Abstract
Clavulanic acid (CLA) exhibits low MICs against some Acinetobacter baumannii strains. The present study evaluates the efficacy of CLA in a murine model of A. baumannii pneumonia. For this purpose, two clinical strains, Ab11 and Ab51, were used; CLA MICs for these strains were 2 and 4 mg/liter, respectively, and the imipenem (IPM) MIC was 0.5 mg/liter for both. A pneumonia model in C57BL/6 mice was used. The CLA dosage (13 mg/kg of body weight given intraperitoneally) was chosen to reach a maximum concentration of the drug in serum similar to that in humans and a time during which the serum CLA concentration remained above the MIC equivalent to 40% of the interval between doses. Six groups (n = 15) were inoculated with Ab11 or Ab51 and were allocated to IPM or CLA therapy or to the untreated control group. In time-kill experiments, CLA was bactericidal only against Ab11 whereas IPM was bactericidal against both strains. CLA and IPM both decreased bacterial concentrations in lungs, 1.78 and 2.47 log10 CFU/g (P ≤ 0.001), respectively, in the experiments with Ab11 and 2.42 and 2.28 log10 CFU/g (P ≤ 0.001), respectively, with Ab51. IPM significantly increased the sterility of blood cultures over that for the controls with both strains (P ≤ 0.005); CLA had the same effect with Ab51 (P < 0.005) but not with Ab11 (P = 0.07). For the first time, we suggest that CLA may be used for the treatment of experimental severe A. baumannii infections.
Acinetobacter baumannii is a gram-negative, nonfermenting, nonmobile, strictly aerobic, oxidase-negative bacterium that is able to grow in general culture media without specific requirements (4) and is often associated with nosocomial infections and outbreaks (16, 21, 22). This pathogen can produce different types of infections, such as septicemia, endocarditis, meningitis, wound and skin and soft-tissue infections, urinary tract infections (4), and nosocomial pneumonia, especially in patients with mechanical ventilation (8, 26). The attributable mortality of A. baumannii infections ranges from 7.8% to 23% (17, 18).
The standard treatment for infections caused by A. baumannii has been imipenem (IPM). This pathogen exhibits a great adaptive capacity and a great ability to acquire numerous effective antibiotic resistance mechanisms, and it may be considered the paradigm of multiresistant nosocomial bacteria. The frequent isolation of strains with resistance to the most commonly used antimicrobials, including IPM, has prompted the evaluation of diverse therapeutic alternatives, such as sulbactam and colistin, which have shown efficacy similar to that of IPM, rifampin (rifampicin), and tigecycline and are under evaluation for the treatment of A. baumannii infections, and antimicrobial peptides, which are still in experimental preclinical studies. Thus, the absence of an optimal treatment necessitates the development of new therapeutic alternatives (44).
The commercial β-lactamase inhibitors (clavulanic acid [CLA], tazobactam, and sulbactam) generally have low antibiotic activity against most microorganisms and therefore are not used alone as antimicrobial agents. Sulbactam shows good bactericidal activity in vitro against A. baumannii, and it has been observed that the efficacy of sulbactam against susceptible strains is similar to that of IPM in an experimental pneumonia model (33). CLA was the first β-lactamase inhibitor to be used commercially (35). It is a suicide inhibitor, leading to an irreversible union with β-lactamase. This inhibitor has high affinity for class A β-lactamases, including TEM, SHV, and CTX enzymes.
Although the activity of CLA against A. baumannii is lower than that of sulbactam, two studies with 100 and 115 genetically different clinical strains have been published recently, showing a range of CLA MICs from 2 to 256 mg/liter, with MICs of ≤8 mg/liter for 29% and 40.9% of strains, respectively (3, 27); the MICs at which 50% of isolates were inhibited (MIC50s) and MIC90s ranged from 16 to 32 and 64 to 512 mg/liter, respectively (3, 27). In another study, the in vitro activities of various β-lactams, along with β-lactamase inhibitors, against 68 strains of Acinetobacter spp. were analyzed, showing that CLA was more active than other antimicrobials, such as ceftriaxone, cefotaxime, and piperacillin (41). In another work, nonclassical combinations of β-lactams and β-lactamase inhibitors were studied, and CLA increased the efficacy of the combinations tested, both in vitro and in vivo (45).
The aim of this study was to compare the efficacy of the β-lactamase inhibitor CLA with that of IPM in an experimental murine pneumonia model using two clinical Acinetobacter baumannii strains.
(This work was presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America, Washington, DC, 25 to 28 October 2008 [29a].)
MATERIALS AND METHODS
Bacterial strains.
Two clinical A. baumannii strains with different susceptibility patterns, Ab11 and Ab51, were selected from 244 Acinetobacter isolates characterized in a previous work (3, 5). The strains were identified phenotypically and were confirmed by amplified rRNA gene restriction analysis (43). The epidemiological relationships of the two Acinetobacter isolates were determined by repetitive extragenic palindromic-PCR (5). The strains were chosen due to their susceptibility to CLA and their ability to produce infections in the murine pneumonia model.
Antimicrobials and susceptibility tests.
