Abstract
The ruthenium NO donors of the group trans-[Ru(NO)(NH3)4L]n+, where the ligand (L) is N-heterocyclic H2O, SO32−, or triethyl phosphite, are able to lyse Trypanosoma cruzi in vitro and in vivo. Using half-maximal (50%) inhibitory concentrations against bloodstream trypomastigotes (IC50try) and cytotoxicity data on mammalian V-79 cells (IC50V79), the in vitro therapeutic indices (TIs) (IC50V79/IC50try) for these compounds were calculated. Compounds that exhibited an in vitro TI of ≥10 and trypanocidal activity against both epimastigotes and trypomastigotes with an IC50try/epi of ≤100 μM were assayed in a mouse model for acute Chagas' disease, using two different routes (intraperitoneal and oral) for drug administration. A dose-effect relationship was observed, and from that, the ideal dose of 400 nmol/kg of body weight for both trans-[Ru(NO)(NH3)4isn](BF4)3 (isn, isonicotinamide) and trans-[Ru(NO)(NH3)4imN](BF4)3 (imN, imidazole) and median (50%) effective doses (ED50) of 86 and 190 nmol/kg, respectively, were then calculated. Since the 50% lethal doses (LD50) for both compounds are higher than 125 μmol/kg, the in vivo TIs (LD50/ED50) of the compounds are 1,453 for trans-[Ru(NO)(NH3)4isn](BF4)3 and 658 for trans-[Ru(NO)(NH3)4imN](BF4)3. Although these compounds exhibit a marked trypanocidal activity and are able to react with cysteine, they exhibit very low activity in T. cruzi-glycosomal glyceraldehyde-3-phosphate dehydrogenase tests, suggesting that this enzyme is not their target. The trans-[Ru(NO)(NH3)4isn](BF4)3 and trans-[Ru(NO)(NH3)4imN](BF4)3 compounds are able to eliminate amastigote nests in myocardium tissue at 400-nmol/kg doses and ensure the survival of all infected mice, thus opening a novel set of therapies to try against trypanosomatids.
Although American trypanosomiasis, or Chagas' disease, has existed on the American continent for more than 9,000 years (3), it still is considered incurable, in view of the fact that the available anti-Trypanosoma cruzi drugs exhibit limited efficacy and undesirable side effects (11). Chagas' disease takes third place, after malaria and leishmaniasis, in mortality and morbidity prevalence due to vector-associated diseases on the American continent (17, 26). Since the parasite can be found in the blood of up to 50% of infected people several years after the primary infection (36), Chagas' disease is also a cause for government concern in several countries where the disease is not endemic (31). This infection can be contracted by infected-blood transfusion and organ transplant from donors originating from areas of Latin America where the disease is endemic (31).
The parasite's biological cycle includes two multiplicative forms (epimastigote and amastigote), one infective form (trypomastigote), and an obligatory passage through vertebrate and invertebrate hosts (14). The trypomastigotes escape to the cytosol of the macrophage and transform into amastigotes, which are released as trypomastigote forms (26, 30). Under these circumstances, the infection triggers interleukin-12 (2) and tumor necrosis factor alpha production (10), which leads to gamma interferon (IFN-γ) synthesis (2) and to inducible nitric oxide (NO) synthase activation (22). As a consequence, NO is synthesized, and this NO production has been thought to be responsible for the trypanocidal effect (53). At this stage of the infection, the parasite also triggers production of transforming growth factor beta and interleukin-10, which are negative regulators of NO production inhibiting IFN-γ-produced macrophage activation and NO synthesis (22). Transforming growth factor beta production, which is described as an anti-inflammatory response, often promotes permissiveness to T. cruzi infection (53). Furthermore, it has been observed that the NO produced during acute infection also plays an important role in at least two of the processes that facilitate parasite evasion from the cellular immune response (26). In addition to mediating resistance against infection, NO can suppress the immune response to T. cruzi via the induction of apoptosis of T cells (25). Thus, this experimental evidence gives support to the idea that the intracellular NO production by host cells plays an essential role in resistance to the parasite infection (51). Indeed, the pharmacological modulation of the host immune response against T. cruzi through control of the NO levels has been recently accepted as a therapeutic target (26).
In this context, the ruthenium species of the group trans-[RuII(NO+)(NH3)4L]n+, where the ligand (L) is N heterocyclic, H2O, sulfite (SO32−), or triethyl phosphite [P(OEt)3], which can deliver NO at a selected rate constant (k−NO) upon reduction of the nitrosyl ligand, can be useful in chemotherapy against T. cruzi and other pathogens. The NO release specific rate constant and the redox potential for the [RuIINO+]/[RuIINO0] couple in these complexes can be controlled through the judicious selection of the trans ligand (44). Additionally, these compounds exhibit low cytotoxicity and good water solubility (up to 10−2 M) and are stable for days under physiological hydrogen ion conditions (43). Certainly there are many other compounds that could be tested for such purposes (4, 47). However, the main advantages of our compounds are that they are kinetically robust and are activated only by reductors (43). Furthermore, they offer the possibility of tuning the redox potential of the nitrosonium ligand and the lability of the formed NO as a function of the trans ligand in a very efficient manner (41, 43). As far as we know, the other compounds in question do not offer the same flexibility, at least to the same extent. In addition, we are now exploring the possibility that our compounds operate on the catalytic cycle converting NO2− into NO+ (28).
As a natural extension of a previous work in which we described the antiproliferative activity of ruthenium NO donors against epimastigote forms (38), this work evaluates the trypanocidal activity of multiplicative forms of T. cruzi. In addition, based on in vitro and in vivo therapeutic indices (TIs), this work also demonstrates that trans-[RuII(NO+)(NH3)4imN](BF4)3, trans-[Ru(NO)(NH3)4py](BF4)3, and trans-[RuII(NO+)(NH3)4isn](BF4) (imN, imidazole; py, pyridine; isn, isonicotinamide) can be successfully used to treat acute murine Chagas' disease and that at nanomolar doses they are able to lyse in vivo the parasite and to ensure the survival of all infected mice.
This work is presently under Brazilian patent of invention deposit request PI 0705849-7 (D. W. Franco et al.).
(Part of this work was conducted by J. J. N. Silva in partial fulfillment of the requirements for a Ph.D. from the Instituto de Química de São Carlos, Universidade de São Paulo [USP], Brazil, 2008.)
MATERIALS AND METHODS
Chemicals, drugs, and reagents.
