Abstract
A sensitive and reliable liquid chromatographic method was developed and validated for the determination of colistin concentrations in mouse brain homogenate. With a mobile phase consisting of acetonitrile-tetrahydrofuran-water (50:25:25 [vol/vol]) at a flow rate of 1 ml/min, a linear correlation between peak area and colistin concentration was observed over the concentration range of 93.8 to 3,000 ng/g in brain tissue (R2 > 0.994). Intra- and interday coefficients of variation were 5.1 to 8.3% and 5.8 to 8.5%, respectively, and the recovery ranged from 85% to 94%. This assay was then utilized to determine the amount of colistin that permeated the blood-brain barrier over a 2-h period following bolus intravenous administration of colistin sulfate to mice. After a single dose of 5 mg/kg of body weight to mice, brain homogenate concentrations of colistin were very low, relative to plasma colistin concentrations, suggesting that colistin permeability across the healthy blood-brain barrier is negligible during this experimental period.
Colistin (also known as polymyxin E) is a cationic polypeptide antibiotic and is bactericidal against multidrug-resistant gram-negative pathogens (8). Like other members of the polymyxin family, colistin consists of two major components (colistins A and B), with colistins A and B differing only in the fatty acid side chain. In studies conducted several decades ago, colistin was shown to result in adverse effects after intramuscular or intravenous administration to patients (6, 14); nephrotoxicity and neurotoxicity were the most commonly observed adverse effects. It is possible that the prevalence of such toxicity may have been exaggerated due to a lack of understanding of colistin pharmacology and the use of inappropriate doses (22). Over the last few years, however, resistance to many other antibiotics and limited development of new antibiotics have resulted in an increasing use of colistin, administered in the form of its inactive prodrug, colistin methanesulfonate (CMS) (1), for the treatment of infections caused by gram-negative pathogens, such as Pseudomonas aeruginosa and Acinetobacter baumannii (5). With its increased use, interest in the pharmacology of this old antibiotic has been rekindled and optimization of dosage regimens is therefore urgently required in order to maximize its efficacy while minimizing potential toxicity.
Gram-negative bacterial species are increasingly reported to cause severe central nervous system (CNS) infections, such as meningitis (11, 24, 26) and ventriculitis (7, 30), which have a high mortality rate if untreated or treated inappropriately. There have been several clinical case reports of successful treatment of meningitis and ventriculitis by parenteral administration of CMS, either alone or in combination with other antibacterials (11, 12, 18), resulting in eradication of the gram-negative pathogens from the cerebrospinal fluid (CSF). These studies suggest that CMS, or colistin that is generated from CMS in vivo, has the potential to permeate the blood-CSF barrier (BCSFB). Indeed, using a microbiological assay, Jiménez-Mejías et al. were able to detect “colistin” in human CSF following intravenous administration of CMS (12). However, less is known about the ability of colistin to penetrate the blood-brain barrier (BBB), the endothelial lining of the cerebral blood vessels—a process which would be required in order to treat infections of the brain parenchyma, such as meningoencephalitis (27). A more thorough understanding of colistin penetration into the brain parenchyma, therefore, would be useful for optimizing treatment of such infections. In addition, the neurotoxicity associated with colistin (2) warrants a better understanding of colistin penetration across the BBB so that more rational dosing regimens can be designed with minimal potential for CNS-mediated toxicity. However, in order to accurately determine brain penetration of colistin, a robust and reliable analytical technique is required.
Numerous methods based on microbiological (15, 16, 19), thin-layer chromatographic (TLC) (32), immunological (13), and liquid chromatography and mass spectrometry (10, 25) techniques have been developed to quantitate the polypeptide antibiotics in plasma and tissues. While liquid chromatography and mass spectrometry methods are quite sensitive, they are often not very accessible, and the other techniques listed above often lack sensitivity or selectivity required for accurate quantitation. With respect to colistin, several methods for assaying this polypeptide in rat or human plasma have been developed utilizing derivatizing reagents such as orthophthalaldehyde (4, 17) and 9-fluorenylmethyl chloroformate (FMOC-Cl) (20, 21). While these assays have been successfully used to measure concentrations of colistin in human and rodent plasma and some tissue samples (34), quantitative assays for measuring the concentration of this antibiotic in animal and human brain tissues are not available.
