Abstract
In an in vitro pharmacodynamic model, linezolid attenuated the activity of aztreonam and ceftazidime against Escherichia coli. Conversely, synergy was detected at 24 and 48 h when daptomycin or vancomycin was added to aztreonam and ceftazidime. We conclude that significant yet underappreciated interactions may occur between gram-positive-spectrum and gram-negative-spectrum antibacterials.
In 2007, the U.S. Food and Drug Administration issued a safety alert regarding the use of linezolid for presumed catheter-related bloodstream infections, noting higher mortality rates in patients treated with linezolid who were infected with gram-negative organisms during phase 3 trials (26; K. J. Tack, M. H. Wilcox, E. Bouza, D. H. Herr, M. M. Ijzerman, R. V. Croos-Dabrera, and C. Knirsch, presented at the 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Chicago, IL, 2007). Linezolid demonstrates minimal activity against gram-negative bacteria, so an explanation for worse outcomes in patients receiving linezolid is not obvious (22). However, there are cases of documented antagonism between beta-lactam agents and chloramphenicol, a drug similar to linezolid, by mechanisms of action (binding to domain V of the 50S subunit of rRNA) and resistance mechanisms (1, 12, 17, 19, 27, 28). Therefore, it is practical to hypothesize that beta-lactam agents may be antagonized in the presence of linezolid or other protein synthesis inhibitors. The aim of this study was to evaluate the in vitro activities of aztreonam and ceftazidime in the presence or absence of linezolid, daptomycin, and vancomycin against two Escherichia coli strains.
(This work was presented in part at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Washington, DC, 24 to 28 October 2008 [15].)
Escherichia coli ATCC 25922 and a clinical sputum isolate (L1035) were tested (13). Stock solutions of linezolid (lot no. 08E14Z99 [purchased commercially]), aztreonam (lot no. 124K1448), ceftazidime (lot no. 117K1286), vancomycin (lot no. 048K1457; Sigma-Aldrich, St Louis, MO), and daptomycin (lot no. CDCX01; Cubist Pharmaceuticals, Inc., Lexington, MA) were freshly prepared and kept frozen at −20°C. All in vitro experiments were performed with cation-adjusted Mueller-Hinton broth (Difco Laboratories, Sparks, MD), except for daptomycin, where the calcium concentration was adjusted to 50 mg/liter. MICs were determined by the Etest methodology and broth microdilution according to the Clinical and Laboratory Standards Institute (6, 7).
E. coli was tested against linezolid, aztreonam, and ceftazidime alone and in combination by using in vitro modeling, time kill analyses, and two-dimensional or checkerboard screening (mean fractional inhibitory concentration [FIC] index [∑FIC]) (5, 9).
Drug concentrations for time kill analyses were equal to one (0.19 mg/ml for aztreonam and 0.19 mg/ml for ceftazidime) and two (0.38 mg/ml for aztreonam and 0.38 mg/ml for ceftazidime) times the MIC of each drug. Linezolid (16 μg/ml) was used to simulate clinically achievable peak serum concentrations in patients administered 600 mg every 12 h (q12h) (10).
Additionally, a previously described one-compartment in vitro pharmacodynamic model (IVPM) provided continuous exposure of bacteria to changing concentrations of antibiotics (3, 29). All model simulations were conducted over 48 h and were performed at minimum in triplicate. Calculated free-drug concentrations were simulated from the following total pharmacokinetic concentrations: for aztreonam, 1 g q8h (half-life [t1/2], 2 h; maximum concentration of drug in serum [Cmax], 75 μg/ml; protein binding, 55%; free maximum concentration (fCmax), 34 μg/ml) (25); for ceftazidime, 1 g q8h (t1/2, 2.3 h; Cmax, 60 μg/ml; protein binding, 10%; fCmax, 54 μg/ml) (4); for daptomycin, 6 mg/kg of body weight q24h (t1/2, 8 h; Cmax, 98.6 μg/ml; protein binding, 92%; fCmax, 7.9 μg/ml) (8); for linezolid, 600 mg q12h; (t1/2, 6 h; Cmax, 18 μg/ml; protein binding, 31%; fCmax, 12.4 μg/ml) (23); and for vancomycin, 1.25 g q12h (t1/2, 6 h; Cmax, 45 μg/ml; minimum concentration of drug in serum, 15 to 20 μg/ml; protein binding, 55%; fCmax, 20.3 μg/ml) (18). A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) was used to continually replace antibiotic-containing medium with fresh Mueller-Hinton broth supplemented with 25 μg/ml calcium and 12.5 μg/ml magnesium (at a rate to simulate the t1/2 of the respective antibiotic). For combination regimens, the elimination rate was set for the drug with the shortest t1/2, and the drug with the longer t1/2 was supplemented (3). All samples were diluted 10-fold before plating in order to minimize antibiotic carryover. High-performance liquid chromatography and bioassays were conducted as previously described (2, 14, 21).
