Abstract
Seventy-one human immunodeficiency virus-infected patients with tuberculosis who were receiving a rifampin (rifampicin)-containing regimen were initiated on treatment with efavirenz at 600 mg/day plus stavudine-lamivudine. Fasting efavirenz concentrations at 12 h after dosing (C12) were monitored. The mean ± standard deviation efavirenz C12 at weeks 6 and 12 and after rifampin discontinuation were 4.5 ± 4.3, 3.8 ± 3.5, and 3.5 ± 2.7 mg/liter, respectively. High body weight was associated with a low efavirenz C12 at weeks 6 and 12 (P = 0.003, r = −0.255). The efavirenz C12 regression prediction line at 1 mg/liter intercepted a mean body weight of 57.5 kg.
N2R was a randomized clinical trial comparing the clinical efficacies of two nonnucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy (HAART) regimens for patients coinfected with human immunodeficiency virus (HIV) and tuberculosis who received rifampin (rifampicin) (9). A secondary objective was to assess body weight as a cutoff for selecting the daily dosage of efavirenz. Patients were enrolled and followed through 60 weeks after HAART. Inclusion criteria were described previously (9). All patients were started on twice-daily stavudine-lamivudine and efavirenz at 600 mg/day at bedtime. The dosages of rifampin were 450 mg/day for body weights of ≤50 kg and 600 mg/day for body weights of >50 kg. Fasting efavirenz concentrations at 12 h after dosing (C12) were measured with a validated high-performance liquid chromatography assay at weeks 6 and 12 and at 6 to 12 weeks after rifampin discontinuation. This assay was developed at the Department of Clinical Pharmacology of the University Medical Center Nijmegen, Nijmegen, The Netherlands (5).
All statistical analyses were performed with SPSS version 11.5 (SPSS Inc., Chicago, IL). Pearson's correlations were used to study the relationships between parameters. Factors possibly predictive of efavirenz C12 and an elevated serum alanine aminotransferase (ALT) activity at week 12 were evaluated with a linear-regression model. Inter- or intrapatient variability was expressed as a coefficient of variation (CV). The institutional ethics committees of the Bamrasnaradura Infectious Diseases Institute and the Thai Ministry of Public Health approved this study.
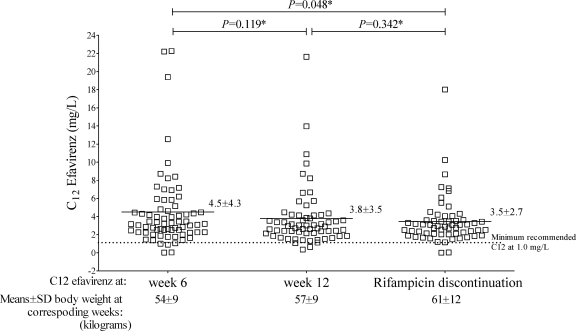
Seventy-one patients were enrolled; 65% were male, and the mean ± standard deviation (SD) baseline body weight was 53 ± 10 kg. The mean ± SD baseline CD4 cell count was 75 ± 68/μl, the median (interquartile range) log plasma HIV type 1 RNA copy number was 5.8 (range, 5.6 to 5.8), and the mean ± SD serum ALT activity was 29 ± 18 U/liter. Six (8.5%) patients were positive for hepatitis B surface antigen, and 18 (25.4%) were positive for hepatitis C antibody. The mean ± SD efavirenz C12 at weeks 6 and 12 and after rifampin discontinuation are shown in Fig. 1. After stratification into four groups by body weight (35 to 45, 46 to 55, 56 to 65, and >66 kg) at week 6, the mean C12 were 7.6 mg/liter, 4.1 mg/liter, 2.4 mg/liter, and 1.8 mg/liter, respectively. Three (4.3%) of 70, 2 (3.1%) of 64, and 2 (3.3%) of 60 patients, respectively, had efavirenz C12 of <1 mg/liter at weeks 6 and 12 and after rifampin discontinuation (P > 0.05). Twenty-four (34.3%), 16 (25.0%), and 14 (23.3%) patients had efavirenz C12 of >4 mg/liter at the corresponding periods (P > 0.05). High interpatient variability at weeks 6 (CV, 105%) and 12 (CV, 107%) and after rifampin discontinuation (CV, 77%), as well as high intrapatient variability between weeks 6 and 12 (CV, 136%) were observed in the efavirenz C12. Figure 2 shows the relationship between efavirenz C12 at the different time points. In the separate multivariate analysis model, at the different time points, low C12 at weeks 6 and 12 were associated with higher body weights (P = 0.028), as shown in Table 1. This correlation was not significant (P = 0.244) after discontinuation of rifampin.