Ruthenium trichloride (Aldrich Chemical Company, St. Louis, MO) was the starting material for the synthesis of all ruthenium complexes described herein. All solvents were purified by following known procedures (29), and doubly distilled water was used throughout. All of the synthesis and manipulations were carried out under an argon atmosphere (37). Benznidazole (Bz) and sodium nitroprusside (SNP) (Aldrich) were used as the reference drug and reference NO donor, respectively.
Synthesis.
All of the ruthenium NO donors of the group trans-[Ru(NO)(NH3)4L]Xn, where L is imN, py, pyrazine (pz), l-histidine (l-hist), isn, P(OEt)3, or SO32− and X is BF4−, Cl−, or PF6−, were synthesized and characterized by following published procedures (6, 21).
Instrumentation.
Elemental analyses of hydrogen, carbon, and nitrogen were carried out using an EA 1110 CHNS-O CE instrument. Analysis of ruthenium was performed according to a published method (12), using a polarized Zeeman atomic absorption spectrophotometer (model Z-8100; Hitachi) with a Hitachi hollow-cathode lamp (12 mA, λ = 349.9 nm). UV-visible-light measurements were performed with a 1.0-cm quartz cell on a model 8452A Hewlett-Packard diode array spectrophotometer. Infrared spectra were recorded using a Bomem model MB-102 Fourier transform infrared spectrophotometer in the 400- to 4,000-cm−1 range, supported in potassium bromide pellets. A polarographic analyzer/stripping voltameter (model 264A; Princeton Applied Research) attached to a microcomputer and employing Microquímica electrochemical software was used for the electrochemical measurements. The electrochemical cell used was a conventional three-electrode type with an aqueous saturated calomel electrode as a reference electrode and glassy-carbon and platinum wires as working and auxiliary electrodes, respectively.
Parasites.
We used the Y strain of T. cruzi, which has been described as partially Bz resistant and highly virulent (24). The Y strain of T. cruzi was obtained from an intermediary strain-matched infected mouse and grown in rhesus monkey kidney epithelial cells (LLC-MK2 line) under culture conditions. Furthermore, among the strains of T. cruzi used, this is the most well described one with respect to its biochemical, genetic, and immunological aspects and also to the host-parasite interaction in various experimental models (wild-type and knockout mice, dog, rabbit, and hamster, etc.). Female Swiss mice were infected by intraperitoneal (i.p.) administration of 1.0 × 103 bloodstream trypomastigote forms of T. cruzi (BT) obtained from an intermediary, strain-matched, infected mouse. Before infection of the intermediary mice, parasites were grown in Schneider's medium and purified from a monkey kidney fibroblast cell line, LLC-MK2. This procedure was realized in the immunoparasitology laboratory of the Departamento de Bioquímica e Imunologia da Faculdade de Medicina de Ribeirão Preto, USP.
Evaluation of trypanocidal activity on epimastigotes.
The experiments were performed according to a previously described procedure (38). Epimastigotes were grown at 28°C in Schneider's medium (Sigma Chemical Co., St. Louis, MO) supplemented with 20% fetal calf serum, harvested during the exponential growth phase, washed in phosphate-buffered saline (PBS), and resuspended to 1.0 × 106 parasites/ml. A volume of 200 μl of parasites was plated onto 96-well plates (in triplicate) and treated with the NO donors diluted in PBS (concentrations from 100 μM up to 1 mM) and incubated at 37°C and 5% CO2. Bz and SNP were used as a reference drug and reference NO donor, respectively. Parasite viability was subsequently tested by determining the number of motile forms in a Newbauer chamber (7), and the percentage of trypanocidal activity (%TA) was calculated as follows: %TA = [1 − (LDt/LCt)] × 100, where LDt is the average of the numbers of motile epimastigotes in wells containing the NO donor at time t and LCt is the average of the numbers of motile epimastigotes in wells in the absence of any compound at time t (negative control) (34). In a previous work, the compound concentration corresponding to the epimastigote 50% antiproliferative activity (50% inhibitory concentration) after 24 h of incubation was expressed as IC50epi (38). Herein, the IC50epi corresponds to the compound concentration with 50% trypanocidal activity after 24 h of incubation. However, the trypanocidal activity was calculated only for the compound concentrations, where the number of motile forms is smaller than in the negative control at time zero.
Evaluation of trypanocidal activity in vivo (acute model).
Female Swiss mice aged 6 to 8 weeks (25 to 30 g) were bred and maintained in microisolator cages at the animal housing facilities of the Faculdade de Medicina de Ribeirão Preto, USP, which observes the local protocols of ethics for animal care. Mice were infected by administration of 1.0 × 103 BT, were housed in temperature-controlled rooms (22 to 25°C), and received water and food ad libitum. The NO donors (10, 50, 100, 400, 1,000, and 3,000 nmol/kg of body weight) were given daily as a single dose by oral or i.p. injection in 100 μl of PBS. The treatment was performed during 15 consecutive days, but the survivor mice were followed up to day 60 postinfection. Six animals per group were used in each experiment [six animals for the control group, six animals for the group treated with the Ru(NO)isn, and six animals for the group treated with the Ru(NO)imN]. Bz was used as the reference trypanocidal drug (100 nmol/kg = 26 μg/kg). All procedures performed during the study described herein were approved by the Ethics Committee on Animal Research of the USP. Moreover, the study was committed to the so-called 3 R's principle (replacement, reduction, refinement), which basically considers alternative methods when animals are used, as well as improved techniques aiming to diminish whenever possible the suffering and the number of animals necessary for the project (55). The course of infection was monitored by counting the number of motile trypomastigotes in the blood samples (5 μl) drawn from the tail veins, as previously described (8).
Acute toxicity.
Female BALB/c mice were treated with the trans-[Ru(NO)(NH3)4imN](BF4)3, trans-[Ru(NO)(NH3)4py](BF4)3, and trans-[Ru(NO)(NH3)4isn](BF4)3 complexes diluted in PBS according to the up-and-down test protocol for acute toxicity (9) and an oral acute toxic class (ATC) method (35). In the up-and-down test protocol, the compound was i.p. administered in a single dose to only one animal, followed by a 48-h observation period. If hyperventilation, tremors, thirst symptoms, or death occurred, another animal would be chosen and would receive one-third the previous dose; if none of these signs and symptoms occurred, the next animal would receive three times the previous dose (9). The starting dose was 25 μmol/kg. At the same time, the oral ATC method was also applied; the starting dose for this test was 2,000 mg/kg body weight, with three animals at each stage. If two or three of the animals die at a defined dose level, another group of three animals is chosen and receives the next lower dose level (300 mg/kg). On the other hand, if one animal or no animals die at a defined dose, this dose level is tested with three additional animals before proceeding to the next higher dose level (35).
