Abstract
A phylogenetic analysis was performed for 34 Aspergillus strains belonging to section Nigri. Molecular methods allowed for the correct classification into three different clades (A. niger, A. tubingensis, and A. foetidus). Correlation with in vitro itraconazole susceptibility distinguished the following three profiles: susceptible, resistant, and showing a paradoxical effect. A number of different species whose morphological features resemble those of A. niger showed unusual MICs to itraconazole that have never been described for the Aspergillus genus.
Black aspergilli are widely distributed in nature (16); they are common food spoilers but are also well used for industrial purposes (15). Among Aspergillus species of the Nigri group, A. niger constitutes the most frequent etiological agent of otomycosis (13) and is considered the third cause of pulmonary aspergillosis (10). Nevertheless, the clinical implications of other species are rarely reported, and they are generally identified as A. niger (14, 22).
Clinically, identification of unknown Aspergillus clinical isolates to the species level may be important given that different species have dissimilar susceptibilities to antifungal drugs. Thus, the knowledge of the species identity may influence the choice of appropriate antifungal therapy (2, 4). Furthermore, since the antifungal susceptibility patterns for most of the species within section Nigri have been poorly investigated, their identification and antifungal susceptibility profiles appear to be of clinical interest for further research.
Black aspergilli belong to one of the most difficult groups concerning classification and identification (18), and so a number of different techniques have been developed in order to solve this issue. Among them, molecular tools are the gold standard (1, 18), as the sequencing of the β-tubulin or calmodulin gene is suitable, and enough, to discriminate between species within section Nigri (3, 18, 21).
Thirty-four Aspergillus section Nigri strains belonging to the Mold Collection of the Centro Nacional de Microbiologia and collected since 2004 were analyzed. Thirty-three strains were independent clinical isolates, and 1 had an environmental origin. All strains were identified as A. niger using conventional methods of morphology at the macroscopic as well as microscopic levels (9). Species identification analysis was addressed using sequences of the β-tubulin gene from all the strains included in this study together with the sequences of different Aspergillus section Nigri type strains and others that were available at GenBank as follows: A. tubingensis AY820007T, AY820009, and AY585527; A. foetidus AY585533T, AY585534, and DQ768454; and A. niger FJ629288T, EF422213, and AY585537. Partial sequences of the β-tubulin gene were amplified using the primer set βtubAniger1 and βtubANiger2 (11) and were carried out according to standard PCR guidelines (Applied Biosystems). Sequences were assembled and edited using the SeqMan II and EditSeq software packages (Lasergene 8.0; DNAStar, Inc., Madison, WI).
All phylogenetic analyses were conducted with InfoQuest FP software, version 4.50 (Bio-Rad). The methodology used was maximum parsimony clustering. Phylogram stability was assessed by using parsimony bootstrapping with 2,000 simulations and by using the Aspergillus clavatus AY214441T sequence as the out-group.
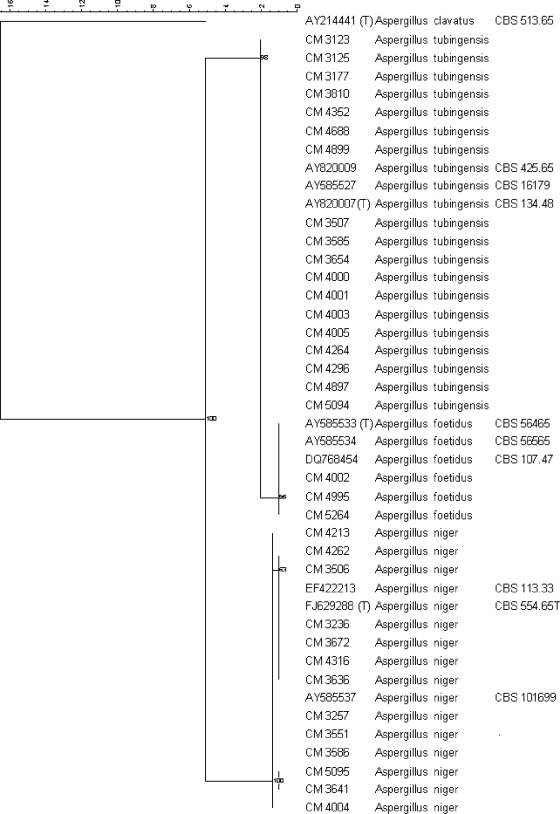
The phylogenetic tree grouped the 34 clinical isolates into three different clades consisting of 13 A. niger isolates, 18 A. tubingensis isolates, and 3 A. foetidus isolates (Fig. 1). Table 1 shows β-tubulin gene identification, as well as the origin and susceptibility profiles of the isolates.
