reductase inhibitory activity by 1.7- and 2.2-fold, respectively, after administration of atorvastatin 10 mg once daily for 14 days (11). The combination of darunavir-ritonavir 300 mg/100 mg twice daily with atorvastatin 10 mg daily resulted in an AUC0-24 that was 15% lower than the AUC0-24 of atorvastatin 40 mg daily alone, suggesting an almost fourfold increase in the atorvastatin AUC0-24 (R. M. W. Hoetelmans, A. Lasure, A. Koester, M. de Pauw, B. van Baelen, M. Peeters, W. Parys, and E. Lefebvre, presented at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 30 October to 2 November 2004).
One of the most serious AEs associated with HMG-CoA reductase inhibitors (statins) is rhabdomyolysis, which can result in significant morbidity and mortality (21). Although rhabdomyolysis may occur in 0.1% to 0.5% of the population when statins are prescribed as monotherapy, the risk is greatly increased when drugs that inhibit CYP3A4-mediated metabolism are used concomitantly (21). Several cases of atorvastatin-associated rhabdomyolysis have been reported in patients using atorvastatin in combination with CYP3A4 inhibitors, such as cyclosporine or delavirdine (3, 18). Although the safety and efficacy of rosuvastatin and atorvastatin could not be assessed by these single-dose studies, to minimize the risk of rhabdomyolysis, careful monitoring for any signs or symptoms of toxicity is recommended. Alternatively, statins with a lower likelihood of interactions could be considered.
In conclusion, we observed a clinically relevant increase in rosuvastatin and atorvastatin concentrations during coadministration of TPV/r 500 mg/200 mg twice daily. Based on these results, a low initial dose of rosuvastatin (5 mg) and atorvastatin (10 mg) is recommended when combined with TPV/r, with careful clinical monitoring of rosuvastatin- or atorvastatin-related AEs, such as myopathy.
Acknowledgments
The study participants and the study nurses are kindly acknowledged for their invaluable help with this study. Jan M. Wruck (Boehringer Ingelheim US) and Peter Jones (Boehringer Ingelheim UK) are gratefully acknowledged for the statistical analyses of these clinical studies.
D.W.C. is supported by a Career Scientist Award from the Ontario HIV Treatment Network of the Ontario Ministry of Health. D.W.C. and C.J.L.L. have received grants and contracts for this and other research from Boehringer Ingelheim US through their institution and employer, the University of Ottawa at The Ottawa Hospital and the Ottawa Health Research Institute. The rosuvastatin study is supported by a GCRC grant (RR-00052) and an investigator-initiated grant from Boehringer Ingelheim Pharmaceuticals, Inc.
Footnotes
Published ahead of print on 10 August 2009.
REFERENCES
- 1.American Heart Association. 2002. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143-3421. [PubMed] [Google Scholar]
- 2.Busti, A. J., A. M. Bain, R. G. Hall II, R. G. Bedimo, R. D. Leff, C. Meek, and R. Mehvar. 2008. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. J. Cardiovasc. Pharmacol. 51:605-610. [DOI] [PubMed] [Google Scholar]
- 3.Castro, J. G., and L. Gutierrez. 2002. Rhabdomyolysis with acute renal failure probably related to the interaction of atorvastatin and delavirdine. Am. J. Med. 112:505. [DOI] [PubMed] [Google Scholar]
- 4.Fetsch, P. A., A. Abati, T. Litman, K. Morisaki, Y. Honjo, K. Mittal, and S. E. Bates. 2006. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 235:84-92. [DOI] [PubMed] [Google Scholar]
- 5.Fichtenbaum, C. J., J. G. Gerber, S. L. Rosenkranz, Y. Segal, J. A. Aberg, T. Blaschke, B. Alston, F. Fang, B. Kosel, and F. Aweeka. 2002. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 16:569-577. [DOI] [PubMed] [Google Scholar]
- 6.Gutmann, H., P. Hruz, C. Zimmermann, C. Beglinger, and J. Drewe. 2005. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem. Pharmacol. 70:695-699. [DOI] [PubMed] [Google Scholar]
- 7.Hagenbuch, B., and P. J. Meier. 2003. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta 1609:1-18. [DOI] [PubMed] [Google Scholar]
- 8.Hermann, M., A. Asberg, H. Christensen, H. Holdaas, A. Hartmann, and J. L. Reubsaet. 2004. Substantially elevated levels of atorvastatin and metabolites in cyclosporine-treated renal transplant recipients. Clin. Pharmacol. Ther. 76:388-391. [DOI] [PubMed] [Google Scholar]
- 9.Hicks, C. B., P. Cahn, D. A. Cooper, S. L. Walmsley, C. Katlama, B. Clotet, A. Lazzarin, M. A. Johnson, D. Neubacher, D. Mayers, and H. Valdez. 2006. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet 368:466-475. [DOI] [PubMed] [Google Scholar]