Statistical analysis.
The observed results presented herein are expressed as means ± standard errors of the means. The Mann-Whitney and Kruskal-Wallis tests were used to determine the statistical significance of the intergroup comparison. Results were considered statistically significant when P was <0.05.
RESULTS
In vitro assays.
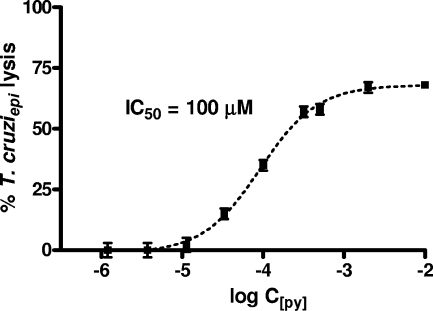
Preliminary experiments carried out to evaluate the in vitro trypanocidal activities of trans-[Ru(NO)(NH3)4L]3+ compounds, where L is imN, py, pz, l-hist, isn, P(OEt)3, or SO32−, were set up using epimastigote culture forms, harvested in the exponential phase of T. cruzi growth as determined through sigmoidal plots, as exemplified in Fig. 1. As shown in Table 1, the compounds where L was pz, isn, py, l-hist, or imN exhibited IC50epi values smaller than that for SNP. However, only trans-[Ru(NO)(NH3)4isn](BF4)3, trans-[Ru(NO)(NH3)4py](BF4)3, trans-[Ru(NO)(NH3)4imN](BF4)3, and trans-[Ru(NO)(NH3)4pz](BF4)3 complexes showed IC50epis of ≤100 μM.
FIG. 1.
Effect of the NO donors trans-[Ru(NO)(NH3)4L]3+, where L is pz, imN, isn, or py, against T. cruzi epimastigote forms (T. cruziepi) (1.0 × 106 parasites/ml) after 24 h of incubation. The concentrations range from 100 μM up to 1 mM. The data are representative of results of three independent experiments with similar results and are shown as percentages of the control results.
TABLE 1.
IC50s against V-79 cells and T. cruzi, in vitro TIs, and chemical properties for SNP and trans-[Ru(NO)(NH3)4L]3+ NO donors
| NO donor | IC50V79 (μM)a | Trypanocidal activity
|
In vitro TI (IC50V79/IC50try) | Chemical propertyc
|
||
|---|---|---|---|---|---|---|
| IC50tryb | IC50epi | ENO+/NO0 | k−NO | |||
| SNP | 51 | 52 | 284 | 1 | −0.195 | ND |
| t-[Ru(NO)(NH3)4pz](BF4)3 | 120 | 50 | 76 | 2 | 0.112 | 0.070 |
| t-[Ru(NO)(NH3)4L-hist](BF4)3 | 414 | 51 | 134 | 8 | −0.108 | 0.140 |
| t-[Ru(NO)(NH3)4imN](BF4)3 | 646 | 52 | 97 | 12 | −0.118 | 0.160 |
| t-[Ru(NO)(NH3)4isn](BF4)3 | 743 | 77 | 85 | 10 | 0.052 | 0.043 |
| t-[Ru(NO)(NH3)4py](BF4)3 | 930 | 75 | 100 | 12 | 0.012 | 0.060 |
| t-[Ru(NO)(NH3)4SO3]Cl | 1,000 | 59 | 300 | 17 | −0.138 | ND |
| t-[Ru(NO)(NH3)4P(OEt)3](PF6)3 | 2,260 | 60 | 423 | 38 | 0.132 | 0.980 |
According to published data (38), the ruthenium NO donor trans-[Ru(NO)(NH3)4L]3+, where L is imN, py, pz, l-hist, isn, P(OEt)3, or SO32−, and the well-known NO donor SNP, tested in vitro against BT, exhibited between 7- and 10-fold-higher activity than did gentian violet. In light of these findings, the IC50s against BT (IC50try) and the cytotoxicity data for mammalian V-79 cells (IC50V79) previously reported (32, 38, 45) were used to estimate the in vitro TI. This would be a reasonable guideline for a safe dose for in vivo experiments (38). The IC50V79 data for the trans-[Ru(NO)(NH3)4P(OEt)3]+3 complex were assessed using three tests: (i) the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, (ii) the neutral red uptake assay, and (iii) the nucleic acid content assay (45). The IC50V79s for the other complexes were measured through the nucleic acid content assay (32, 38). Table 1 also summarizes these data and exhibits only the respective in vitro TI values for the compounds with IC50trys of ≤100 μM.
SNP and the ruthenium NO donor trans-[Ru(NO)(NH3)4pz](BF4)3 exhibited excellent activities after 24 h of incubation at 37°C (IC50try = 52 and 50 μM, respectively). However, their use as chemoprophylaxis agents is discouraged since their in vitro TIs are 1 and 2, respectively. The trans-[Ru(NO)(NH3)4SO3]Cl and trans-[Ru(NO)(NH3)4P(OEt)3](PF6)3 compounds also exhibited very good activities against the infective forms of T. cruzi (IC50trys = 59 and 60 μM, respectively). Nevertheless, these compounds were considered inadequately trypanocidal against the multiplicative epimastigote forms (IC50epis ≥ 300 μM) (Table 1). Thus, despite the good in vitro TI values for these ruthenium NO donors, we decided not to use these compounds for in vivo experiments in the present study. The trans-[Ru(NO)(NH3)4L]3+ compounds in which L is imN, py, or isn are shown to exhibit excellent trypanocidal activities against both epimastigotes and trypomastigotes (IC50epi/trys ≤ 100 μM) (Table 1) and were then selected to evaluate the optimal dose and to calculate the median effective dose for chemotherapy in acute murine Chagas' disease. Furthermore, among all the ruthenium NO donors dealt with in this study that exhibited IC50epi/trys values of ≤100 μM for multiplicative and infective forms, only these three compounds exhibited an in vitro TI that was ≥10 (Table 1).
In vivo assays.