FIG. 1.
Phylogenetic tree using maximum parsimony phylogenetic analysis and 2,000 bootstrap simulations based on β-tubulin gene sequences from all the Aspergillus section Nigri strains included in the study. Percentages indicate the bootstrap support for each group of sequences. (T), type strain.
TABLE 1.
Source, molecular identification, MICs, and MECs for species of Aspergillus section Nigria
| Isolate | Source | Molecular identification (β-tubulin gene) | MIC (mg/liter)b
|
MEC (mg/liter)c
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMB | ITC | VCZ | RVC | POS | TRB | CAS | MICA | |||
| Isolates of Aspergillus section Nigri showing low ITC MICs | ||||||||||
| CM-3236 | Respiratory | A. niger | 0.19 | 0.5 | 0.5 | 1.0 | 0.12 | 1.0 | 0.25 | 0.03 |
| CM-3257 | Respiratory | A. niger | 0.25 | 1.0 | 1.0 | 1.0. | 0.25 | 1.0 | 0.25 | 0.03 |
| CM-3506 | Respiratory | A. niger | 0.19 | 0.5 | 0.75 | 1.0 | 0.12 | 0.31 | 0.15 | 0.03 |
| CM-3507 | Respiratory | A. tubingensis | 0.19 | 0.5 | 1.0 | 1.5 | 0.15 | 0.62 | 0.06 | 0.03 |
| CM-3585 | Environmental | A. tubingensis | 0.19 | 0.5 | 1.0 | 1.67 | 0.12 | 0.42 | 0.37 | 0.03 |
| CM-3586 | Catheter | A. niger | 0.25 | 0.5 | 1.0 | 2.0 | 0.12 | 0.12 | 1.0 | 0.03 |
| CM-3636 | Respiratory | A. niger | 0.25 | 0.5 | 0.5 | 1.0 | 0.19 | 1.0 | 0.25 | 0.03 |
| CM-3641 | Respiratory | A. niger | 0.25 | 0.5 | 1.0 | 1.0 | 0.125 | 0.25 | 0.5 | 0.03 |
| CM-3672 | Cutaneous | A. niger | 0.12 | 0.5 | 1.0 | 1.5 | 0.19 | 0.07 | 0.15 | 0.03 |
| CM-4004 | Unknown | A. niger | 0.25 | 1.0 | 1.0 | 1.67 | 0.25 | 0.13 | 0.10 | 0.03 |
| CM-4213 | Respiratory | A. niger | 0.33 | 0.14 | 0.33 | 0.58 | 0.03 | 0.22 | 0.39 | 0.03 |
| CM-4264 | Blood culture | A. tubingensis | 0.12 | 0.5 | 1.0 | 1.5 | 0.12 | 0.50 | 0.03 | 0.03 |
| CM-4296 | Respiratory | A. tubingensis | 0.12 | 0.75 | 1.0 | 1.5 | 0.19 | 0.62 | 0.25 | 0.03 |
| CM-4316 | Respiratory | A. niger | 0.19 | 0.5 | 0.5 | 1.0 | 0.125 | 0.5 | 0.25 | 0.03 |
| CM-5094 | Respiratory | A. tubingensis | 0.12 | 0.5 | 0.75 | 2.0 | 0.06 | 0.62 | 0.19 | 0.03 |
| CM-5095 | Respiratory | A. niger | 0.19 | 0.5 | 0.75 | 1.5 | 0.12 | 0.62 | 0.25 | 0.03 |
| GM for group | 0.20 | 0.56 | 0.82 | 1.31 | 0.14 | 0.50 | 0.28 | 0.03 | ||