Synergy was defined as a ≥2-log10 decrease in number of CFU per milliliter between the combination and its most active constituent and the point at which the number of surviving organisms in the presence of the combination was ≥2 log10 CFU/ml below the level for the starting inoculum (11). Indifference was defined as a 1- to 2-log10-CFU/ml increase in kill in comparison to the level for the most active single agent. Combinations that resulted in >2-log10-CFU bacterial growth in comparison to the level for the most active single agent were considered antagonistic (11).
Samples taken from the model were also immediately plated onto tryptic soy agar, and susceptibility was determined using an Etest. In addition, a population analysis was conducted with aztreonam and ceftazidime against E. coli (≥109 CFU/ml) as previously described (20).
Changes in number of CFU/ml at 24 and 48 h and time to 99.9% kill were compared by two-way analysis of variance with Tukey's post hoc test, using SPSS Statistical Software (release 14; SPSS, Inc., Chicago, IL). A P value of ≤0.05 was considered significant.
Both E. coli strains were susceptible to aztreonam and ceftazidime, with MICs of <0.25 μg/ml and 0.125 μg/ml, respectively. The daptomycin, linezolid, and vancomycin MICs were >256 μg/ml. Population analyses (starting inoculum, 9.98 log10 CFU/ml) demonstrated homogeneous susceptibility to aztreonam and ceftazidime, with a population analysis profile MIC of ≤0.25 μg/ml.