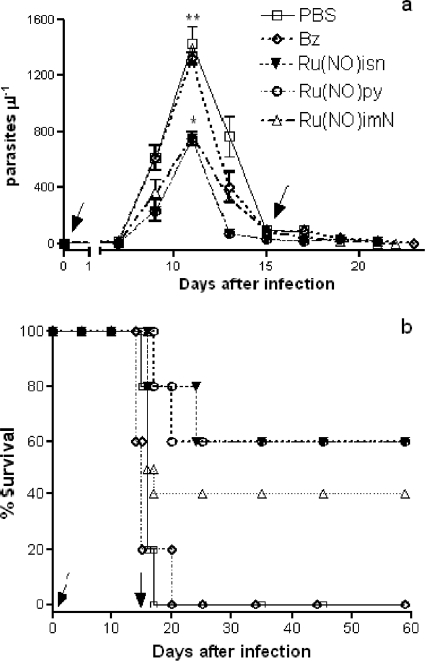
Using an up-and-down test protocol, which was developed as an alternative to replace the oral 50% lethal dose (LD50) test (9), the acute toxicity of the trans-[Ru(NO)(NH3)4imN](BF4)3, trans-[Ru(NO)(NH3)4py](BF4)3, and trans-[Ru(NO)(NH3)4isn](BF4)3 complexes was evaluated. The animals did not show any toxic symptoms during the 7-day period when doses up to 125 μmol/kg were i.p. administered (9). However, at 200-μmol/kg doses, hyperventilation, tremors, or thirst symptoms were observed, and at 250-μmol/kg doses, the mice died on the second day. Therefore, it is reasonable to assume that the true LD50 is between 125 and 250 μmol/kg for the three complexes evaluated. Thus, according to the ATC method, the trans-[Ru(NO)(NH3)4imN](BF4)3, trans-[Ru(NO)(NH3)4py](BF4)3, and trans-[Ru(NO)(NH3)4isn](BF4)3 complexes are in the class 3 toxicity group and hence have been classified as moderately toxic (35). Next, two sets of assays were performed to evaluate in vivo trypanocidal activity. In the first, the trans-[Ru(NO)(NH3)4L](BF4)3 compounds which exhibited in vitro TIs higher than 10, where L is imN, py, or isn, hereinafter referred to as Ru(NO)imN, Ru(NO)py, and Ru(NO)isn, respectively, were assayed in a murine model of acute Chagas' disease. According to Fig. 2, the three compounds, administered orally at a 100-nmol/kg dose for 15 consecutive days, are able to decrease the parasitemic peak by 40 to 50% compared to parasite peaks in the nontreated group (receiving only PBS) and even the group treated with Bz at the same dose (100 nmol/kg = 26 μg/kg). The Ru(NO)py compound, despite its very good in vitro and in vivo activities against trypomastigote forms, was not used in the other in vivo experiments because its specific rate constant for NO release (k−NO = 6.0 × 10−2s−1), reduction potential for the [RuIINO+]/[RuIINO0] couple (ERuIINO0/RuIINO+ = 0.012 V), and in vitro and in vivo trypanocidal activities (IC50try = 75 μM; percent survival = 60%) were similar to those of the Ru(NO)isn compound (k−NO = 4.3 × 10−2s−1 and ERuIINO0/RuIINO+ = 0.052 V versus those for NHE, IC50try = 77 μM, and percent survival = 60%).
FIG. 2.
Parasitemia and survival of Swiss mice infected with T. cruzi and treated with Ru(NO)isn, Ru(NO)py, or Ru(NO)imN compounds. The mice were infected with T. cruzi (Y strain, 1.0 × 103 BT/mouse) and treated by a 100-nmol oral dose of Ru(NO)isn, Ru(NO)py, or Ru(NO)imN per kg of body weight for 15 consecutive days. Another group of mice received only PBS (control group) or Bz at a 100-nmol/kg dose (26 μg/kg). (a) Parasitemia levels; (b) survival curves. The data are representative of results of three independent experiments with similar results (six mice per group). Arrows indicate the beginnings and the ends of the treatment periods. *, results considered statistically significant.
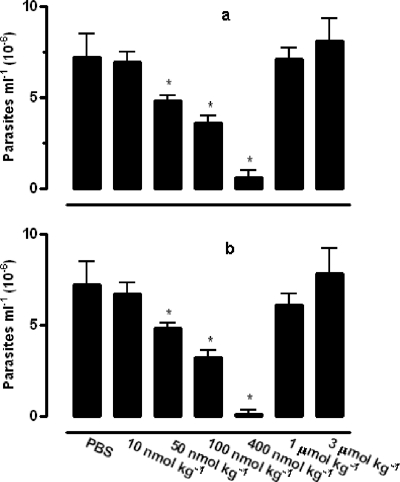
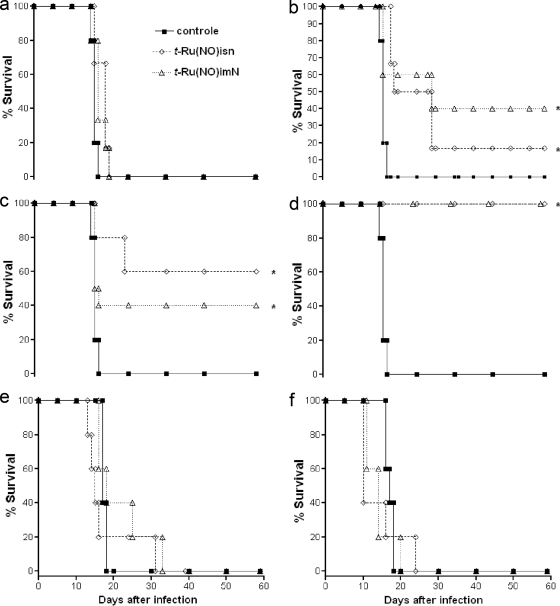
In the second set, the Ru(NO)isn and Ru(NO)imN compounds, which exhibit distinct ENO+/NO0 and k−NO (Table 1), were tested in vivo using different concentrations. The 10-, 50-, 100-, 400-, 1,000-, and 3,000-nmol/kg doses of Ru(NO)isn or Ru(NO)imN were administered by the i.p. route for 15 consecutive days. These two compounds were able to reduce the number of parasites throughout the course of the infection. For example, Fig. 3 shows only the data for the parasitemic peak (11th day after inoculation). According to our experimental data, the course of the infection was reduced by administering ruthenium NO donors in nanomolar concentrations, with the ideal dose being 400 nmol/kg. However, at micromolar concentrations, both Ru(NO)isn and Ru(NO)imN were shown to exhibit opposing effects, even increasing, at some concentrations (e.g., 3,000 nmol/kg), the number of parasites per ml over that found in the control group (which received only PBS). As a consequence, the survival rates at 60 days after infection for Ru(NO)imN were 0, 40, 40, 100, 0, and 0%, respectively, for the concentrations of 10, 50, 100, 400, 1,000, and 3,000 nmol/kg, whereas for Ru(NO)isn, these numbers were 0, 20, 60, 100, 0, and 0%, respectively, for the same concentrations (Fig. 4). The median effective doses for the Ru(NO)isn and Ru(NO)imN compounds calculated from the sigmoid dose-response curve are 86 and 190 nmol/kg, respectively, and therefore the in vivo TI values for these compounds are 1,453 and 658, respectively. Additionally, infected mice treated with a 400-nmol/kg dose of Bz did not exhibit any protective effect against death (data not shown).