CLA was obtained from GlaxoSmithKline (Madrid, Spain), IPM from Merck, Sharp & Dohme (Madrid, Spain), sulbactam from Pfizer (Orsay, France), and tazobactam from Wyeth Pharmaceuticals (Philadelphia, PA). For these antibiotics, MICs were determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) methods (9). The activities of CLA and IPM were tested using three different inocula: 105, 106, and 107 CFU/ml. For ampicillin, piperacillin, piperacillin-tazobactam, cefotaxime, ceftazidime, cefepime, meropenem, ciprofloxacin, trimethoprim-sulfamethoxazole, gentamicin, amikacin, and colistin, MICs were determined by Etest (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions.
Minimal bactericidal concentrations (MBCs) were determined by subculturing 100-μl aliquots from wells containing antimicrobial concentrations greater than or equal to the MIC of CLA or IPM onto antimicrobial-free Mueller-Hinton agar. Plates were incubated at 35°C for 24 to 48 h, and viable colonies were counted. The MBC was determined as the concentration that killed ≥99.9% of the initial inoculum.
Time-kill curves.
The in vitro bactericidal activities of CLA and IPM were measured using the time-kill method as described by the NCCLS (30). Briefly, 20 ml of MHBCA (Mueller-Hinton broth, cation adjusted; Becton Dickinson Microbiology Systems, Cockeysville, MD) was incubated at 37°C with a concentration of antibiotic equivalent to the MIC, twice the MIC, or four times the MIC (1×, 2×, or 4× MIC) and an inoculum of 5 × 105 CFU of strain Ab11 or Ab51/ml. As a control, tubes containing 20 ml of medium broth with the inoculum and without antibiotic were used. The bacterial concentration (expressed in log10 CFU per milliliter) was determined at 0, 2, 4, 8, and 24 h. An antibiotic was considered bactericidal when it produced a decrease of ≥3 log10 CFU/ml from the initial inoculum.
PAE.
The in vitro postantibiotic effect (PAE) was determined by exposing both strains to CLA and IPM at concentrations of 1×, 2×, and 4× MIC for 60 min in MHBCA. Antibiotic was removed by centrifuging three times at 4,500 × g for 10 min, removing the supernatant, and resuspending the pellet in prewarmed broth. The number of CFU per milliliter was counted at 0, 2, 4, 8, and 24 h. A growth control was performed in the same way without exposure to the antibiotic (12).
In vitro inactivation of CLA.
Twenty milliliters of MHBCA with 1×, 2×, and 4× MIC of CLA was inoculated with 5 × 105 CFU/ml of Ab11 or Ab51. The cultures were incubated at 37°C, and the concentration of CLA was determined at 0, 2, 4, 8, and 24 h. Aliquots (50 μl) of each culture were collected and centrifuged at 4,500 × g for 10 min, and the bacterium-free supernatant was analyzed. The CLA concentration was calculated with a bioassay using Klebsiella pneumoniae ATCC 29665 (31) as an indicator. Two 10-μl aliquots from each time point were loaded onto blank disks, and the size of the inhibition zone was measured. Concentrations were estimated by extrapolation from the standard curve. The intraday and interday variations of the assays are described below.
Studies of AmpC enzymes.
For the kinetics experiments, a total-protein extract was prepared by sonication of each strain. The steady-state kinetics parameters (Km- and maximum rate of metabolism [Vmax]-like parameters) for CLA were determined at 25°C using a Nicolette Evolution 300 spectrophotometer (Thermo Electron Corporation, Waltham, MA). Each rate was determined three times in phosphate-buffered saline, with quartz cuvettes with a 1-cm path length. The Km values were calculated as Ki values in competitive assays with nitrocefin (Oxoid Ltd., Basingstoke, Hampshire, England). The Vmax was calculated by considering a CLA concentration four times the Km (28).
Animals.
Immunocompetent C57BL/6 female mice, weighing 16 to 18 g, were obtained from the University of Seville; they had a sanitary status of MPF (murine pathogen free) and were assessed for genetic authenticity. Animals were housed in regulation cages with food and water ad libitum. The study was approved by the Ethics and Clinical Research Committee of the University Hospitals Virgen del Rocío.
Drug pharmacokinetics.
Several doses of CLA were assayed in mice in order to produce serum CLA concentrations similar to the maximum concentration in serum (Cmax) for humans (data not shown). Finally, the serum pharmacokinetics of CLA was determined after intraperitoneal administration of a single dose of 13 mg/kg of body weight. The dose of IPM (30 mg/kg given intramuscularly) was chosen because of its known efficacy against A. baumannii in this experimental pneumonia model (34). In both cases, after 5, 10, 15, 30, 60, 90, 120, and 240 min, blood was extracted from the periorbital plexuses of three anesthetized mice per time point. The serum drug concentrations were calculated by a bioassay using K. pneumoniae ATCC 29665 for CLA and Micrococcus luteus ATCC 9341 for IPM. The parameters determined were Cmax (expressed in milligrams per liter), the area under the concentration-time curve (AUC, expressed in micrograms·hour/liter), the terminal half-life (t1/2, expressed in hours) (37), and the time during which the serum CLA concentration remained above the MIC (TMIC, expressed in hours), which was estimated by extrapolation from the regression line of serum elimination using the MIC obtained (20). The intraday and interday variations of the assays were 3.21% ± 1.28% and 2.96% ± 2.92% for CLA and 2.62% ± 2.44% and 3.22% ± 1.91% for IPM; the linearity (r2) of the assay was 0.99 ± 0.01 and 0.97 ± 0.02, respectively; the lower limits of detection were 0.25 and 0.01 mg/liter.