For combinations of linezolid plus aztreonam or ceftazidime, FIC indices demonstrated indifference (FIC index = 1). Static time kill assays run at 1× and 2× the MIC with linezolid demonstrated mean decreases of +1.15 and +1.42 log10 kill for ceftazidime and aztreonam, respectively, in comparison to the level for the most active single agent, thus demonstrating indifference.
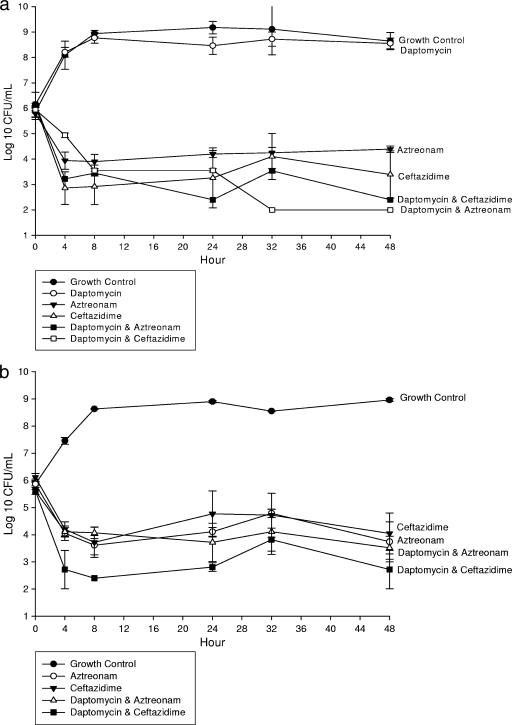
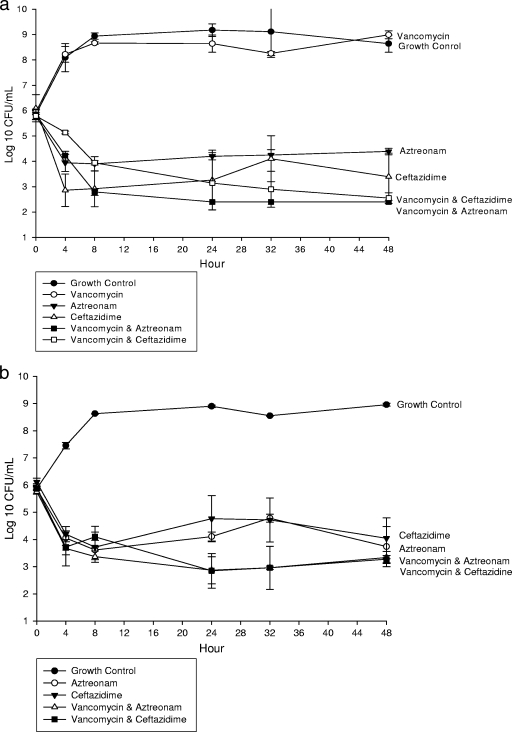
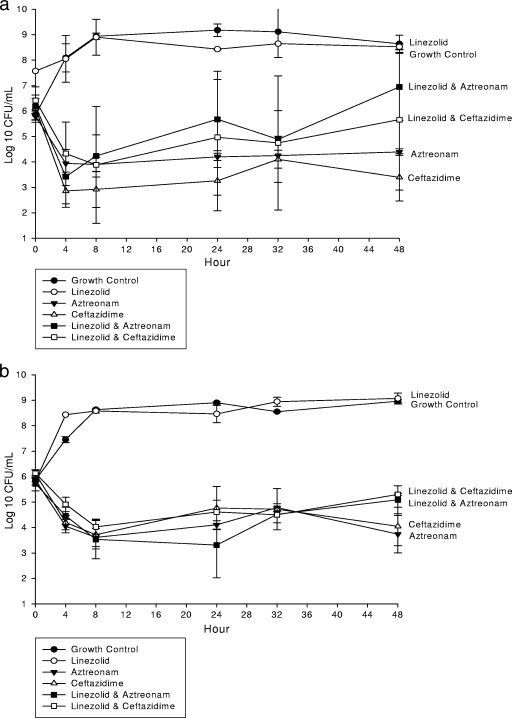
In an IVPM at clinically free therapeutic concentrations, aztreonam and ceftazidime alone and combined with daptomycin (Fig. 1) or vancomycin (Fig. 2) demonstrated significant (P ≤ 0.05) activity at 24 and 48 h. However, the combination of linezolid with aztreonam (Table 1 and Fig. 3a) or ceftazidime (Fig. 3b) resulted in decreased activity at 24 and 48 h and represented antagonism against ATCC 25922. Indifference was noted at 24 h. Daptomycin, vancomycin, and linezolid monotherapy demonstrated no significant activity against E. coli ATCC 25922 at any time point. However, daptomycin and vancomycin enhanced the activities of aztreonam and ceftazidime at 24 and 48 h. All concentrations achieved within the IVPM were within 15% of the targeted level.
FIG. 1.
In vitro activity of daptomycin alone and combined with aztreonam or ceftazidime against E. coli ATCC 25922 (a) and a clinical isolate (b). Results show log10 numbers of CFU/ml of activity ± standard deviations.
FIG. 2.
In vitro activity of linezolid alone and combined with aztreonam or ceftazidime against E. coli ATCC 25922 (a) and a clinical isolate (b).
TABLE 1.