The purpose of this study, therefore, was to develop a reliable and sensitive method to quantify colistin in mouse brain homogenate. Our method consists of protein precipitation with trichloroacetic acid (TCA), solid-phase extraction (SPE) of colistin from brain tissue, and derivatization with FMOC-Cl, followed by liquid chromatography with fluorescence detection. This method was then employed in our laboratory to determine the concentration of colistin in mouse brain homogenate following intravenous administration of colistin sulfate, to determine the potential of colistin to penetrate the BBB in healthy mice.
MATERIALS AND METHODS
Chemicals and reagents.
Colistin sulfate was purchased from Zhejiang Shenghua Biok Biology Co., Ltd. (EP5 grade; Zhejiang, China). FMOC-Cl, TCA, boric acid, and sodium hydroxide were all of analytical grade and obtained from Sigma-Aldrich (Castle Hill, New South Wales, Australia), and sodium hydrogen carbonate (analytical grade) was from Merck Chemicals (Kilsyth, Victoria, Australia). Acetonitrile (ACN), methanol, and acetone of high-performance liquid chromatography (HPLC) grade were all obtained from Merck KGaA (Darmstadt, Germany), and tetrahydrofuran was purchased from Honeywell Burdick & Jackson (Muskegon, MI). Water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA). Carbonate buffer (1% [wt/wt], pH 10) was prepared as described previously (20). SPE cartridges (C18 Sep-Pak; 100 mg) were purchased from Waters Corporation, Milford, MA.
Preparation of stock solutions, working standards, and QC solutions.
A stock solution (1.00 mg/ml) of colistin sulfate was prepared in water and stored at 4°C. Working standard solutions with concentrations of 0.47, 0.94, 1.88, 3.75, 7.50, and 15.0 μg/ml were prepared by serial dilution of the stock solution with water. Quality control (QC) solutions were prepared separately at 0.47, 1.88, and 15.0 μg/ml, using an independently prepared stock solution (1.00 mg/ml).
Choice of protein precipitant: ACN versus TCA.
While ACN is often used to precipitate brain homogenate proteins, extraction recovery of the compound of interest may be low, and so we compared the efficiency of both ACN and TCA as brain homogenate protein precipitants for this assay. Blank mouse brains were homogenized in a volume of Milli-Q water (in ml) equal to twice the weight (in g) of the tissue using a Kinematica Polytron PT-DA 3007/2EC homogenizer (Luzernerstrasse, Switzerland). A 3,000-ng/g colistin-spiked homogenate was prepared by adding 20 μl of a 15.0-μg/ml working standard into 280 μl of blank brain homogenate. Aliquots of this homogenate sample were subjected to either an ACN or TCA-ACN pretreatment to precipitate proteins, as follows. To 300 μl of 3,000 ng/g colistin-spiked brain homogenate was added ACN at various volumes (300, 600, 900, or 1,200 μl) or various TCA solutions (750 μl of 2% [wt/vol] TCA, 300 μl of 5% TCA, 150 μl of 10% TCA, or 75 μl of 20% TCA). To TCA-treated homogenates, ACN was added at the same volume of TCA. Both ACN and TCA-ACN-treated brain homogenates underwent tissue processing as detailed below.
Preparation of calibration and QC samples.
Calibration and QC samples were prepared by adding a 20-μl aliquot of each working standard or QC solution into 280 μl of blank brain homogenate to achieve concentrations of 93.8, 188, 375, 750, 1,500, and 3,000 ng/g. To each mixture was added 300 μl of 5% TCA, given that this was found to be the most appropriate tissue precipitant (see Results). This mixture was vortex mixed for 5 min, and another 300 μl of ACN was added prior to further vortex mixing and centrifugation at 16,100 × g for 15 min. The entire supernatant was transferred to an SPE cartridge which had been conditioned by washing with 1 ml of acetone and 1 ml of methanol, followed by 1 ml of carbonate buffer. An aliquot (30 μl) of 100 mM FMOC-Cl and 80 μl of methanol were then added to the SPE cartridge, and after 10 min, colistin-FMOC derivatives were eluted with 900 μl of acetone. The eluent was vortex mixed for 1 min, and 40 μl of this solution was injected onto the HPLC column. The peak areas of the FMOC derivatives of colistins A and B were summed and employed for calibration (20). The low-, medium-, and high-QC samples of 93.8, 375, and 3,000 ng/g were prepared in the same manner, and intraday accuracy and precision were assessed. Intraday assay performance was determined by replicate analysis of six QC samples at each concentration, and interday assay parameters were determined by analysis of the QC samples on 3 separate days.