Animal model.
An experimental murine pneumonia model (34) was used to evaluate the efficacies of CLA and IPM against A. baumannii strains. The animals were anesthetized intraperitoneally with 5% (wt/vol) sodium thiopental (Braun) and were inoculated with 50 μl of the bacterial suspension, for which the bacteria had been grown for 18 h in MHBCA at 37°C and had then been mixed 1:1 with a saline solution of porcine mucin at 10% (wt/vol). The final inoculum was 8.6 log10 CFU/ml for Ab11 and 8.58 log10 CFU/ml for Ab51. Treatments were begun 4 h after inoculation. Prior to the use of the pneumonia model, a group of 10 uninfected mice were treated with the dose regimen selected for CLA to evaluate its toxicity.
To ascertain the efficacy of CLA, 45 mice were inoculated with each strain and were randomly allocated to three groups of 15 animals. The first group did not receive antimicrobial treatment and was used as a control. The other two groups were treated with CLA (13 mg/kg, given intraperitoneally) or IPM (30 mg/kg, given intramuscularly). Because both antimicrobials assayed are β-lactams, the dose regimen was calculated to produce serum concentrations above the MIC during 40% of the interval between doses (11); thus, the intervals between doses for CLA were 2.5 h and 2 h for strains Ab11 and Ab51, respectively, and the intervals between doses for IPM were 3.5 h for both strains. The animals were observed for mortality over 24 h, and all the animals were analyzed immediately after death. Blood and lung samples were obtained and processed as described previously (34). The results are expressed as means ± standard deviations of the log10 CFU per gram of lung and as frequencies of sterile blood cultures.
Statistical analysis.
The mean log10 CFU per gram of lung for the different treatment groups were compared by analysis of variance (ANOVA). If the differences were significant, comparisons among groups were made using Dunnett and Tukey post hoc tests. Frequencies of sterile tissues, sterile blood cultures, and survival were analyzed by chi-square tests. The SPSS (version 15.0) statistical package was used (SPSS Inc., Chicago, IL).
RESULTS
In vitro studies.
The MICs and MBCs of CLA and IPM against Ab11 and Ab51 are shown in Table 1. For both strains and both antibiotics, a low inoculum effect was observed.
TABLE 1.
MICs and MBCs of CLA and IPM against two strains of A. baumannii at three different inocula
| Strain, antimicrobial, and inoculum (CFU/ml) | MIC (mg/liter) | MBC (mg/liter) |
|---|---|---|
| Ab11 | ||
| CLA | ||
| 105 | 2 | 2 |
| 106 | 4 | 4 |
| 107 | 4 | 8 |
| IPM | ||
| 105 | 0.5 | 0.5 |
| 106 | 0.5 | 0.5 |
| 107 | 1 | 2 |
| Ab51 | ||
| CLA | ||
| 105 | 4 | 4 |
| 106 | 8 | 8 |
| 107 | 8 | 16 |
| IPM | ||
| 105 | 0.5 | 0.5 |
| 106 | 0.5 | 0.5 |
| 107 | 1 | 2 |
Sulbactam MICs were 1 and 2 mg/liter, respectively. Tazobactam had a MIC of 16 mg/liter against both strains. For these two β-lactamase inhibitors, only one inoculum (105) was tested.
Strains Ab11 and Ab51 were susceptible to piperacillin, piperacillin-tazobactam, meropenem, and colistin and were resistant to ampicillin, cefotaxime, and cefepime. Moreover, against ceftazidime, ciprofloxacin, trimethoprim-sulfamethoxazole, gentamicin, and amikacin, Ab11 was susceptible and Ab51 was resistant.
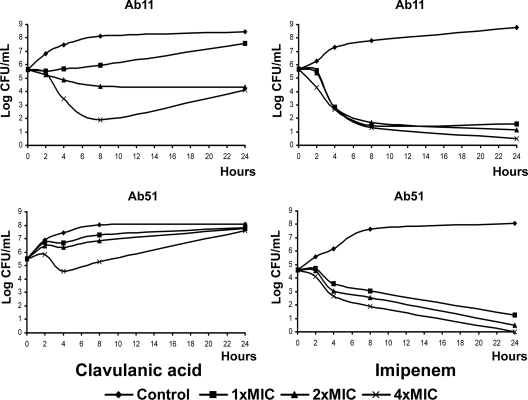
Time-kill curves are represented in Fig. 1. CLA at 4× MIC showed bactericidal activity at 8 h against Ab11; against Ab51, CLA was not bactericidal. IPM was bactericidal at 1×, 2×, and 4× MIC against Ab11 at 8 h; against Ab51, IPM was bactericidal at 1×, 2×, and 4× MIC at 24 h. No PAE of CLA or IPM was observed with either of the strains.