Activity of each antibiotic alone and combined with aztreonam or ceftazidime in an IVPM over 24 and 48 h
| Antibiotic | Bacterial strain | Change in no. of CFU/ml relative to 0-h levela at:
|
|
|---|---|---|---|
| 24 h | 48 h | ||
| Growth control | ATCC 25922 | +3.33 ± 0.18 | +2.79 ± 0.33 |
| L1035 | +3.03 ± 0.05 | +3.09 ± 0.04 | |
| Aztreonam | ATCC 25922 | −1.59 ± 0.50 | −1.40 ± 0.52 |
| L1035 | −1.76 ± 0.50 | −2.12 ± 0.52 | |
| Ceftazidime | ATCC 25922 | −2.83 ± 0.29 | −2.70 ± 0.24 |
| L1035 | −1.35 ± 0.31 | −2.07 ± 0.30 | |
| Linezolid | ATCC 25922 | −0.86 ± 0.47 | +0.95 ± 0.41 |
| L1035 | +2.64 ± 0.16 | +3.25 ± 0.13 | |
| Daptomycin | ATCC 25922 | +2.31 ± 0.12 | +2.40 ± 0.10 |
| L1035 | NA | NA | |
| Vancomycin | ATCC 25922 | +2.70 ± 0.15 | +3.05 ± 0.13 |
| L1035 | NA | NA | |
| Aztreonam-linezolid | ATCC 25922 | −0.51 ± 0.66 (inhibited by 1.48 CFU/ml) | +0.77 ± 0.53 (inhibited by 2.56 CFU/ml; antagonism) |
| L1035 | −2.41 ± 0.46 (inhibited by 0.80 CFU/ml) | −0.63 ± 0.45 (inhibited by 0.60 CFU/ml) | |
| Ceftazidime-linezolid | ATCC 25922 | −1.45 ± 0.86 (inhibited by 1.71 CFU/ml) | −0.75 ± 0.88 (inhibited by 2.27 CFU/ml; antagonism) |
| L1035 | −1.53 ± 0.13 (inhibited by 0.16 CFU/ml) | −0.84 ± 0.15 (inhibited by 1.25 CFU/ml) | |
| Aztreonam-daptomycin | ATCC 25922 | −3.75 ± 0.03 (enhanced by 1.80 CFU/ml) | −4.15 ± 0.04 (enhanced by 2.39 CFU/ml; synergy) |
| Ceftazidime-daptomycin | L1035 | −1.89 ± 0.27 (enhanced by 0.39 CFU/ml) | −2.09 ± 0.30 (enhanced by 0.97 CFU/ml) |
| ATCC 25922 | −2.39 ± 0.07 (inhibited by 0.30 CFU/ml) | −3.94 ± 0.08 (enhanced by 1.39 CFU/ml) | |
| L1035 | −2.80 ± 0.31 (enhanced by 2.1 CFU/ml; synergy) | −2.89 ± 0.31 (enhanced by 1.33 CFU/ml) | |
| Aztreonam-vancomycin | ATCC 25922 | −3.45 ± 0.09 (enhanced by 1.80 CFU/ml) | −3.45 ± 0.10 (enhanced by 2.0 CFU/ml; synergy) |
| L1035 | −2.89 ± 0.25 (enhanced by 1.24 CFU/ml) | −2.42 ± 0.28 (enhanced by 1.15 CFU/ml) | |
| Ceftazidime-vancomycin | ATCC 25922 | −2.65 ± 0.48 (enhanced by 0.12 CFU/ml) | −3.24 ± 0.41 (enhanced by 0.84 CFU/ml) |
| L1035 | −3.02 ± 0.23 (enhanced by 1.92 CFU/ml) | −2.60 ± 0.22 (enhanced by 0.77 CFU/ml) | |
NA, not applicable.
FIG. 3.
In vitro activity of vancomycin alone and combined with aztreonam or ceftazidime against E. coli ATCC 25922 (a) and a clinical isolate (b).
In one of the eight experiments evaluating linezolid and aztreonam in the IVPM, aztreonam-resistant subpopulations of E. coli (MIC of 64 μg/ml) were detected at 4 and 24 h. This resistant phenotype was unstable, with three serial passages on antibiotic-free tryptic soy agar yielding E. coli with aztreonam MICs decreasing from 64 to 12 to 6 μg/ml. Subpopulations of E. coli with increased ceftazidime MICs were detected in one ceftazidime in vitro model experiment at 24 and 48 h (MICs of 0.125 to 2.0 μg/ml). This MIC shift was also unstable but accounts for the larger standard deviations observed in these experiments.
The 2007 Food and Drug Administration MedWatch alert and a recent publication highlighted that patients with catheter-related bloodstream infection treated with linezolid had an 84-day mortality rate of 21.5% (78/363), versus the comparator group rate of 16% (28/269) (26; K. J. Tack et al., presented at the 47th Annual ICAAC, Chicago, IL, 2007). It was found that baseline bacteremia caused by gram-negative organisms was a predictor of death (K. J. Tack et al., presented at the 47th Annual ICAAC, Chicago, IL, 2007).