FIG. 3.
Parasitemia levels at the parasitemic peak (11th day after inoculation) of Swiss mice infected with T. cruzi and treated with Ru(NO)isn or Ru(NO)imN compounds. Each group of six mice was infected with T. cruzi (Y strain, 1.0 × 103 BT/mouse) and treated i.p. with PBS (control group) or 10, 50, 100, 400, 1,000, or 3,000 nmol/kg of one of the tested compounds for 15 consecutive days. (a and b) Parasitemia levels with Ru(NO)isn (a) and Ru(NO)imN (b). The data are representative of results of three independent experiments with similar results (six mice per group). *, values significantly lower than the control value (P < 0.05).
FIG. 4.
Survival curves of Swiss mice infected with T. cruzi and treated with Ru(NO)isn or Ru(NO)imN. The mice were infected with T. cruzi (Y strain, 1.0 × 103 BT/mouse) and treated i.p. with 10 (a), 50 (b), 100 (c), 400 (d), 1,000 (e), or 3,000 (f) nmol/kg of Ru(NO)isn or Ru(NO)imN. Another group of mice received only PBS (control). The data are representative of results of three independent experiments with similar results (six mice per group). *, results considered statistically significant.
Histological analysis.
All the experiments conducted for this study were performed according to a previously described protocol (38). Swiss mice were i.p. infected with 1.0 × 103 BT/mouse and treated with Ru(NO)imN or Ru(NO)isn at a dose of 400 nmol/kg for 15 consecutive days. On the 15th day after infection, the survivor mice of the control group (treated only with PBS) and of the group treated with the nitrosyl complexes were euthanized and the hearts processed for staining with hematoxylin and eosin.
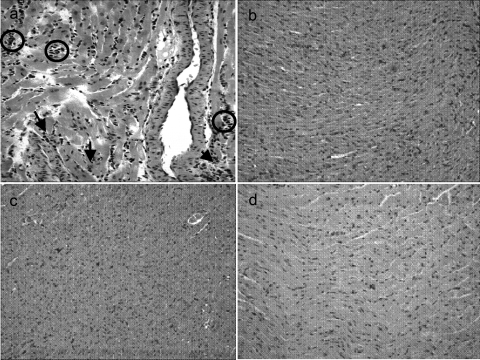
The microscopy analysis revealed that the control mice exhibited several nests of amastigotes (intracellular forms of T. cruzi) in their hearts, whereas no nests were observed in the hearts of the mice treated with the Ru(NO)imN or Ru(NO)isn compounds. Furthermore, the histological analysis also showed that chemotherapy with these compounds decreases the occurrence of myocarditis (Fig. 5).
FIG. 5.
Histological patterns of heart sections of Swiss mice infected with T. cruzi (1.0 × 103 BT/mouse) and treated with PBS (a), Ru(NO)imN (b), or Ru(NO)isn (c) for 15 consecutive days. (d) Noninfected mice. On the 15th day after infection, the mice were sacrificed and their hearts processed for staining with hematoxylin and eosin. Note the intensity of the inflammatory process with mononuclear cell infiltrates in panel a but not in panel b or c. Arrows indicate the nests of amastigotes; circles indicate inflammatory infiltrates. Photomicrographs are representative of results of three independent experiments with similar results. Final magnification, ×200.
DISCUSSION
NO has emerged as an important cytotoxic and cytostatic effector for a number of pathogens, including viruses, bacteria, fungi, and parasites (13). The NO produced by IFN-γ-activated macrophages is the effector mechanism that kills T. cruzi (16, 51). NO donors are capable of blocking the life cycles of Plasmodium, Trypanosoma, and Leishmania species by inactivating parasite enzymes, e.g., cysteine proteinases (13). Therefore, NO-based therapies against T. cruzi have been an interesting alternative (27), especially those involving the use of NO donor compounds capable of controlling NO levels in vivo (38). Indeed, ruthenium NO donors are able to decrease T. cruzi infection at nanomolar concentrations when doses of 50 to 400 nmol/kg are administered for 15 consecutive days. Whereas Bz is unable to reduce the course of infection at 100 nmol/kg, the Ru(NO)isn, Ru(NO)py, and Ru(NO)imN compounds showed 40 to 50% less parasitemia than the control group and 40 to 60% of the protective effect against death at 100-nmol/kg doses. Furthermore, at a 400-nmol/kg dose, Ru(NO)isn and Ru(NO)imN exhibit 100% of the protective effect against death; thus, this dose can be considered optimal for chemotherapy of T. cruzi infection in mice. On the other hand, unregulated NO production during parasite infection promotes inflammation, induces cell and tissue dysfunction (5, 25), and leads to less host resistance to T. cruzi infection (53). Moreover, NO has been reported to also play a role in apoptosis induction during the acute phase of T. cruzi infection in mice (1, 24, 36), in the suppression of host immunity (1, 25, 39), and in the pathogenesis of Chagas' disease of the heart (19). In addition, there are reports in the literature suggesting that the presence of NO increases the toxicity of reactive oxygen species, yielding oxidizing agents such as peroxynitrite (18, 42). This opposing effect was also observed in the present study, since the administration of Ru(NO)isn or Ru(NO)imN compounds in doses of 1,000 to 3,000 nmol/kg for 15 consecutive days leads to increased parasitemia levels and reduces the survival rate with respect to that of the control group (Fig. 4e and f). Thus, we took the precaution of not exceeding the IC50 and LD50 limits for these compounds. However, the possibility that at this concentration level (1,000 to 3,000 nmol/kg) these nitrosyl complexes exhibit some vasodilator effect could not be ruled out (38). This would have a negative effect on infected animals, whose hearts are already fragile. Therefore, all the experiments (except the ones using 1,000 to 3,000 nmol/kg) were performed in such a way as to avoid the nitrosyl complex hypotensive-effect manifestation.