FIG. 1.
Time-kill curves of CLA and IPM against strains Ab11 and Ab51. The control has no antibiotic.
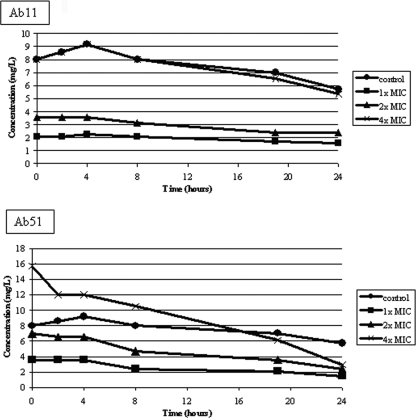
The increase in the growth of strain Ab51 at 24 h in the time-kill curve experiment with CLA was remarkable, reaching the growth level of the antibiotic-free control (Fig. 1). Figure 2 shows the decrease in the CLA concentration in the culture medium during the assay with strain Ab51 but not with strain Ab11. As stated above, one possible explanation is that somehow CLA is either sequestered or inactivated by β-lactamase enzymes. In this respect, the relative enzymatic efficiency (Vmax/Km-like) parameters of the Ab51 AmpC enzyme (0.02597 min−1) were twice that of the Ab11 AmpC enzyme (0.01255 min−1), which can explain the decrease in the CLA drug concentration in MHBCA along the time-kill curves. On the other hand, the presence of additional β-lactamases was discarded by isoelectric focusing gel experiments (data not shown).
FIG. 2.
CLA concentrations during the time-kill curves with strains Ab11 and Ab51. The control has no bacteria and 8 mg of CLA/liter.
In vivo studies. (i) Pharmacokinetics/pharmacodynamics.
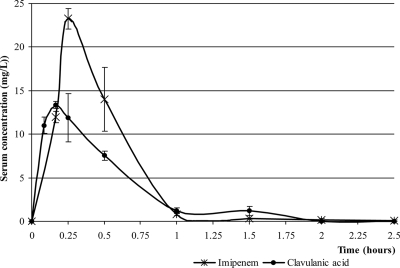
The t1/2 values of CLA and IPM after doses of 13 mg/kg and 30 mg/kg, respectively, were 0.24 and 0.26 h. The TMICs were 0.93 and 0.79 h for CLA with Ab11 and Ab51, respectively, and 1.27 h for IPM with both strains. The pharmacokinetics curves are shown in Fig. 3. The calculated pharmacokinetic/pharmacodynamic parameters are detailed in Table 2.
FIG. 3.
Serum CLA and IPM concentrations. CLA was administered at 13 mg/kg, and IPM was administered at 30 mg/kg.
TABLE 2.
Pharmacokinetic/pharmacodynamic parameters of CLA (13 mg/kg) and IPM (30 mg/kg)
| Antimicrobial and strain | MIC (mg/liter) | Pharmacokinetic parameter
|
Pharmacodynamic parameter
|
||||
|---|---|---|---|---|---|---|---|
| Cmax (mg/liter) | AUC (mg·h/liter) | t1/2 (h) | AUC/MIC (h) | Cmax/MIC | TMIC (h) | ||
| CLA | 13.38 | 8.17 | 0.24 | ||||
| Ab11 | 2 | 4.03 | 6.69 | 0.93 | |||
| Ab51 | 4 | 2.02 | 3.35 | 0.79 | |||
| IPM | 0.5a | 23.25 | 11.29 | 0.26 | 22.57 | 46.5 | 1.27 |
The IPM MIC is the same for strains Ab11 and Ab51, and consequently the pharmacodynamic parameters for the two strains are the same.
(ii) Efficacy of treatment in experimental pneumonia.
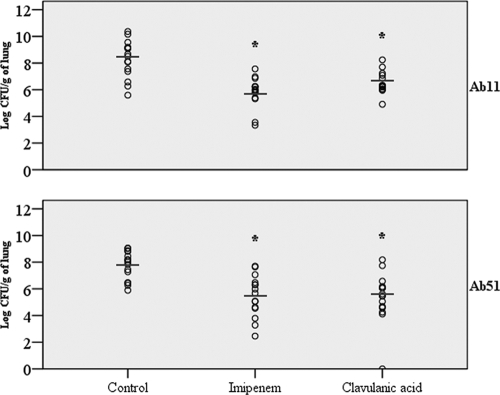
In groups inoculated with strain Ab11, both treatments (CLA and IPM) were effective compared to the untreated control group, decreasing the bacterial load by 1.78 log10 CFU/g lung and 2.47 log10 CFU/g lung, respectively (P < 0.001). The frequency of sterile blood cultures increased to 80% in the IPM group (P = 0.005) and 60% in the CLA group (P = 0.07) compared with the control group (26.67%). In the groups inoculated with Ab51, both treatments (CLA and IPM) were effective compared to the control group, decreasing the bacterial load by 2.41 log10 CFU/g lung (P < 0.001) and 2.28 log10 CFU/g lung, respectively (P = 0.001). The IPM and CLA groups had higher frequencies of sterile blood cultures (100% and 93.3%, respectively; P < 0.001) than the control group (20%). The results are detailed in Table 3 and Fig. 4. No toxicity was observed with CLA in the group of uninfected mice.