Chromatographic conditions.
All chromatographic analyses were performed on a Shimadzu HPLC system consisting of an LC-10ATvp pump, a SIL-10ADvp autoinjector, a DGU-14A degasser, and an RF-10AXL fluorescence detector (Shimadzu, Kyoto, Japan) connected to a data acquisition and processing system (Shimadzu CLASS VP 6.14 SP1). The analyses were performed at 25°C. Samples were injected onto a Phenomenex Onyx monolithic C18 column (5 by 4.6 mm) using a mobile phase of ACN-tetrahydrofuran-water (50:25:25 [vol/vol]). The flow rate was set at 1 ml/min, and colistin-FMOC was detected at an excitation wavelength of 265 nm and an emission wavelength of 315 nm. For the linear least-squares regression analysis of the calibration curve, data were weighted according to 1/response.
Determination of recovery.
Duplicate sets of colistin-spiked samples were prepared in either 300 μl of blank brain homogenate or the same volume of water. The concentrations used for determining recovery were 93.8, 375, and 3,000 ng/g. The recovery was calculated by comparing the peak areas of colistin derivatives extracted from brain homogenate-spiked samples to those obtained from water-spiked samples.
To mimic the extraction scenario that would be encountered with samples obtained from in vivo mouse brain uptake studies, another set of colistin-spiked brain homogenate samples were prepared and the recovery of colistin was measured after a 4-h incubation at 37°C. The reason for incubating colistin for 4 h in brain homogenate is that this would provide an opportunity for colistin to diffuse into the brain tissue and more closely resemble what would be expected in an in vivo brain uptake study. Brain homogenate (300 μl) at 93.8, 375, and 3,000 ng/g colistin was incubated in a 37°C shaking water bath for 4 h (120 rpm). After this period, water-spiked and brain homogenate-spiked samples underwent the extraction procedure described above and recovery was calculated in the same manner.
Mouse brain uptake study.
Animal experiments were approved by the Faculty of Pharmacy and Pharmaceutical Sciences Animal Ethics Committee and performed in accordance with the National Health and Medical Research Council guidelines for the care and use of animals for scientific purposes. On the day of the study, a colistin sulfate dosing solution was prepared by dissolving colistin sulfate in saline, which was filtered with a 0.2-μm-pore syringe filter (Minisart; Sartorius). The brain uptake study was carried out with 16 Swiss Outbred male mice (6 to 8 weeks of age and weighing 22 to 28 g). A 50-μl bolus dose of colistin was administered to each mouse by tail vein injection at a dose of 5 mg/kg. Approximately 4 min prior to tissue harvesting, mice were anesthetized with an intraperitoneal dose of 133 mg/kg ketamine and 10 mg/kg xylazine. At 5, 30, 60, or 120 min (n = 4 mice at each time point), blood was collected by cardiac puncture and centrifuged immediately to obtain plasma (∼200 μl per sample). Animals were humanely killed by cervical dislocation, and then the whole brain was removed. Plasma and whole brain were stored at −20°C until analysis.
On the day of analysis, the brain was thawed and homogenized as described above. A 280-μl sample was taken, and 20 μl of water was added, to match the homogenate standards. These brain homogenate samples were then processed as detailed above. Concentrations of colistin in mouse plasma were determined using the reported HPLC method (21), which was validated for mouse plasma in the present study. Precision and accuracy results from this assay validation (data not shown) demonstrate that the method for assaying colistin in rat plasma is transferable to mouse plasma.
RESULTS AND DISCUSSION
Choice of precipitant (ACN versus TCA) and chromatography.