Using three different methods, we examined the effects of daptomycin, vancomycin, and linezolid on the activities of aztreonam and ceftazidime against E. coli in vitro and found attenuation of the activities of aztreonam and ceftazidime against E. coli by linezolid and enhancement of aztreonam activity against E. coli by daptomycin and vancomycin. The reasons for these observations are not immediately clear. A partial explanation may lie in the well-accepted notion that bacteriostatic agents inhibit the activities of bactericidal agents. For example, one well-known clinical study (16) demonstrated that the addition of chlortetracycline attenuated the activity of penicillin in the treatment of patients with pneumococcal meningitis. Patients receiving combination penicillin-tetracycline had twofold-increased mortality compared with patients who received the same penicillin dose as monotherapy.
Antagonism has been observed with antibiotics that inhibit cell wall synthesis (e.g., beta-lactam) in combination with agents that inhibit protein synthesis. In the case of linezolid, the cell wall agent may be increasing the permeability of the cell wall, allowing more linezolid to enter and accumulate intracellularly in the E. coli, inhibiting protein synthesis appreciably and consequently antagonizing the beta-lactam agent. Previous studies demonstrate linezolid's activity via protein synthesis inhibition against gram-negative bacteria, including E. coli, when there is inhibition of RND-type efflux pumps (22).
While intriguing, the findings of this study must be interpreted with caution and viewed as exploratory. Other Enterobacteriaceae spp. and other antimicrobial classes must also be evaluated. It is noteworthy that in the study by Wilcox et al. (26), there was no apparent difference in microbiological outcome between treatment arms and there were no documented microbiological failures in either arm due to infection with gram-negative bacteria, raising the question of whether or not the different outcomes were related to the treatment issues which generated the hypothesis of this study.
Nevertheless, despite these limitations, the findings of this study have potential profound clinical implications. The use of combination therapy in empirical regimens or those targeting polymicrobial infections would require greater scrutiny and point toward potential pitfalls of the “more is better” approach that is sometimes employed clinically without supporting data. These findings also demonstrate inconsistencies in in vitro evaluations of the pharmacodynamic effects of antibiotic combinations. Finally, these data show the limitations of extrapolating results obtained from traditional in vitro methods, like checkerboards and simple time kill studies using broth, to antimicrobial pharmacodynamics in vivo.
In summary, this study provides preliminary evidence that antibiotics with spectra of activity limited to gram-positive bacteria may influence the bactericidal activities of aztreonam and ceftazidime against E. coli. Specifically, linezolid may attenuate the activities of cell wall agents against E. coli and may enhance the development of resistance to these agents. Further investigation of the effect of linezolid on the activities of other antibiotics used against other gram-negative bacteria is warranted.
Acknowledgments
We thank Suzanne Woodmansee for technical assistance.
This work was unfunded. Both authors have current and previous relationships with Pfizer, the makers of linezolid, and Cubist Pharmaceuticals, the makers of daptomycin.