| Isolates of Aspergillus section Nigri showing much higher ITC MICs | ||||||||||
| CM-3123 | Respiratory | A. tubingensis | 0.25 | 11 | 1.67 | 2.67 | 0.25 | 1.17 | 0.25 | 0.03 |
| CM-3810 | Respiratory | A. tubingensis | 0.25 | 4.0 | 2.0 | 2.0 | 0.12 | 1.0 | 0.5 | 0.03 |
| CM-4003 | Unknown | A. tubingensis | 0.12 | 16 | 2.0 | 4.0 | 0.25 | 1.0 | 0.25 | 0.03 |
| CM-4005 | Unknown | A. tubingensis | 0.12 | 16 | 2.0 | 4.0 | 0.5 | 0.25 | 0.5 | 0.03 |
| CM-4688 | Respiratory | A. tubingensis | 0.21 | 3.67 | 2.0 | 3.33 | 0.25 | 1.50 | 0.18 | 0.03 |
| CM-5264 | Respiratory | A. foetidus | 0.12 | 16 | 2.0 | 8.0 | 0.5 | 0.5 | 0.06 | 0.03 |
| GM for group | 0.18 | 11.11 | 1.95 | 4.0 | 0.31 | 0.90 | 0.29 | 0.03 | ||
| Isolates of Aspergillus section Nigri showing paradoxical effect against ITC | ||||||||||
| CM-3125 | Respiratory | A. tubingensis | 0.12 | 0.5 | 1 | 1.67 | 0.12 | 0.5 | 0.05 | 0.03 |
| CM-3177 | Respiratory | A. tubingensis | 0.16 | 1 | 2 | 3.33 | 0.25 | 0.67 | 0.05 | 0.03 |
| CM-3551 | Respiratory | A. niger | 0.5 | 4.75 | 1 | 2 | 0.12 | 0.25 | 0.03 | 0.03 |
| CM-3654 | Blood culture | A. tubingensis | 0.19 | 1 | 2 | 2.50 | 0.25 | 0.63 | 0.14 | 0.03 |
| CM-4000 | Unknown | A. tubingensis | 0.16 | 1 | 2 | 2 | 0.25 | 0.33 | 0.10 | 0.03 |
| CM-4001 | Unknown | A. tubingensis | 0.19 | 1 | 1.75 | 2.50 | 0.25 | 0.56 | 0.11 | 0.03 |
| CM-4002 | Unknown | A. foetidus | 0.25 | 1 | 2 | 2.67 | 0.12 | 0.33 | 0.14 | 0.03 |
| CM-4262 | Ophthalmic | A. niger | 0.25 | 1 | 2 | 2 | 0.25 | 0.29 | 0.13 | 0.03 |
| CM-4352 | Respiratory | A. tubingensis | 0.28 | 1 | 0.88 | 2 | 0.25 | 0.31 | 0.15 | 0.03 |
| CM-4897 | Blood culture | A. tubingensis | 0.16 | 1 | 2 | 2 | 0.25 | 0.42 | 0.10 | 0.03 |
| CM-4899 | Respiratory | A. tubingensis | 0.16 | 1 | 2 | 2.67 | 0.25 | 0.33 | 0.10 | 0.05 |
| CM-4995 | Prosthesis | A. foetidus | 0.21 | 1 | 2 | 2 | 0.16 | 0.33 | 0.14 | 0.03 |
| GM for group | 0.2 | 1.3 | 1.72 | 2.28 | 0.21 | 0.41 | 0.10 | 0.03 | ||
GM, geometric means of MICs and MECs for the strains within each group.
MIC geometric mean of amphotericin B (AMB), itraconazole (ITC), voriconazole (VCZ), ravuconazole (RVC), posaconazole (POS), and terbinafine (TRB).
MEC geometric mean of caspofungin (CAS) and micafungin (MICA).