Previously, HPLC methods for the measurement of colistin in rat and human plasma have been developed and validated using ACN to precipitate the proteins in plasma (20, 21). However, we were unable to use ACN for brain homogenate protein precipitation due to poor recovery of colistin, even at the highest concentration of colistin (3,000 ng/g) and the largest volume of ACN (1,200 μl). TCA, a widely used precipitating reagent in brain homogenate assays (3, 35), was consequently assessed for its ability to extract colistin at different concentrations and volumes, and the extents of recovery of colistin from each of the volumes and concentrations of TCA are shown in Table 1. An aliquot of 75 μl of 20% (wt/vol) TCA provided the lowest extraction of colistin, whereas 750 μl of 2% (wt/vol) TCA provided the highest extraction. However, to minimize the liquid handling on the SPE cartridge, it was deemed most appropriate to use 300 μl of 5% (wt/vol) TCA combined with the same volume of ACN, and this protocol was applied to carry out the assay validation. This extraction method provided satisfactory recovery at low, medium, and high concentrations of colistin (see below).
TABLE 1.
Average HPLC peak areas (mvolts · min) of colistin obtained following addition of TCA and ACN to colistin-spiked brain homogenatea
| TCA concn (% [wt/vol]) | Vol (μl) of:
|
Mean ± SD peak area of colistin (107)b | |
|---|---|---|---|
| TCA | ACN | ||
| 2 | 750 | 750 | 3.2 ± 0.3 |
| 5 | 300 | 300 | 3.1 ± 0.1 |
| 10 | 150 | 150 | 3.0 ± 0.4 |
| 20 | 75 | 75 | 1.9 ± 0.1 |
Shown are results from various combinations of TCA and ACN added to 300 μl of 3,000 ng/g colistin-spiked brain homogenate.
n = 3.
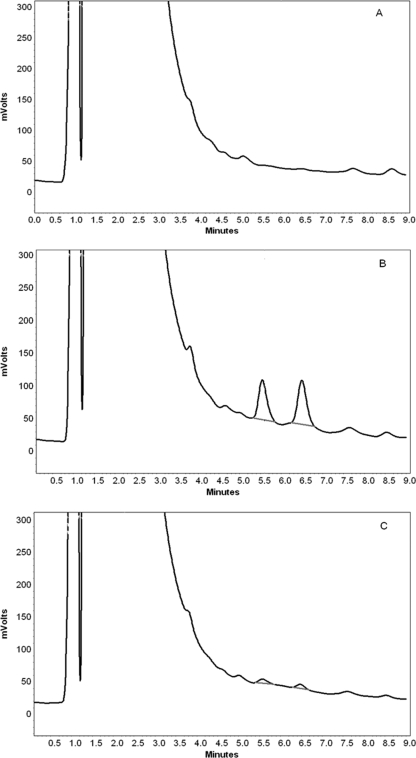
Under the current chromatographic conditions, well-resolved and symmetric peaks of FMOC derivatives of colistin A and colistin B were observed and there was no interference from components of the brain homogenate. The average retention times of FMOC derivatives of colistin B and colistin A were 5.6 and 6.5 min, respectively, while the run time was 9 min. Figure 1 shows representative chromatograms of blank brain homogenate, a colistin-spiked brain sample, and a brain homogenate sample obtained following intravenous administration of colistin sulfate (5 mg/kg) to a mouse.
FIG. 1.
Chromatograms of blank brain homogenate (A), brain homogenate spiked with 3,000 ng/g colistin (B), and brain homogenate at 5 min after intravenous administration of colistin sulfate to a mouse at a dose of 5 mg/kg (C). The colistin concentration in brain homogenate in this mouse was 220 ng/g.
Linearity, precision, and accuracy.
The calibration curve relating colistin response to brain homogenate concentration exhibited good linearity (R2 > 0.994) over the range of 93.8 to 3,000 ng/g. The lower limit of quantitation was 93.8 ng/g, as demonstrated by the satisfactory precision and accuracy values presented in Table 2. Intraday and interday levels of precision for QCs at three different concentrations were less than 8.3% and 8.5%, respectively, and the accuracy ranged from 92 to 108%. These performance characteristics demonstrate that this novel method was robust and reliable for the determination of colistin concentration in brain homogenate following in vivo administration.
TABLE 2.
Precision and accuracy values of the assay quantitating colistin in mouse brain homogenate
| Parameter and target colistin concn (ng/g) | Mean ± SD measured colistin concn (ng/g) | Precision (%) | Accuracy (%) |
|---|---|---|---|
| Intraday (n = 6) | |||
| 93.8 | 91.6 ± 7.2 | 7.9 | 97.7 |
| 375 | 384 ± 31.8 | 8.3 | 102 |
| 3,000 | 3,264 ± 167 | 5.1 | 108 |
| Interday (n = 3) | |||
| 93.8 | 97.5 ± 8.3 | 8.5 | 103.9 |
| 375 | 345 ± 27.6 | 8.0 | 92 |
| 3,000 | 3,210 ± 186 | 5.8 | 107 |
Recovery.