FIG. 1.
Distributions of efavirenz C12 after 6 and 12 weeks of HAART (in patients concurrently receiving efavirenz and rifampin) and after rifampin discontinuation. *, P value for comparison of efavirenz C12 means between time points by repeated-measurement analysis.
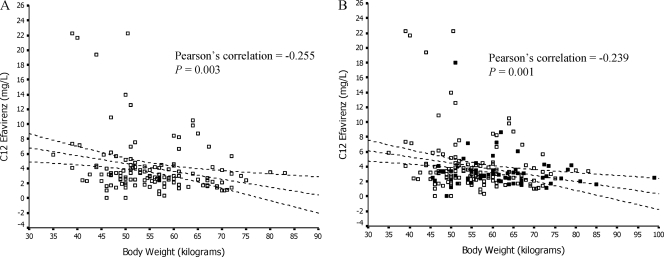
FIG. 2.
Relationship between body weight at timing of concentration and pooled efavirenz C12 at weeks 6 and 12 (in patients receiving efavirenz and rifampin) (A) and at weeks 6 and 12 and after rifampin discontinuation (B). Broken lines represent regression prediction and 95% confidence intervals for the mean. Open squares represent C12 at weeks 6 and 12, and filled squares represent C12 after rifampin discontinuation. There appears to be a relationship between high body weight and low combined efavirenz C12 at weeks 6 and 12 (P = 0.003, r = −0.255). Conversely, when the regression line was plotted by defining efavirenz C12 as an independent variable, efavirenz C12 at 1 mg/liter intercepted body weight at a mean of 57.5 (95% CI, 54.9 to 60.1) kg. The same trends were found at weeks 6 (P = 0.033, r = −0.257) and 12 (P = 0.058, r = −0.234) and were also found in efavirenz C12 at weeks 6 and 12 and after rifampin discontinuation, as shown in panel B (P = 0.001, r = −0.239).
TABLE 1.
Summary of multivariate analysis of efavirenz concentration and patients' parameters at weeks 6 and 12
| Parameter | Efavirenz C12 at:
|
|||||
|---|---|---|---|---|---|---|
| Wk 6
|
Wk12
|
|||||
| Beta | 95% CI | P value | Beta | 95% CI | P value | |
| Body wt | −0.289 | −0.254 to − 0.015 | 0.028 | −0.310 | −0.207 to − 0.019 | 0.020 |
| Baseline serum creatinine | 0.122 | −3.988 to 10.284 | 0.381 | 0.192 | −0.006 to 0.049 | 0.125 |
| Age | −0.095 | −0.183 to 0.081 | 0.440 | 0.176 | −0.030 to 0.175 | 0.162 |
| Serum ALT | −0.008 | −0.006 to 0.005 | 0.947 | 0.103 | −3.388 to 7.821 | 0.432 |
By intent-to-treat and on-treatment analyses at 60 weeks, 70.4% (50 of 71) and 81.9% (50 of 61) of the patients achieved HIV type 1 RNA levels of <50 copies/ml, respectively. Two (66.7%) of 3 patients with efavirenz C12 of <1 mg/liter at week 6 and 18 (16.9%) of 67 patients with efavirenz C12 of ≥1 mg/liter at week 6 developed treatment failure. The mean ± SD serum ALT activity increased from the baseline at week 12 (38 ± 32 U/liter; P = 0.046, paired t test). At 12 weeks, two patients developed grade III hepatotoxicity. Multivariate analysis of ALT activity-predictive factors at week 12 showed that positivity for hepatitis B surface antigen was a predictor of high serum ALT activity during concurrent efavirenz and rifampin administration (P = 0.005). Three patients developed grade II/III cutaneous reactions.