Antifungal susceptibility testing (AST) was performed following the EUCAST Definitive Document E.DEF 9.1 method for the determination of broth dilution MICs of antifungal agents for conidium-forming molds (17). Antifungal ranges used in the microdilution assays have been described previously (2). Endpoints were determined at 48 h. The endpoint for MEC determination was the minimal antifungal concentration that produced morphological alterations of hyphal growth at 48 h. The paradoxical effect to itraconazole was defined as an increase in growth occurring at least 2 drug dilutions above the MIC. AST was repeated at least twice on different days.
Three different antifungal patterns were clearly distinguishable based on the itraconazole MIC values (Table 1): low and high itraconazole MICs and a third group (12 strains) showing an uncommon paradoxical effect of this antifungal (5). Either those strains classified as paradoxical strains or those showing much higher itraconazole MICs also had higher MIC values to voriconazole and ravuconazole.
Posaconazole showed better activity in vitro. Moreover, all strains were susceptible to the rest of the following antifungals tested: amphotericin B, terbinafine, and echinocandins.
In summary, A. niger MICs for itraconazole, voriconazole, and ravuconazole were slightly higher than A. fumigatus MICs and even more so for A. tubingensis and A. foetidus MICs. Identification of clinical isolates belonging to Aspergillus section Nigri and involved in proven or probable infections should be to the species level because it is the only way to monitor the development of secondary resistances of these molds (7, 8).
The paradoxical effect or “Eagle effect” (12) has been previously described for yeasts or A. fumigatus but always in relation to echinocandins (5, 6, 19, 20). This is the first report showing the paradoxical effect of azole drugs against Aspergillus spp. The link between the paradoxical effect against itraconazole and a molecular mechanism responsible for it is yet to be determined, as is the clinical impact of those findings. Therefore, further studies including experimental models of aspergillosis to address any in vitro/in vivo correlations are warranted.
Nucleotide sequence accession numbers.
GenBank accession numbers for β-tubulin gene fragment sequences from all the strains used in this work are as follows: CM-3123:FJ828892, CM-3125:FJ828893, CM-3177:FJ828894, CM-3236:FJ828895, CM-3257:FJ828896, CM-3506:FJ828897, CM-3507:FJ828898, CM-3551:FJ828899, CM-3585:FJ828900, CM-3586:FJ828901, CM-3636:FJ828902, CM-3641:FJ828903, CM-3654:FJ828904, CM-3672:FJ828905, CM-3810:FJ828906, CM-4000:FJ828907, CM-4001:FJ828908, CM-4002:FJ828909, CM-4003:FJ828910, CM-4004:FJ828911, CM-4005:FJ828912, CM-4213:FJ828913, CM-4262:FJ828914, CM-4264FJ828915, CM-4296:FJ828916, CM-4316:FJ828917, CM-4352:FJ828918, CM-4688:FJ828919, CM-4897:FJ828920, CM-4899:FJ828921, CM-4995:FJ828922, CM-5094:FJ828923, CM-5095:FJ82892, and CM-5264:FJ828925.
Acknowledgments
This work was funded by SAF2008-04143 from the Ministerio de Ciencia e Innovacion (MICINN). L. Alcazar-Fuoli held a postdoctoral contract from the EU-STREP project (LSHM-CT-2005-518199). A. Alastruey-Izquierdo held a predoctoral fellowship (grant FI05/00856) from Fondo de Investigación Sanitaria (FIS) ISCIII.