TABLE 3.
Effects of antibiotic therapy on the clearance of Ab11 and Ab51 strains from mouse lungs, the frequency of sterile blood cultures, and mortality
| Treatment group (n) | Ab11
|
Ab51
|
||||
|---|---|---|---|---|---|---|
| Bacterial load (mean log10 CFU/g of lung ± SD) | % Sterile blood cultures | % Mortality | Bacterial load (mean log10 CFU/g of lung ± SD) | % Sterile blood cultures | % Mortality | |
| Control (15) | 8.28 ± 1.40 | 26.7 | 40 | 7.75 ± 1.07 | 20 | 93.3 |
| IPM (15) | 5.81 ± 1.13a | 80a | 53.3 | 5.46 ± 1.55a | 100a | 40a |
| CLA (15) | 6.50 ± 0.81a | 60 | 26.7 | 5.33 ± 1.88a | 93.3a | 33.3a |
P < 0.01 relative to the control group (by ANOVA, post hoc Tukey and Dunnett tests, and chi-square tests).
FIG. 4.
Effect of antibiotic therapy with CLA or IPM on the clearance of A. baumannii from mouse lungs. *, P ≤ 0.001 relative to the control group (by ANOVA and Tukey and Dunnett post hoc tests).
DISCUSSION
The results of the present study show, for the first time, that CLA is effective in the treatment of A. baumannii in experimental murine pneumonia, in terms of reduction of lung bacterial concentrations, sterilization of blood cultures, and survival, with the last two effects being strain dependent. The results with CLA are similar to those with IPM, which is the gold standard for the treatment of clinical A. baumannii infections and has shown high efficacy in the treatment of experimental pneumonia with this bacterium (34), although CLA was slightly less efficacious in the sterilization of blood cultures than IPM with one of the strains assayed.
Due to the high frequency of antimicrobial multiresistance among clinical isolates of A. baumannii, new therapeutic alternatives are being evaluated (44). Among the β-lactamase inhibitors, sulbactam has shown good efficacy in treating A. baumannii infections, both in experimental murine pneumonia and in clinical infections (10, 29, 33), but less is known about the activities of the other β-lactamase inhibitors, such as tazobactam or CLA. CLA has wide antibacterial activity (19), especially against pathogens such as Neisseria spp., Chlamydia spp., Legionella pneumophila, and Enterobacteriaceae. In an in vitro study with 68 isolates of Acinetobacter spp., CLA was more active than other β-lactams, such as ceftriaxone, cefotaxime, and piperacillin; the MIC50 and MCI90 of CLA were 15.4 and 28.4 mg/liter, respectively (41). In two studies with 100 and 115 epidemiologically defined strains of Acinetobacter spp., the CLA MIC was ≤8 mg/liter for 29% and 40.9% of the strains, respectively, and CLA MIC50s were 32 and 16 mg/liter (3, 27).
In the time-kill curves, a bactericidal effect was observed against strain Ab11, but not against Ab51, at 4× MIC. To explain this unexpected result, the CLA concentration in the culture medium was determined, and a significant decrease was observed with strain Ab51, from 16 to 2.5 mg/liter after 24 h of incubation at 4× MIC, but not with Ab11. This effect could be due to hydrolysis or a sequestration-like effect of the AmpC enzyme that decreases the CLA concentration in the medium. Indeed, it has been shown previously that CLA can inhibit AmpC from A. baumannii, which demonstrates the interaction of this inhibitor with the naturally occurring AmpC enzyme from this microorganism (6). Another possible explanation could be an increase in CLA MICs during the time-kill experiments; however, we determined CLA MICs against both strains before and after a 24-h incubation with a CLA concentration of 8 mg/liter, and we observed no changes in the MICs (data not shown).
As occurs with other β-lactams against gram-negative bacilli, except in the case of IPM (39), we have found absent or minimal PAE with CLA against the strains studied. Previously, it has been shown that the addition of CLA to amoxicillin (amoxicilline) produced an extended PAE against β-lactamase-negative strains of Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae, Moraxella catarrhalis, and Escherichia coli (24). Other authors (42) found a lack of enhancement of the amoxicillin PAE by CLA, and they explained the findings of the previous work (24) by the high concentrations of CLA used, which have antibacterial activity per se. We have found no PAE with IPM, in spite of the results of other authors (39).
Different in vitro studies of pathogens such as S. pneumoniae (15, 36), L. pneumophila (40), E. coli (25), and Acinetobacter spp. (39) suggest that CLA has affinity for penicillin-binding proteins, although the activity can differ greatly depending on the microorganism. It seems that an important factor in considering the activity of CLA, especially when it is combined with other β-lactams, such as amoxicillin or ticarcillin, is the interaction with the immune system. In two works in which Klebsiella pneumoniae infections in renal transplant patients (13) and patients with chronic hemodialysis (14) were studied, addition of amoxicillin-clavulanate at 0.5× MIC restored the activity of human polymorphonuclear cells to levels similar to those in healthy subjects. In agreement with these results, the association of amoxicillin and CLA resulted in a synergistic potentiation of the activity of both drugs on polymorphonuclear cell activity against S. pneumoniae in such a manner that the bacteria became more susceptible either to phagocytosis or to the microbicidal activities of phagocytes (15). The use of subinhibitory concentrations of amoxicillin-clavulanate may increase the expression of the proinflammatory cytokines interleukin-8 and interleukin-1β (32). The interaction between amoxicillin-clavulanate and the immune cells seems clear, but further studies are necessary to determine the involvement of CLA in this interaction.