- 10.Hirano, M., K. Maeda, Y. Shitara, and Y. Sugiyama. 2004. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J. Pharmacol. Exp. Ther. 311:139-146. [DOI] [PubMed] [Google Scholar]
- 11.Hsyu, P. H., M. D. Schultz-Smith, J. H. Lillibridge, R. H. Lewis, and B. M. Kerr. 2001. Pharmacokinetic interactions between nelfinavir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and simvastatin. Antimicrob. Agents Chemother. 45:3445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, L., Y. Wang, and S. Grimm. 2006. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab. Dispos. 34:738-742. [DOI] [PubMed] [Google Scholar]
- 13.Keskitalo, J. E., O. Zolk, M. F. Fromm, K. J. Kurkinen, P. J. Neuvonen, and M. Niemi. 2009. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 86:197-203. [DOI] [PubMed] [Google Scholar]
- 14.Kiser, J. J., J. G. Gerber, J. A. Predhomme, P. Wolfe, D. M. Flynn, and D. W. Hoody. 2008. Drug/drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. J. Acquir. Immune Defic. Syndr. 47:570-578. [DOI] [PubMed] [Google Scholar]
- 15.Lennernäs, H. 2003. Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 42:1141-1160. [DOI] [PubMed] [Google Scholar]
- 16.MacGregor, T. R., J. P. Sabo, S. H. Norris, P. Johnson, L. Galitz, and S. McCallister. 2004. Pharmacokinetic characterization of different dose combinations of coadministered tipranavir and ritonavir in healthy volunteers. HIV Clin. Trials 5:371-382. [DOI] [PubMed] [Google Scholar]
- 17.Maliepaard, M., G. L. Scheffer, I. F. Faneyte, M. A. van Gastelen, A. C. Pijnenborg, A. H. Schinkel, M. J. van De Vijver, R. J. Scheper, and J. H. Schellens. 2001. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61:3458-3464. [PubMed] [Google Scholar]
- 18.Maltz, H. C., D. L. Balog, and J. S. Cheigh. 1999. Rhabdomyolysis associated with concomitant use of atorvastatin and cyclosporine. Ann. Pharmacother. 33:1176-1179. [DOI] [PubMed] [Google Scholar]
- 19.Matsson, P., G. Englund, G. Ahlin, C. A. Bergstrom, U. Norinder, and P. Artursson. 2007. A global drug inhibition pattern for the human ATP-binding cassette transporter breast cancer resistance protein (ABCG2). J. Pharmacol. Exp. Ther. 323:19-30. [DOI] [PubMed] [Google Scholar]
- 20.Merino, G., J. W. Jonker, E. Wagenaar, A. E. van Herwaarden, and A. H. Schinkel. 2005. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol. Pharmacol. 67:1758-1764. [DOI] [PubMed] [Google Scholar]
- 21.Omar, M. A., and J. P. Wilson. 2002. FDA adverse event reports on statin-associated rhabdomyolysis. Ann. Pharmacother. 36:288-295. [DOI] [PubMed] [Google Scholar]
- 22.Penzak, S. R., J. M. Shen, R. M. Alfaro, A. T. Remaley, V. Natarajan, and J. Falloon. 2004. Ritonavir decreases the nonrenal clearance of digoxin in healthy volunteers with known MDR1 genotypes. Ther. Drug Monit. 26:322-330. [DOI] [PubMed] [Google Scholar]
- 23.Posvar, E. L., L. L. Radulovic, D. D. Cilla, Jr., L. R. Whitfield, and A. J. Sedman. 1996. Tolerance and pharmacokinetics of single-dose atorvastatin, a potent inhibitor of HMG-CoA reductase, in healthy subjects. J. Clin. Pharmacol. 36:728-731. [DOI] [PubMed] [Google Scholar]
- 24.Simonson, S. G., A. Raza, P. D. Martin, P. D. Mitchell, J. A. Jarcho, C. D. Brown, A. S. Windass, and D. W. Schneck. 2004. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin. Pharmacol. Ther. 76:167-177. [DOI] [PubMed] [Google Scholar]
- 25.Tirona, R. G., B. F. Leake, A. W. Wolkoff, and R. B. Kim. 2003. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J. Pharmacol. Exp. Ther. 304:223-228. [DOI] [PubMed] [Google Scholar]
- 26.van Heeswijk, R. P., A. Veldkamp, J. W. Mulder, P. L. Meenhorst, J. M. Lange, J. H. Beijnen, and R. M. Hoetelmans. 2001. Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience. Antivir. Ther. 6:201-229. [PubMed] [Google Scholar]
- 27.Weiss, J., J. Rose, C. H. Storch, N. Ketabi-Kiyanvash, A. Sauer, W. E. Haefeli, and T. Efferth. 2007. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J. Antimicrob. Chemother. 59:238-245. [DOI] [PubMed] [Google Scholar]
- 28.Ye, Z. W., P. Augustijns, and P. Annaert. 2008. Cellular accumulation of cholyl-glycylamido-fluorescein in sandwich-cultured rat hepatocytes: kinetic characterization, transport mechanisms, and effect of human immunodeficiency virus protease inhibitors. Drug Metab. Dispos. 36:1315-1321. [DOI] [PubMed] [Google Scholar]