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Abarca, M. L., F. Accensi, J. Cano, and F. J. Cabanes. 2004. Taxonomy and significance of black aspergilli. Antonie van Leeuwenhoek 86:33-49. [DOI] [PubMed] [Google Scholar]
- 2.Alcazar-Fuoli, L., E. Mellado, A. Alastruey-Izquierdo, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52:1244-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balajee, S. A., A. M. Borman, M. E. Brandt, J. Cano, M. Cuenca-Estrella, E. Dannaoui, J. Guarro, G. Haase, C. C. Kibbler, W. Meyer, K. O'Donnell, C. A. Petti, J. L. Rodriguez-Tudela, D. Sutton, A. Velegraki, and B. L. Wickes. 2009. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47:877-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balajee, S. A., J. Gribskov, M. Brandt, J. Ito, A. Fothergill, and K. A. Marr. 2005. Mistaken identity: Neosartorya pseudofischeri and its anamorph masquerading as Aspergillus fumigatus. J. Clin. Microbiol. 43:5996-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamilos, G., R. E. Lewis, N. Albert, and D. P. Kontoyiannis. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons, K. V., M. Espiritu, R. Parmar, and D. A. Stevens. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 50:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, A. Monzon, M. J. Buitrago, and J. L. Rodriguez-Tudela. 2009. Activity profile in vitro of micafungin against Spanish clinical isolates of common and emerging species of yeasts and molds. Antimicrob. Agents Chemother. 53:2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, M. J. Buitrago, A. Monzon, and J. L. Rodriguez-Tudela. 2006. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 50:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Hoog, G. S., J. Guarro, C. S. Tan, R. G. F. Wintermans, and J. Gené. 1995. Hyphomycetes, p. 308-1007. In G. S. de Hoog and J. Guarro (ed.), Atlas of clinical fungi. Facultat de Medicina, Universitat Rovira i Virgili, Reus, Spain.
- 10.Denning, D. W. 2006. Aspergillus spp. and aspergillosis—progress on many fronts. Med. Mycol. 44(Suppl. 1):S1-S2. [DOI] [PubMed] [Google Scholar]
- 11.Glass, N. L., and G. C. Donaldson. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, C. C. 1996. In vitro testing: correlation of bacterial susceptibility, body fluids levels and effectiveness of antibacterial therapy, p. 813-834. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams and Wilkins, Baltimore, MD.
- 13.Kaya, A. D., and N. Kiraz. 2007. In vitro susceptibilities of Aspergillus spp. causing otomycosis to amphotericin B, voriconazole and itraconazole. Mycoses 50:447-450. [DOI] [PubMed] [Google Scholar]
- 14.Paldrok, H. 1965. Report on a case of subcutaneous dissemination of Aspergillus niger, type awamori. Acta Derm. Venereol. 45:275-282. [PubMed] [Google Scholar]
- 15.Raper, K. B., and D. I. Fennell (ed.). 1965. The genus Aspergillus. Williams and Wilkins, Baltimore, MD.
- 16.Reiss, J. 1986. Physiologie, p. 33-41. In U. Kück, M. Nowrousian, B. Hoff, I. Engh, and J. Reiß (ed.), Schimmelpilze—Lebensweise, Nutzen, Schaden, Be-kämpfung. Centralbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 17.Rodriquez-Tudela, J.-L., J. P. Donnelly, M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Danaoui, D. Denning, W. Fegeler, P. Gaustad, C. Lass-Flörl, C. Moore, M. Richardson, A. Schmalreck, J. A. Velegraki, P. Verweij, and the Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2008. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982-984. [DOI] [PubMed] [Google Scholar]
- 18.Samson, R. A., P. Noonim, M. Meijer, J. Houbraken, J. C. Frisvad, and J. Varga. 2007. Diagnostic tools to identify black aspergilli. Stud. Mycol. 59:129-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens, D. A., M. J. McCullough, K. V. Clemons, and M. C. Martinez. 2006. Candida dubliniensis, a species with an extremely high frequency of paradoxical effect with caspofungin, abstr. M1756. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006.
- 20.Stevens, D. A., T. C. White, D. S. Perlin, and C. P. Selitrennikoff. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51:173-178. [DOI] [PubMed] [Google Scholar]
- 21.Susca, A., G. Stea, G. Mule, and G. Perrone. 2007. Polymerase chain reaction (PCR) identification of Aspergillus niger and Aspergillus tubingensis based on the calmodulin gene. Food Addit. Contam. 24:1154-1160. [DOI] [PubMed] [Google Scholar]
- 22.Williams, B., B. Popoola, and S. K. Ogundana. 1984. A possible new pathogenic Aspergillus isolation and general mycological properties of the fungus. Afr. J. Med. Med. Sci. 13:111-115. [PubMed] [Google Scholar]