Animal infection models are very useful for the observation of new potential uses of antibiotics already established in clinical practice. For example, good in vitro activity of CLA was observed against Chlamydia trachomatis (7). Later, the efficacy of treatment with CLA was demonstrated in experimental murine pneumonia caused by C. trachomatis, in which CLA protected 75% of mice, amoxicillin protected 90%, and the combination of the two protected 100% (2). In the treatment of L. pneumophila in neutropenic rats, CLA was highly efficacious, similar to the gold standard, erythromycin; amoxicillin-clavulanate was not more effective, while amoxicillin alone was ineffective (38). This correlates with the results obtained in vitro, in which the CLA MIC was 0.1 to 0.25 mg/liter (40).
In the murine model, both CLA and IPM treatments were efficacious in pulmonary bacterial clearance, as determined by comparison with clearance in the group without treatment, and increased the number of sterile blood cultures. There were no significant differences between two treatments in these parameters, although the number of sterile blood cultures was higher (and significantly different from that for the control) with IPM than with CLA. The model was not used beyond 24 h because of the high number of doses that had to be administered to the animals to reach a serum concentration above the MIC for at least 40% of the time between doses. In spite of this, CLA decreased the mortality rate in the model with strain Ab51, which produced a mortality rate of 93.3% in the control group.
In an experimental A. baumannii pneumonia model in neutropenic mice, the addition of CLA to ticarcillin, using a strain for which ticarcillin and ticarcillin-clavulanate MICs were 32 mg/liter, decreased the lung bacterial concentration, whereas ticarcillin alone was not efficacious in comparison with the control group. In this work, IPM alone was also efficacious in comparison with the control group, showing no differences from ticarcillin plus CLA (45).
Data about the pharmacokinetics of CLA in humans are scarce. In the present experiments, the dose of CLA was chosen to produce a Cmax similar to that in humans. Thus, the Cmax of CLA after a dose of 200 mg in humans is 11.4 mg/liter (1), and in the mice it was 13.38 mg/liter. The t1/2 in humans receiving the same dose is 0.8 to 1 h (1), higher than the t1/2 found in our experiments with mice, which was 0.24 h, as is the rule for small animals. Also, in human bronchial mucosae, the levels of CLA are 118% of the corresponding levels in serum (23). These data suggest that CLA may be used to treat human lung infections with A. baumannii strains for which the CLA MIC is ≤8 mg/liter, such as those in the present study, taking into account that a similar Cmax is obtained with a 200-mg dose and that the interval between doses may be the same as that obtained when CLA is used as a β-lactamase inhibitor because of its higher t1/2 in humans than in mice.
In summary, the present study suggests that CLA is effective in the treatment of experimental murine pneumonia caused by A. baumannii and that it thus warrants future assessment of its efficacy with A. baumannii strains showing higher CLA MICs than those in the present study. Studies of humans, such as those performed with sulbactam, are necessary to confirm these results.
Acknowledgments
This study was partially supported by the Spanish Network for Research in Infectious Diseases (REIPI RD06/0008), the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III-FEDER, Fondo de Investigación Sanitaria (PI061368, PI081613), and Conselleria de Sanidad PS07/90. A.B. has an Angeles Alvariño research contract from Xunta de Galicia.
None of the authors has a conflict of interest to declare.
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Bamberger, D. M., J. W. Foxworth, D. L. Bridwell, C. S. Shain, and D. N. Gerdin. 2005. Extravascular antimicrobial distribution and the respective blood and urine concentrations in humans, p. 719-814. In V. Lorian (ed.), Antibiotics in laboratory medicine. Lippincott Williams & Wilkins, Philadelphia, PA.