SNP is a well-known NO donor and has been clinically used for over 70 years to reduce blood pressure in hypertensive emergencies despite the concomitant CN− liberation (23). Although SNP has the ability to inhibit the catalytic activity of cruzipain by 94% at 10 μM (48), its use as a chemoprophylaxis agent would not be recommended, since the concentration necessary to inhibit trypomastigotes is equal to their in vitro cytotoxicity value (in vitro TI = 1). Furthermore, this iron NO donor is in vivo 10- to 17-fold more toxic than the ruthenium NO donors (45).
On the other hand, it has been reported that T. cruzi's principal mechanisms of defense against oxidative stress are reduced trypanothione [T(SH)2] and glutathione (26, 46). Since the ruthenium nitrosyl complexes are able to react efficiently with sulphydryl groups such as cysteine and glutathione (33) and glutathione is a precursor of trypanothione synthesis, it is reasonable to suppose that a possible mechanism of action for the nitrosyl compounds is through thiol metabolism, with oxidation of sulfhydryl groups (as in parasites) and NO release to trypanocidal effect through oxidative stress. Furthermore, trans-[Ru(NH3)4L(SO4)]+ and trans-[Ru(H2O)(NH3)4P(OEt)3]2+, which are not able to lyse both trypomastigotes and epimastigotes (38), react with sulfhydryl groups (15) but are unable to act as NO donors.
In this sense, glycolysis has been claimed as another promising target for the development of new drugs against T. cruzi, since trypomastigotes are highly dependent on glycolysis as a source of ATP production (50). In this metabolic pathway, there are at least three enzymes in trypanosomatid protozoa whose three-dimensional structures have been determined (40). One of these proteins in T. cruzi is the glycosomal glyceraldehyde-3-phosphate dehydrogenase (gGAPDH), which shows potential target sites with significant differences from those of the homologous human enzyme (49). This enzyme catalyzes the oxidative phosphorylation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate in the presence of NAD+ and inorganic phosphate (54). It has been reported that NO is able to inhibit rabbit muscle GAPDH activity by modifying the thiols (e.g., cysteine) in the active site that are essential for its catalytic activity (20). However, no such result has been found for T. cruzi-gGAPDH. Thus, the ability of the ruthenium NO donors trans-[Ru(NO)(NH3)4isn](BF4)3, trans-[Ru(NO)(NH3)4py](BF4)3, and trans-[Ru(NO)(NH3)4imN](BF4)3 to inhibit the catalytic activity of T. cruzi-gGAPDH has been investigated by using a previously reported procedure (52). No significant inhibitory activity was found when the enzyme was assayed in these compounds in concentrations up to 350 μM, not even in the presence of reducing agents such as ascorbic acid and cysteine. Since the ICs for trypomastigotes of trans-[Ru(NO)(NH3)4isn](BF4)3, trans-[Ru(NO)(NH3)4py](BF4)3, and trans-[Ru(NO)(NH3)4imN](BF4)3 are lower than 100 μM, it is likely that, at least for the compounds considered in this study, the mechanism of action will not be T. cruzi-gGAPDH inhibition.
Conclusions.
The trans-[Ru(NO)(NH3)4L]3+ ruthenium complexes in which L is N-heterocyclic H2O, SO32−, or P(OEt)3 are potent trypanocidal compounds able to lyse epimastigotes and trypomastigotes in vitro. However, only the compounds in which L is P(OEt)3, SO32−, py, imN, and isn showed in vitro TIs higher than 10. The Ru(NO)py compound exhibited an in vivo trypanocidal activity similar to that of the Ru(NO)isn compound, and this was ascribed to the similarity of their chemical properties. According to our experiments, the true LD50s for the Ru(NO)isn, Ru(NO)imN, and Ru(NO)py compounds are in the range of 125 to 250 μmol/kg. The optimal dose for Ru(NO)isn and Ru(NO)imN compounds in the chemotherapy of T. cruzi infection in mice is 400 nmol/kg, and their median effective doses are 86 and 190 nmol/kg, respectively. Thus, the in vivo TIs are 1,453 for Ru(NO)isn and 658 for Ru(NO)imN. Additionally, these compounds are able to eliminate amastigote nests at 400-nmol/kg doses. Although these compounds exhibit very good trypanocidal activities and are able to react with cysteine, they exhibit very low activities against T. cruzi-gGAPDH.
TABLE 2.
Half-maximal effective concentrations from the in vivo experiments with the trans-[Ru(NO)(NH3)4L]3+ compounds in which L is imN, isn, or SNP
Acknowledgments
We are indebted to CAPES, MIVDT, CNPq, and FAPESP for their financial support.
We are also grateful to Glaucius Oliva (IFSC-USP) for providing the facilities for the T. cruzi-gGAPDH assays.