The values of recovery for colistin at concentrations of 93.8, 375, and 3,000 ng/g ranged from 85% to 94%. After a 4-h incubation of colistin sulfate in brain homogenate at 37°C, the values of recovery for the three corresponding concentrations were 87%, 86%, and 96%, respectively, indicating that colistin could be adequately recovered from brain tissue obtained from in vivo studies. While several HPLC methods have been reported for measuring colistin concentrations in human and rodent plasma (20, 21) and some tissue samples (34), the present method allows for excellent recovery and quantitative analysis of colistin in brain tissue. With some minor modification, this method may also be utilized to quantify the concentration of colistin (and other polymyxins) in other tissues from rodents.
Brain uptake of colistin following intravenous administration to mice.
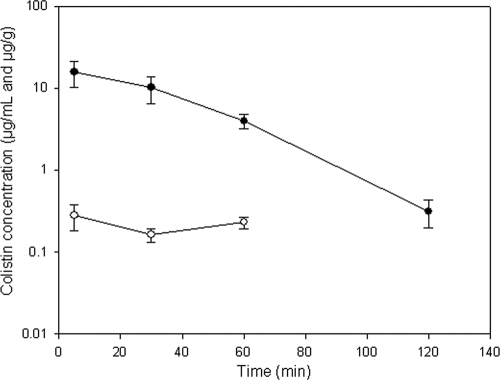
The brain and plasma concentrations of colistin at various times after intravenous administration of colistin sulfate to mice at a nominal dose of 5 mg/kg are shown in Fig. 2. Mice appeared to tolerate this dose with no observed toxicity during the whole experimental period, which is consistent with earlier findings (31). While the concentration of colistin in plasma declined rapidly over the 2-h experiment, the concentration of colistin in the brain homogenate remained relatively constant over a 90-min period, albeit the concentrations were relatively low. This resulted in an increasing brain/plasma ratio with time (ranging from 0.02 to 0.06), suggestive of potential accumulation of colistin within the brain. However, further studies with multiple dosing or intravenous infusions would be required to more clearly identify the potential of colistin to accumulate within the brain, as there are limited data in the present study to conclusively confirm this hypothesis.
FIG. 2.
Plasma (•; μg/ml) and brain homogenate (○; μg/g) concentrations of colistin after intravenous administration of colistin sulfate to Swiss Outbred mice at a dose of 5 mg/kg. At 120 min, the concentration of colistin in brain homogenate was below the lower limit of quantitation. Data are presented as means ± standard deviations (n = 4).
To accurately determine if a compound has permeated the BBB using brain homogenate studies, it is common to account for the concentration of compound remaining within the brain microvasculature (33). Our group has determined the brain microvascular volume in Swiss Outbred mice to be 0.026 ml/g, using [14C]inulin as a vascular marker (unpublished data), and when this volume is taken into account, the concentrations of colistin within the brain parenchyma are close to zero. This indicates that any colistin detected in the mouse brain homogenate following a single intravenous dose of colistin sulfate is likely to be restricted within the brain microvessels, suggesting poor penetration across the healthy BBB. However, as mentioned above, multiple doses of colistin (as prescribed clinically) may result in appreciable accumulation of this compound within the brain parenchyma, and future studies may be directed at addressing this issue.
The limited penetration of colistin into brain parenchyma following a single intravenous dose could be due to several factors. First, the average molecular mass of colistin (1,163 Da) far exceeds the “threshold” for optimum BBB permeability (450 Da) (9). Second, the logP values (where P is the octanol-to-water partition coefficient) of the two main components of colistin (i.e., colistin A and colistin B) are −3.15 and −3.68 (28), respectively, which are below the range of the logP values recommended for passive permeation of the BBB (9). Third, like many large cationic compounds, colistin may be actively excluded from the brain parenchyma by active efflux systems, such as P-glycoprotein (23). The mechanisms limiting the BBB penetration of colistin warrant further investigation so that appropriate measures can be taken not only to enhance colistin exposure for the treatment of life-threatening CNS infections, but also to minimize the potential for CNS-induced neurotoxicity when colistin is being used for the treatment of systemic infections.