- 2.Beale, A. S., E. Faulds, S. E. Hurn, J. Tyler, and B. Slocombe. 1991. Comparative activities of amoxycillin, amoxycillin/clavulanic acid and tetracycline against Chlamydia trachomatis in cell culture and in an experimental mouse pneumonitis. J. Antimicrob. Chemother. 27:627-638. [DOI] [PubMed] [Google Scholar]
- 3.Beceiro, A., F. Fernandez-Cuenca, A. Ribera, L. Martinez-Martinez, A. Pascual, J. Vila, J. Rodriguez-Bano, J. M. Cisneros, J. Pachon, and G. Bou. 2008. False extended-spectrum beta-lactamase detection in Acinetobacter spp. due to intrinsic susceptibility to clavulanic acid. J. Antimicrob. Chemother. 61:301-308. [DOI] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bou, G., G. Cervero, M. A. Dominguez, C. Quereda, and J. Martinez-Beltran. 2000. PCR-based DNA fingerprinting (REP-PCR, AP-PCR) and pulsed-field gel electrophoresis characterization of a nosocomial outbreak caused by imipenem- and meropenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 6:635-643. [DOI] [PubMed] [Google Scholar]
- 6.Bou, G., and J. Martinez-Beltran. 2000. Cloning, nucleotide sequencing, and analysis of the gene encoding an AmpC beta-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 44:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowie, W. R. 1986. In vitro activity of clavulanic acid, amoxicillin, and ticarcillin against Chlamydia trachomatis. Antimicrob. Agents Chemother. 29:713-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cefai, C., J. Richards, F. K. Gould, and P. McPeake. 1990. An outbreak of Acinetobacter respiratory tract infection resulting from incomplete disinfection of ventilatory equipment. J. Hosp. Infect. 15:177-182. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI document M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Corbella, X., J. Ariza, C. Ardanuy, M. Vuelta, F. Tubau, M. Sora, M. Pujol, and F. Gudiol. 1998. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J. Antimicrob. Chemother. 42:793-802. [DOI] [PubMed] [Google Scholar]
- 11.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 12.Craig, W. A., and S. Gudmundsson. 1991. Postantibiotic effect, p. 403-431. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, MD.
- 13.Cuffini, A. M., V. Tullio, F. Giacchino, A. Bonino, N. Mandras, N. Bianchi, J. Roana, D. Scalas, F. Bonello, and N. A. Carlone. 2001. Improved phagocyte response by co-amoxiclav in renal transplant recipients. Transplantation 71:575-577. [DOI] [PubMed] [Google Scholar]
- 14.Cuffini, A. M., V. Tullio, F. Giacchino, N. Mandras, D. Scalas, P. Belardi, C. Merlino, and N. A. Carlone. 2001. Impact of co-amoxiclav on polymorphonuclear granulocytes from chronic hemodialysis patients. Am. J. Kidney Dis. 37:1253-1259. [DOI] [PubMed] [Google Scholar]
- 15.Cuffini, A. M., V. Tullio, A. Ianni Palarchio, A. Bonino, G. Paizis, and N. A. Carlone. 1998. Enhanced effects of amoxycillin/clavulanic acid compared with amoxycillin and clavulanic acid alone on the susceptibility to immunodefenses of a penicillin-resistant strain of Streptococcus pneumoniae. Drugs Exp. Clin. Res. 24:173-184. [PubMed] [Google Scholar]
- 16.Dijkshoorn, L., H. M. Aucken, P. Gerner-Smidt, M. E. Kaufmann, J. Ursing, and T. L. Pitt. 1993. Correlation of typing methods for Acinetobacter isolates from hospital outbreaks. J. Clin. Microbiol. 31:702-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falagas, M. E., I. A. Bliziotis, and I. I. Siempos. 2006. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit. Care 10:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falagas, M. E., and P. I. Rafailidis. 2007. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit. Care 11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay, J., L. Miller, and J. A. Poupard. 2003. A review of the antimicrobial activity of clavulanate. J. Antimicrob. Chemother. 52:18-23. [DOI] [PubMed] [Google Scholar]
- 20.Frimodt-Moller, N., M. W. Bentzon, and V. F. Thomsen. 1986. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J. Infect. Dis. 154:511-517. [DOI] [PubMed] [Google Scholar]
- 21.Gerner-Smidt, P., and W. Frederiksen. 1993. Acinetobacter in Denmark: I. Taxonomy, antibiotic susceptibility, and pathogenicity of 112 clinical strains. APMIS 101:815-825. [DOI] [PubMed] [Google Scholar]
- 22.Gerner-Smidt, P., and I. Tjernberg. 1993. Acinetobacter in Denmark. II. Molecular studies of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. APMIS 101:826-832. [PubMed] [Google Scholar]
- 23.Gould, I. M., G. Harvey, D. Golder, T. M. Reid, S. J. Watt, J. A. Friend, J. S. Legge, and J. G. Douglas. 1994. Penetration of amoxycillin/clavulanic acid into bronchial mucosa with different dosing regimens. Thorax 49:999-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould, I. M., K. Milne, and C. Jason. 1991. Post antibiotic effect of coamoxyclav, abstr. 1360. Progr. abstr. 5th Eur. Cong. Clin. Microbiol. Infect. Dis.