The range of acceptable efavirenz C12 is currently proposed to be 1 to 4 mg/liter (7). To date, clinical trials evaluating weight-based cutoffs for efavirenz dosing during coadministration of rifampin are limited (1, 2, 6-8, 10, 12). In the present study, almost one-third of the patients had body weights of ≥60 kg during monitoring. Nevertheless, most achieved concentrations above the minimum recommended level. The efavirenz C12 decreased 16% relative to that at week 6, as patients gained an average of 3 kg of body weight, but this decrease did not change the percentage of patients with a C12 of <1 mg/liter. Interestingly, an efavirenz concentration of 1 mg/liter intercepted a mean body weight of 60 kg at the upper limit of the 95% confidence interval (CI) of the beta value of the correlation coefficient. Therefore, increasing efavirenz dosing, practically to 800 mg/day, in patients weighting >60 kg to compensate for the effect of rifampin coadministration may be considered.
Efavirenz concentrations persistently decreased from 25% to 46% for every 10-kg increase in body weight. In addition, a high body weight was found to be an important predictive factor of a low drug concentration. These models did not include gender because this parameter had a highly positively correlated with body weight (P < 0.001). A cross-sectional study showed that body weight was an independent predictive factor for efavirenz concentration (16). Furthermore, marked interpatient and intrapatient variabilities were observed in this study during concurrent the use of both drugs, and this trend was found to be consistent over time. The previous studies showed low intrapatient variability in patient receiving efavirenz alone (11). This may be explained by the extensive difference of genetic polymorphism that influences CYP2B6 enzyme expression (17).
A number of limitations should be acknowledged. First, this study collected C12 instead of the minimal drug concentration to assess drug exposure. A number of studies attempted to demonstrate the ability of C12 to predict clinical efficacy and adverse reactions (3, 11). Second, there are no studies that clearly show that therapeutic drug monitoring improved patients' clinical outcomes. Third, a persons with the CYP2B6 polymorphism 516G>T had higher efavirenz concentrations (4, 13, 15). The frequency of this allele ranges from 15% in Asians to 50% in Africans (4, 15). Ultimately, previous studies demonstrated that many factors and issues contribute to pharmacokinetic variability, including biological factors, environmental factors, and genetic issues (14).
A standard-dose efavirenz-based regimen is appropriate for HIV patients with tuberculosis who are receiving rifampin and have body weights of <60 kg. In clinical practice, a weight-based cutoff for efavirenz dosing is a practical therapeutic approach. However, further study is needed to explore the appropriate escalating daily efavirenz dosage in patients with higher body weights. In addition, the issue of ethnic pharmacogenetic differences should be taken into account.
Acknowledgments
This study was funded by grants from the Thailand Research Fund (TRF); the Ministry of Public Health, Thailand; and the Bamrasnaradura Infectious Diseases Institute, Thailand.
We thank all of the patients in this study, as well as Achara Chaovavanich, Palakorn Srinithiwat, Somsit Tansuphaswadikul, Samruay Nilkamhang, Phatchara Tunteerapat, Putthiporn Limpanadusadee, and all of the other staff of the Bamrasnaradura Infectious Diseases Institute, for their support and assistance in conducting this study. We also acknowledge David M. Butler for his comments and help in reviewing the manuscript.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print on 10 August 2009.