While colistin has been reported to penetrate the human BCSFB (12), the current study demonstrates that colistin has minimal penetration into the brain parenchyma of healthy mice. The reason for this observation could be due to differences between the BBB and BCSFB transport of colistin, species differences associated with CNS exposure, or the fact that the BBB and BCSFB are intact in the healthy mice, which during infection may become compromised to allow greater CNS exposure of colistin (29). Therefore, further studies may be performed to determine whether the penetration of colistin into the brain parenchyma is enhanced during meningitis and/or meningoencephalitis, which may be considered beneficial when treating meningoencephalitis and other infections of the brain parenchyma.
The use of protein precipitation with TCA and derivatization with FMOC-Cl has allowed for the accurate and reproducible quantitation of colistin in mouse brain homogenate. This HPLC method is reliable and has been successfully applied to evaluate colistin penetration across the BBB in healthy mice. This method can be conveniently applied to determine whether the brain uptake of colistin is altered during meningitis and meningoencephalitis, which more closely mirrors the scenario encountered in patients, and may assist in the understanding of colistin efficacy and/or neurotoxicity in these infections.
Footnotes
Published ahead of print on 10 August 2009.
REFERENCES
- 1.Bergen, P. J., J. Li, C. R. Rayner, and R. L. Nation. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beringer, P. 2001. The clinical use of colistin in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 7:434-440. [DOI] [PubMed] [Google Scholar]
- 3.Castel-Branco, M. M., A. M. Almeida, A. C. Falcao, T. A. Macedo, M. M. Caramona, and F. G. Lopez. 2001. Lamotrigine analysis in blood and brain by high-performance liquid chromatography. J. Chromatogr. B 755:119-127. [DOI] [PubMed] [Google Scholar]
- 4.Decolin, D., P. Leroy, A. Nicolas, and P. Archimbault. 1997. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J. Chromatogr. Sci. 35:557-564. [DOI] [PubMed] [Google Scholar]
- 5.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960-967. [DOI] [PubMed] [Google Scholar]
- 6.Falagas, M. E., and S. K. Kasiakou. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit. Care. 10:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gump, W. C., and J. W. Walsh. 2005. Intrathecal colistin for treatment of highly resistant Pseudomonas ventriculitis. Case report and review of the literature. J. Neurosurg. 102:915-917. [DOI] [PubMed] [Google Scholar]
- 8.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hitchcock, S. A. 2008. Blood-brain barrier permeability considerations for CNS-targeted compound library design. Curr. Opin. Chem. Biol. 12:318-323. [DOI] [PubMed] [Google Scholar]
- 10.Jansson, B., M. Karvanen, O. Cars, D. Plachouras, and L. E. Friberg. 2009. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J. Pharm. Biomed. Anal. 49:760-767. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Mejías, M. E., B. Becerril, F. J. Marquez-Rivas, C. Pichardo, L. Cuberos, and J. Pachon. 2000. Successful treatment of multidrug-resistant Acinetobacter baumannii meningitis with intravenous colistin sulfomethate sodium. Eur. J. Clin. Microbiol. Infect. Dis. 19:970-971. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez-Mejías, M. E., C. Pichardo-Guerrero, F. J. Marquez-Rivas, D. Martin-Lozano, T. Prados, and J. Pachon. 2002. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 21:212-214. [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa, T., W. Ohtani, Y. Maeno, K. Fujiwara, and Y. Kimura. 1985. Sensitive enzyme immunoassay of colistin and its application to detect residual colistin in rainbow trout tissue. J. Assoc. Off. Anal. Chem. 68:661-664. [PubMed] [Google Scholar]
- 14.Koch-Weser, J., V. W. Sidel, E. B. Federman, P. Kanarek, D. C. Finer, and A. E. Eaton. 1970. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med. 72:857-868. [DOI] [PubMed] [Google Scholar]
- 15.Kunin, C. M. 1970. Binding of antibiotics to tissue homogenates. J. Infect. Dis. 121:55-64. [DOI] [PubMed] [Google Scholar]