- 25.Greenwood, D., F. O'Grady, and P. Baker. 1979. An in vitro evaluation of clavulanic acid, a potent, broad-spectrum beta-lactamase inhibitor. J. Antimicrob. Chemother. 5:539-547. [DOI] [PubMed] [Google Scholar]
- 26.Hartstein, A. I., A. L. Rashad, J. M. Liebler, L. A. Actis, J. Freeman, J. W. Rourke, Jr., T. B. Stibolt, M. E. Tolmasky, G. R. Ellis, and J. H. Crosa. 1988. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am. J. Med. 85:624-631. [DOI] [PubMed] [Google Scholar]
- 27.Higgins, P. G., H. Wisplinghoff, D. Stefanik, and H. Seifert. 2004. In vitro activities of the beta-lactamase inhibitors clavulanic acid, sulbactam, and tazobactam alone or in combination with beta-lactams against epidemiologically characterized multidrug-resistant Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 48:1586-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hujer, K. M., N. S. Hamza, A. M. Hujer, F. Perez, M. S. Helfand, C. R. Bethel, J. M. Thomson, V. E. Anderson, M. Barlow, L. B. Rice, F. C. Tenover, and R. A. Bonomo. 2005. Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 beta-lactamase: defining a unique family of class C enzymes. Antimicrob. Agents Chemother. 49:2941-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez-Mejias, M. E., J. Pachon, B. Becerril, J. Palomino-Nicas, A. Rodriguez-Cobacho, and M. Revuelta. 1997. Treatment of multidrug-resistant Acinetobacter baumannii meningitis with ampicillin/sulbactam. Clin. Infect. Dis. 24:932-935. [DOI] [PubMed] [Google Scholar]
- 29a.López-Rojas, R., A. Beceiro, J. Domínguez-Herrera, F. Docobo-Pérez, G. Bou, and J. Pachón. 2008. In vitro activity and in vivo efficacy of clavulanic acid against Acinetobacter baumannii, poster B-066. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC. [DOI] [PMC free article] [PubMed]
- 30.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 31.Reading, C., and M. Cole. 1977. Clavulanic acid: a beta-lactamase-inhibiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 11:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reato, G., A. M. Cuffini, V. Tullio, A. I. Palarchio, A. Bonino, R. Foa, and N. A. Carlone. 1999. Co-amoxiclav affects cytokine production by human polymorphonuclear cells. J. Antimicrob. Chemother. 43:715-718. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Hernandez, M. J., L. Cuberos, C. Pichardo, F. J. Caballero, I. Moreno, M. E. Jimenez-Mejias, A. Garcia-Curiel, and J. Pachon. 2001. Sulbactam efficacy in experimental models caused by susceptible and intermediate Acinetobacter baumannii strains. J. Antimicrob. Chemother. 47:479-482. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Hernandez, M. J., J. Pachon, C. Pichardo, L. Cuberos, J. Ibanez-Martinez, A. Garcia-Curiel, F. J. Caballero, I. Moreno, and M. E. Jimenez-Mejias. 2000. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J. Antimicrob. Chemother. 45:493-501. [DOI] [PubMed] [Google Scholar]
- 35.Rolinson, G. N. 1994. A review of the microbiology of amoxycillin/clavulanic acid over the 15 year period 1978-1993. J. Chemother. 6:283-318. [DOI] [PubMed] [Google Scholar]
- 36.Severin, A., E. Severina, and A. Tomasz. 1997. Abnormal physiological properties and altered cell wall composition in Streptococcus pneumoniae grown in the presence of clavulanic acid. Antimicrob. Agents Chemother. 41:504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shumaker, R. C. 1986. PKCALC: a BASIC interactive computer program for statistical and pharmacokinetic analysis of data. Drug Metab. Rev. 17:331-348. [DOI] [PubMed] [Google Scholar]
- 38.Smith, G. M., K. H. Abbott, and R. Sutherland. 1992. Bactericidal effects of co-amoxiclav (amoxycillin clavulanic acid) against a Legionella pneumophila pneumonia in the immunocompromised weanling rat. J. Antimicrob. Chemother. 30:525-534. [DOI] [PubMed] [Google Scholar]
- 39.Spivey, J. M. 1992. The postantibiotic effect. Clin. Pharm. 11:865-875. [PubMed] [Google Scholar]
- 40.Stokes, D. H., B. Slocombe, and R. Sutherland. 1989. Bactericidal effects of amoxycillin/clavulanic acid against Legionella pneumophila. J. Antimicrob. Chemother. 23:43-51. [DOI] [PubMed] [Google Scholar]
- 41.Suh, B., T. Shapiro, R. Jones, V. Satishchandran, and A. L. Truant. 1995. In vitro activity of beta-lactamase inhibitors against clinical isolates of Acinetobacter species. Diagn. Microbiol. Infect. Dis. 21:111-114. [DOI] [PubMed] [Google Scholar]
- 42.Thorburn, C. E., S. J. Molesworth, R. Sutherland, and S. Rittenhouse. 1996. Postantibiotic and post-beta-lactamase inhibitor effects of amoxicillin plus clavulanate. Antimicrob. Agents Chemother. 40:2796-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaneechoutte, M., L. Dijkshoorn, I. Tjernberg, A. Elaichouni, P. de Vos, G. Claeys, and G. Verschraegen. 1995. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J. Clin. Microbiol. 33:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila, J., and J. Pachon. 2008. Therapeutic options for Acinetobacter baumannii infections. Expert Opin. Pharmacother. 9:587-599. [DOI] [PubMed] [Google Scholar]
- 45.Wolff, M., M. L. Joly-Guillou, R. Farinotti, and C. Carbon. 1999. In vivo efficacies of combinations of beta-lactams, beta-lactamase inhibitors, and rifampin against Acinetobacter baumannii in a mouse pneumonia model. Antimicrob. Agents Chemother. 43:1406-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]