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Asmar, B. I., M. Prainito, and A. S. Dajani. 1988. Antagonistic effect of chloramphenicol in combination with cefotaxime or ceftriaxone. Antimicrob. Agents Chemother. 32:1375-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechard, D. L., S. S. Hawkins, R. Dhruv, and L. T. Friedhoff. 1985. Penetration of aztreonam into human bronchial secretions. Antimicrob. Agents Chemother. 27:263-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh, M., H. Lode, K. Borner, G. Hoffken, J. Wagner, and P. Koeppe. 1988. Pharmacokinetics and serum bactericidal activity of vancomycin alone and in combination with ceftazidime in healthy volunteers. Antimicrob. Agents Chemother. 32:92-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonapace, C. R., J. A. Bosso, L. V. Friedrich, and R. L. White. 2002. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 44:363-366. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; 7th ed. Approved guideline M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th edition. Approved informational supplement M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliopoulos, G. M. 1989. Synergism and antagonism. Infect. Dis. Clin. N. Am. 3:399-406. [PubMed] [Google Scholar]
- 10.Gee, T., R. Ellis, G. Marshall, J. Andrews, J. Ashby, and R. Wise. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 45:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 12.Jawetz, E., J. B. Gunnison, R. S. Speck, and V. R. Coleman. 1951. Studies on antibiotic synergism and antagonism; the interference of chloramphenicol with the action of penicillin. AMA Arch. Intern. Med. 87:349-359. [DOI] [PubMed] [Google Scholar]
- 13.Lamp, K. C., and M. K. Vickers. 1998. Pharmacodynamics of ampicillin-sulbactam in an in vitro infection model against Escherichia coli strains with various levels of resistance. Antimicrob. Agents Chemother. 42:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaPlante, K. L., S. Woodmansee, and G. Sakoulas. 2008. Evaluation of antagonistic activity between linezolid & aztreonam or ceftazidime against Escherichia coli in an in vitro pharmacodynamic model, abstr. A-038, p. 10. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 16.Lepper, M. H., and H. F. Dowling. 1951. Treatment of pneumococcic meningitis with penicillin compared with penicillin plus aureomycin; studies including observations on an apparent antagonism between penicillin and aureomycin. AMA Arch. Intern. Med. 88:489-494. [DOI] [PubMed] [Google Scholar]
- 17.Lin, A. H., R. W. Murray, T. J. Vidmar, and K. R. Marotti. 1997. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 41:2127-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matzke, G. R., G. G. Zhanel, and D. R. Guay. 1986. Clinical pharmacokinetics of vancomycin. Clin. Pharmacokinet. 11:257-282. [DOI] [PubMed] [Google Scholar]
- 19.McKee, E. E., M. Ferguson, A. T. Bentley, and T. A. Marks. 2006. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 50:2042-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeltz, R. F., J. L. Schmidt, and B. J. Wilkinson. 2001. A microdilution plating method for population analysis of antibiotic-resistant staphylococci. Microb. Drug Resist. 7:289-295. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt, C. A., M. Carazzo, L. V. Laporta, C. F. Bittencourt, M. R. Santos, and M. Friedrich. 2008. Development and validation of an agar diffusion assay for determination of ceftazidime in pharmaceutical preparations. J. AOAC Int. 91:59-66. [PubMed] [Google Scholar]
- 22.Schumacher, A., R. Trittler, J. A. Bohnert, K. Kummerer, J. M. Pages, and W. V. Kern. 2007. Intracellular accumulation of linezolid in Escherichia coli, Citrobacter freundii and Enterobacter aerogenes: role of enhanced efflux pump activity and inactivation. J. Antimicrob. Chemother. 59:1261-1264. [DOI] [PubMed] [Google Scholar]
- 23.Stalker, D. J., G. L. Jungbluth, N. K. Hopkins, and D. H. Batts. 2003. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 51:1239-1246. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Vinks, A. A., R. N. van Rossem, R. A. Mathot, H. G. Heijerman, and J. W. Mouton. 2007. Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using Monte Carlo simulation. Antimicrob. Agents Chemother. 51:3049-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcox, M. H., K. J. Tack, E. Bouza, D. L. Herr, B. R. Ruf, M. M. Ijzerman, R. V. Croos-Dabrera, M. J. Kunkel, and C. Knirsch. 2009. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin. Infect. Dis. 48:203-212. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, D. N., F. Schluenzen, J. M. Harms, A. L. Starosta, S. R. Connell, and P. Fucini. 2008. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl. Acad. Sci. USA 105:13339-13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolter, N., A. M. Smith, D. J. Farrell, W. Schaffner, M. Moore, C. G. Whitney, J. H. Jorgensen, and K. P. Klugman. 2005. Novel mechanism of resistance to oxazolidinones, macrolides, and chloramphenicol in ribosomal protein L4 of the pneumococcus. Antimicrob. Agents Chemother. 49:3554-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinner, S. H., J. Blaser, B. B. Stone, and M. C. Groner. 1985. Use of an in-vitro kinetic model to study antibiotic combinations. J. Antimicrob. Chemother. 15(Suppl. A):221-226. [DOI] [PubMed] [Google Scholar]