- 16.Kunin, C. M., and A. Bugg. 1971. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body. J. Infect. Dis. 124:394-400. [DOI] [PubMed] [Google Scholar]
- 17.Le Brun, P. P., A. I. de Graaf, and A. A. Vinks. 2000. High-performance liquid chromatographic method for the determination of colistin in serum. Ther. Drug Monit. 22:589-593. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. Y., J. W. Lee, D. C. Jeong, S. Y. Chung, D. S. Chung, and J. H. Kang. 2008. Multidrug-resistant Acinetobacter meningitis in a 3-year-old boy treated with i.v. colistin. Pediatr. Int. 50:584-585. [DOI] [PubMed] [Google Scholar]
- 19.Leroy, P., D. Decolin, S. Nicolas, P. Archimbault, and A. Nicolas. 1989. Residue determination of two co-administered antibacterial agents—cephalexin and colistin—in calf tissues using high-performance liquid chromatography and microbiological methods. J. Pharm. Biomed. Anal. 7:1837-1846. [DOI] [PubMed] [Google Scholar]
- 20.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, K. Coulthard, and D. W. Johnson. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B 761:167-175. [DOI] [PubMed] [Google Scholar]
- 21.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, T. C. Smeaton, and K. Coulthard. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J., R. L. Nation, J. D. Turnidge, R. W. Milne, K. Coulthard, C. R. Rayner, and D. L. Paterson. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589-601. [DOI] [PubMed] [Google Scholar]
- 23.Löscher, W., and H. Potschka. 2005. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2:86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowman, W., T. Kalk, C. N. Menezes, M. A. John, and M. P. Grobusch. 2008. A case of community-acquired Acinetobacter baumannii meningitis—has the threat moved beyond the hospital? J. Med. Microbiol. 57:676-678. [DOI] [PubMed] [Google Scholar]
- 25.Ma, Z., J. Wang, J. P. Gerber, and R. W. Milne. 2008. Determination of colistin in human plasma, urine and other biological samples using LC-MS/MS. J. Chromatogr. B 862:205-212. [DOI] [PubMed] [Google Scholar]
- 26.Metan, G., E. Alp, B. Aygen, and B. Sumerkan. 2007. Acinetobacter baumannii meningitis in post-neurosurgical patients: clinical outcome and impact of carbapenem resistance. J. Antimicrob. Chemother. 60:197-199. [DOI] [PubMed] [Google Scholar]
- 27.Michelet, C., S. L. Leib, D. Bentue-Ferrer, and M. G. Täuber. 1999. Comparative efficacies of antibiotics in a rat model of meningoencephalitis due to Listeria monocytogenes. Antimicrob. Agents Chemother. 43:1651-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrick, G. L. 2005. An introduction to medicinal chemistry, 3rd ed. Oxford University Press, Oxford, United Kingdom.
- 29.Quagliarello, V. J., and W. M. Scheld. 1993. New perspectives on bacterial meningitis. Clin. Infect. Dis. 17:603-608. [DOI] [PubMed] [Google Scholar]
- 30.Quinn, A. L., J. P. Parada, J. Belmares, and J. P. O'Keefe. 2005. Intrathecal colistin and sterilization of resistant Pseudomonas aeruginosa shunt infection. Ann. Pharmacother. 39:949-952. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz, B. S., M. R. Warren, F. A. Barkley, and L. Landis. 1959. Microbiological and pharmacological studies of colistin sulfate and sodium colistin methanesulfonate. Antibiot. Annu. 7:41-60. [PubMed] [Google Scholar]
- 32.Thomas, A. H., and J. M. Thomas. 1980. Use of the image analyser Optomax for the quantitative evaluation of antibiotics separated by gel electrophoresis and by thin-layer chromatography. J. Chromatogr. 195:297-302. [DOI] [PubMed] [Google Scholar]
- 33.Van Bree, J. B., A. G. De Boer, M. Danhof, and D. D. Breimer. 1992. Drug transport across the blood-brain barrier. II. Experimental techniques to study drug transport. Pharm. Weekbl. Sci. 14:338-348. [DOI] [PubMed] [Google Scholar]
- 34.Wan, E. C., C. Ho, D. W. Sin, and Y. C. Wong. 2006. Detection of residual bacitracin A, colistin A, and colistin B in milk and animal tissues by liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 385:181-188. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, Y., and W. M. Pardridge. 2001. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J. Neuroimmunol. 114:168-172. [DOI] [PubMed] [Google Scholar]