REFERENCES
- 1.Brennan-Benson, P., R. Lyus, T. Harrison, M. Pakianathan, and D. Macallan. 2005. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS 19:1541-1543. [DOI] [PubMed] [Google Scholar]
- 2.Friedland, G., S. Khoo, C. Jack, and U. Lalloo. 2006. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J. Antimicrob. Chemother. 58:1299-1302. [DOI] [PubMed] [Google Scholar]
- 3.González de Requena, D., O. Gallego, A. Corral, I. Jimenez-Nacher, and V. Soriano. 2004. Higher efavirenz concentrations determine the response to viruses carrying non-nucleoside reverse transcriptase resistance mutations. AIDS 18:2091-2094. [DOI] [PubMed] [Google Scholar]
- 4.Haas, D. W., H. J. Ribaudo, R. B. Kim, C. Tierney, G. R. Wilkinson, R. M. Gulick, D. B. Clifford, T. Hulgan, C. Marzolini, and E. P. Acosta. 2004. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 18:2391-2400. [PubMed] [Google Scholar]
- 5.Hollanders, R. M., E. W. van Ewijk-Beneken Kolmer, D. M. Burger, E. W. Wuis, P. P. Koopmans, and Y. A. Hekster. 2000. Determination of nevirapine, an HIV-1 non-nucleoside reverse transcriptase inhibitor, in human plasma by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 744:65-71. [DOI] [PubMed] [Google Scholar]
- 6.Lopéz-Cortés, L. F., R. Ruiz-Valderas, P. Viciana, A. Alarcon-Gonzalez, J. Gomez-Mateos, E. Leon-Jimenez, M. Sarasanacenta, Y. Lopez-Pua, and J. Pachon. 2002. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin. Pharmacokinet. 41:681-690. [DOI] [PubMed] [Google Scholar]
- 7.Manosuthi, W., S. Kiertiburanakul, S. Sungkanuparph, K. Ruxrungtham, A. Vibhagool, S. Rattanasiri, and A. Thakkinstian. 2006. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS 20:131-132. [DOI] [PubMed] [Google Scholar]
- 8.Manosuthi, W., W. Mankatitham, A. Lueangniyomkul, S. Chimsuntorn, and S. Sungkanuparph. 2008. Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicin. HIV Med. 9:294-299. [DOI] [PubMed] [Google Scholar]
- 9.Manosuthi, W., S. Sungkanuparph, P. Tantanathip, A. Lueangniyomkul, W. Mankatitham, W. Prasithsirskul, S. Burapatarawong, S. Thongyen, S. Likanonsakul, U. Thawornwa, V. Prommool, and K. Ruxrungtham. 2009. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R Study. Clin. Infect. Dis. 48:1752-1759. [DOI] [PubMed] [Google Scholar]
- 10.Manosuthi, W., S. Sungkanuparph, A. Thakkinstian, A. Vibhagool, S. Kiertiburanakul, S. Rattanasiri, W. Prasithsirikul, J. Sankote, A. Mahanontharit, and K. Ruxrungtham. 2005. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS 19:1481-1486. [DOI] [PubMed] [Google Scholar]
- 11.Marzolini, C., A. Telenti, L. A. Decosterd, G. Greub, J. Biollaz, and T. Buclin. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 12.Matteelli, A., M. Regazzi, P. Villani, G. De Iaco, M. Cusato, A. C. Carvalho, S. Caligaris, L. Tomasoni, M. Manfrin, S. Capone, and G. Carosi. 2007. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr. HIV Res. 5:349-353. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran, G., A. K. Hemanth Kumar, S. Rajasekaran, P. Kumar, K. Ramesh, S. Anitha, G. Narendran, P. Menon, C. Gomathi, and S. Swaminathan. 2009. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob. Agents Chemother. 53:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotger, M., C. Csajka, and A. Telenti. 2006. Genetic, ethnic, and gender differences in the pharmacokinetics of antiretroviral agents. Curr. HIV/AIDS Rep. 3:118-125. [DOI] [PubMed] [Google Scholar]
- 15.Rotger, M., H. Tegude, S. Colombo, M. Cavassini, H. Furrer, L. Decosterd, J. Blievernicht, T. Saussele, H. F. Gunthard, M. Schwab, M. Eichelbaum, A. Telenti, and U. M. Zanger. 2007. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin. Pharmacol. Ther. 81:557-566. [DOI] [PubMed] [Google Scholar]
- 16.Stöhr, W., D. Back, D. Dunn, C. Sabin, A. Winston, R. Gilson, D. Pillay, T. Hill, J. Ainsworth, A. Pozniak, C. Leen, L. Bansi, M. Fisher, C. Orkin, J. Anderson, M. Johnson, P. Easterbrook, S. Gibbons, and S. Khoo. 2008. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir. Ther. 13:675-685. [PubMed] [Google Scholar]
- 17.Veldkamp, A. I., G. J. Weverling, J. M. Lange, J. S. Montaner, P. Reiss, D. A. Cooper, S. Vella, D. Hall, J. H. Beijnen, and R. M. Hoetelmans. 2001. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS 15:1089-1095. [DOI] [PubMed] [Google Scholar]