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Abrahamsohn, I. A., and R. L. Coffman. 1995. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J. Immun. 155:3955-3963. [PubMed] [Google Scholar]
- 2.Aliberti, J. C., F. S. Machado, J. T. Souto, A. P. Campanelli, M. M. Teixeira, R. T. Gazzinelli, and J. S. Silva. 1999. β-Chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect. Immun. 67:4819-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aufderheide, A. C., W. Salo, M. Madden, J. Streitz, J. Buikstra, F. Guhl, B. Arriaza, C. Renier, L. E. Wittmers, Jr., G. Fornaciari, and M. Allison. 2004. A 9000-year record of Chagas' disease. Proc. Natl. Acad. Sci. USA 101:2034-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bocedi, A., L. Gradoni, E. Menegatti, and P. Ascenzi. 2004. Kinetics of parasite cysteine proteinase inactivation by NO-donors. Biochem. Biophys. Res. Commun. 315:710-718. [DOI] [PubMed] [Google Scholar]
- 5.Bonavida, B., S. Khineche, S. Huerta-Yepez, and H. Garbán. 2006. Therapeutic potential of nitric oxide in cancer. Drug Resist. Updat. 9:157-173. [DOI] [PubMed] [Google Scholar]
- 6.Borges, S. S. S., C. U. Davanzo, E. E. Castellano, J. Z-Schpector, S. C. Silva, and D. W. Franco. 1998. Ruthenium nitrosyl complexes with N-heterocyclic ligands. Inorg. Chem. 37:2670-2677. [DOI] [PubMed] [Google Scholar]
- 7.Brener, Z. 1962. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 4:389-396. [PubMed] [Google Scholar]
- 8.Brener, Z. 1969. The behavior of slender and stout forms of Trypanosoma cruzi in the blood-stream of normal and immune mice. Ann. Trop. Med. Parasitol. 63:215-220. [DOI] [PubMed] [Google Scholar]
- 9.Bruce, R. D. 1985. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 5:151-157. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo, F., J. C. Voltarelli, S. G. Reed, and J. S. Silva. 1996. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect. Immun. 64:128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerecetto, H., and M. González. 2002. Chemotherapy of Chagas' disease: status and new development. Curr. Top. Med. Chem. 2:1187-1213. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, M. J. 1978. Electrochemistry, synthesis, and spectra of pentaammineruthenium(III) complexes of cytidine, adenosine, and related ligands. J. Am. Chem. Soc. 100:5068-5075. [Google Scholar]
- 13.Colasanti, M., L. Gradoni, M. Mattu, T. Persichini, L. Salvati, G. Venturini, and P. Ascenzi. 2002. Molecular bases for the anti-parasitic effect of NO. Int. J. Mol. Med. 9:131-134. [PubMed] [Google Scholar]
- 14.Coura, J. R., and S. L. Castro. 2002. A critical review on Chagas' disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3-24. [DOI] [PubMed] [Google Scholar]
- 15.Filho, J. R. O., W. C. Silva, J. C. M. Pereira, and D. W. Franco. 2006. Binding of cysteine and glutathione to Ru(II) and Ru(III) centers: formation and products reactivities. Inorg. Chim. Acta 359:2888-2895. [Google Scholar]
- 16.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 22:2501-2506. [DOI] [PubMed] [Google Scholar]
- 17.Gelb, M. H., and W. G. J. Hol. 2002. Drugs to combat tropical protozoan parasites. Science 297:343-344. [DOI] [PubMed] [Google Scholar]
- 18.Gutteridge, J. M., and B. Halliwell. 2000. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann. N. Y. Acad. Sci. 899:136-147. [DOI] [PubMed] [Google Scholar]
- 19.Huang, H., J. Chan, M. Wittner, L. A. Jelicks, S. A. Morris, S. M. Factor, L. M. Weiss, V. L. Braunstein, C. J. Bacchi, N. Yarlett, M. Chandra, J. Shirani, and H. B. Tanowitz. 1999. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J. Mol. Cell. Cardiol. 31:75-88. [DOI] [PubMed] [Google Scholar]
- 20.Ishii, T., O. Sunami, H. Nakajima, H. Nishio, T. Takeuchi, and F. Hata. 1999. Critical role of sulfenic acid formation of thiols in the inactivation of flyceraldehyde-3-phosphate dehydrogenase by nitric oxide. Biochem. Pharmacol. 58:133-143. [DOI] [PubMed] [Google Scholar]
- 21.Lopes, L. G. F., E. E. Castellano, A. G. Ferreira, C. U. Davanzo, M. J. Clarke, and D. W. Franco. 2004. Reactivity of trans-[Ru(NH3)4P(OEt)3NO]X3, (X = PF6−, CF3COO−): modulation of the release of NO by the trans-effect. Inorg. Chim. Acta 358:2883-2890. [Google Scholar]
- 22.Machado, F. S., G. A. Martins, J. C. S. Aliberti, F. L. A. C. Mestriner, F. Q. Cunha, and J. S. Silva. 2000. Trypanosoma cruzi-infected cardiomuocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation 102:3003-3008. [DOI] [PubMed] [Google Scholar]
- 23.Marks, G. S., B. E. McLaughlin, S. L. Jimmo, M. Poklewska-Koziell, J. F. Bien, and K. Nakatsu. 1995. Time-dependent increase in nitric oxide formation concurrent with vasodilation induced by sodium nitroprusside, 3-morpholinosydnonimine, and S-nitroso-N-acetylpenicillamine but not by glyceryl trinitrate. Drug Metab. Dispos. 23:1248-1252. [PubMed] [Google Scholar]
- 24.Martinez-Díaz, R. A., J. A. Escario, J. J. Nogal-Ruiz, and A. Gómez-Barrio. 2001. Biological characterization of Trypanosoma cruzi strains. Mem. Inst. Oswaldo Cruz 96:53-59. [DOI] [PubMed] [Google Scholar]
- 25.Martins, G. A., A. G. Cardoso, J. C. S. Aliberti, and J. S. Silva. 1998. Nitric oxide-induced apoptotic cell death in the acute phase of Trypanosoma cruzi infection in mice. Immunol. Lett. 63:113-120. [DOI] [PubMed] [Google Scholar]
- 26.Maya, J. D., B. K. Cassels, P. Iturriaga-Vásquez, J. Ferreira, M. Faúdez, N. Galanti, A. Ferreira, and A. Morello. 2007. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with mammalian host. Comp. Biochem. Physiol. A 146:601-620. [DOI] [PubMed] [Google Scholar]
- 27.Napoli, C., and L. J. Ignarro. 2003. Nitric oxide-releasing drugs. Annu. Rev. Pharmacol. Toxicol. 43:97-123. [DOI] [PubMed] [Google Scholar]
- 28.Osti, R. Z., and D. W. Franco. 2007. Aspects of nitrite association with trans-[Ru(NH3)4P(OEt)3(H2O)]2+. Polyhedron 26:4746-4750. [Google Scholar]
- 29.Perrin, D. D., W. L. F. Armarego, and D. R. Perrin. 1980. Purification of laboratory chemicals. Pergamon Press, Elmsford, NY.
- 30.Petray, P., E. Castaños-Velez, S. Grinstein, A. Orn, and M. E. Rottenberg. 1995. Role of nitric oxide in resistance and histopathology during experimental infection with Trypanosoma cruzi. Immunol. Lett. 47:121-126. [DOI] [PubMed] [Google Scholar]
- 31.Reesink, H. W. 2004. European strategies against the parasite transfusion risk risque parasitaire, quelles stratégies en Europe? Transfus. Clin. Biol. 12:1-4. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez, J. A., A. Souza-Torsoni, D. W. Franco, and M. Haun. 1997. Abstr. 24th Reun. Anual Soc. Bras. Bioquím. Biol. Mol. (SBBq), Caxambu, Brazil, 3 to 6 May 1997, abstr. S-32.
- 33.Roncaroli, F., and J. A. Olabe. 2005. The reactions of nitrosyl complexes with cysteine. Inorg. Chem. 44:4719-4727. [DOI] [PubMed] [Google Scholar]
- 34.Saraiva, J., C. Vega, M. Rolon, R. Silva, M. L. A. Silva, P. M. Donate, J. K. Bastos, A. Gómez-Barrio, and S. Albuquerque. 2007. In vitro and in vivo activity of lignan lactones derivatives against Trypanosoma cruzi. Parasitol. Res. 100:791-795. [DOI] [PubMed] [Google Scholar]
- 35.Schlede, E., E. Genschow, H. Spielmann, G. Stropp, and D. Kayser. 2005. Oral acute toxic class: a successful alternative to the oral LD50 test. Regulat. Toxicol. Pharmacol. 42:15-23. [DOI] [PubMed] [Google Scholar]
- 36.Schmunis, G. A. 1991. Trypanosoma cruzi, the etiologic agent of Chagas' disease: status in the blood supply in endemic and nonendemic countries. Transfusion 31:547-557. [DOI] [PubMed] [Google Scholar]
- 37.Shriver, D. F. 1969. The manipulation of air-sensitive compounds. McGraw-Hill, New York, NY.
- 38.Silva, J. J. N., A. L. Osakabe, W. R. Pavanelli, J. S. Silva, and D. W. Franco. 2007. In vitro and in vivo antiproliferative and trypanocidal activities of ruthenium NO donors. Br. J. Pharmacol. 152:112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva, J. S., F. S. Machada, and G. A. Martins. 2003. The role of nitric oxide in the pathogenesis of Chagas disease. Front. Biosci. 1:314-315. [DOI] [PubMed] [Google Scholar]
- 40.Souza, D. H. F., R. C. Garratt, A. P. U. Araújo, B. G. Guimarães, W. D. P. Jesus, P. A. M. Michels, V. Hannaert, and G. Oliva. 1998. Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase: structure, catalytic mechanism and targeted inhibitor design. FEBS Lett. 424:131-135. [DOI] [PubMed] [Google Scholar]
- 41.Stefaneli, E. V. 2008. M. D. Sobre a redução do óxido nítrico em complexos de tetraaminas de rutênio (II). University of São Paulo, São Carlos, Brazil.
- 42.Sun, J., L. J. Druhan, and J. L. Zweier. 2008. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch. Biochem. Biophys. 471:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tfouni, E., M. Krieger, B. McGarvey, and D. W. Franco. 2003. Structure, chemical and photochemical reactivity and biological activity of some ruthenium nitrosyl complexes. Coord. Chem. Rev. 236:57-69. [Google Scholar]
- 44.Toledo, J. C., H. A. S. Silva, M. Scarpellini, V. Mori, A. J. Camargo, M. Bertoti, and D. W. Franco. 2004. Ruthenium tetraamine as a model of nitric oxide donor compounds. Eur. J. Inorg. Chem. 9:1879-1885. [Google Scholar]
- 45.Torsoni, A. S., B. F. Barros, J. C. Toledo, M. Haun, M. H. Krieger, E. Tfouni, and D. W. Franco. 2002. Hypotensive properties and acute toxicity of trans-[Ru(NH3)4P(OEt)3(NO)](PF6)3, a new nitric oxide donor. Nitric Oxide 6:247-354. [DOI] [PubMed] [Google Scholar]
- 46.Turrens, J. F. 2004. Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol. Aspects Med. 25:211-220. [DOI] [PubMed] [Google Scholar]
- 47.Valdez, C. A., J. E. Saavedra, B. M. Showalter, K. M. Davies, T. C. Wilde, M. L. Citro, J. J. Barchi, Jr., J. R. Deschamps, D. Parrish, S. El-Gayar, U. Schleicher, C. Bogdan, and L. K. Keefer. 2008. Hydrolytic reactivity trends among potential prodrugs of the O2-glycosylated diazeniumdiolate family. Targeting nitric oxide to macrophages for antileishmanial activity. J. Med. Chem. 51:3961-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venturini, G., L. Salavati, M. Muolo, M. Colasanti, L. Gradoni, and P. Ascenzi. 2000. Nitric oxide inhibits cruzipain, the major papain-like cysteine proteinase from Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 270:437-441. [DOI] [PubMed] [Google Scholar]
- 49.Verlinde, C. L. M. J., M. Callens, S. Van Calenbergh, A. Van Aerschot, P. Herdewijn, V. Hannaert, P. A. M. Michels, F. R. Opperdoes, and W. G. J. Hol. 1994. Selective inhibition of trypanosomal glyceraldehyde-3-phosphate dehydrogenase by protein structure-based design: toward new drugs for the treatment of sleeping sickness. J. Med. Chem. 37:3605-3613. [DOI] [PubMed] [Google Scholar]
- 50.Verlinde, C. L. M. J., V. Hannaert, C. Blonski, M. Willson, J. J. Périé, L. A. Fathergill-Gilmore, F. R. Opperdoes, M. H. Gelb, W. G. J. Hol, and P. A. M. Michels. 2001. Glycolysis as target for the design of new anti-trypanosome drugs. Drug Resist. Updat. 4:1-14. [DOI] [PubMed] [Google Scholar]
- 51.Vespa, G. N. R., F. Q. Cunha, and J. S. Silva. 1994. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect. Immun. 62:5177-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vieira, P. C., J. Mafezoli, M. T. Pupo, J. B. Fernande, M. F. G. F. Silva, S. Albuquerque, G. Oliva, and F. Pavão. 2001. Strategies for the isolation and identification of trypanocidal compounds from the rutales. Pure Appl. Chem. 73:617-622. [Google Scholar]
- 53.Waghabi, M. C., M. Keramidas, J. J. Feige, T. C. Araujo-Jorge, and S. Bailly. 2005. Activation of transforming growth factor beta by Trypanosoma cruzi. Cell. Microbiol. 7:511-517. [DOI] [PubMed] [Google Scholar]
- 54.Yun, M., C. G. Park, J. Y. Kim, and H. W. Park. 2000. Structural analysis of glyceraldehyde 3-phosphate dehydrogenase from Escherichia coli: direct evidence of substrate binding and cofactor-induced conformational changes. Biochemistry 39:10702-10710. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann, M. 1983. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109-110. [DOI] [PubMed] [Google Scholar]