Abstract
V-073, an enterovirus capsid inhibitor, was evaluated for its spectrum of antipoliovirus activity. V-073 inhibited all 45 polioviruses tested in a virus-induced cytopathic effect protection assay, with 50% effective concentration (EC50) values ranging from 0.003 to 0.126 μM. Ninety percent of the polioviruses tested were inhibited at EC50s of ≤0.076 μM (MIC90 = 32 ng/ml). V-073 is a promising antiviral candidate for the posteradication management of poliovirus incidents.
Polio eradication.
The World Health Organization (WHO), Rotary International, the U.S. Centers for Disease Control and Prevention (CDC), and the United Nations International Children's Emergency Fund launched the Global Polio Eradication Initiative in 1988. The initiative is approaching its goal and expects the world to be certified polio free in the near future. Protecting the 25-year and more than $7 billion investment in polio eradication will depend on the policies, tools, and tactics available during the final stages of eradication and in the posteradication era. The global public health community must be equipped to protect against virus reintroduction and, in the event of reintroduction, to rapidly contain, control, and eliminate the virus.
In November 2005, the National Research Council of the (U.S.) National Academies considered the potential role for antipoliovirus drugs in the eradication and posteradication management of poliovirus incidents. Currently, there are no antiviral drugs approved for treatment of poliovirus. The National Research Council concluded that there is indeed an important role for antivirals and recommended their immediate development (3). The case for developing antiviral drugs against poliovirus has been reviewed recently (2).
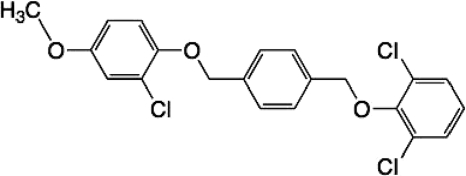
Compound V-073.
V-073 (Fig. 1), previously designated SCH 48973 (1), is a member of the picornavirus antiviral mechanistic class called capsid inhibitors. Antiviral compounds in this class inhibit picornaviruses by a virus-specific mode of action. These compounds integrate into the viral capsid at a specific site. Upon doing so, they prevent virus “uncoating” and the release of the viral RNA from the capsid, thereby blocking the initiation of the viral infection cycle (4). Other notable members of this class are pleconaril, disoxaril, and pirodavir. Unfortunately, these latter compounds lack the necessary antiviral potency and spectrum across poliovirus serotypes (2).
FIG. 1.
Chemical structure of V-073.
V-073 was previously reported to have broad-spectrum antienterovirus activity, including activity against poliovirus type 2, both in cell culture and in a poliovirus challenge model with mice (1). However, since the compound was then being developed for nonpolio enterovirus indications, its spectrum of antipoliovirus activity was not explored. Here, we present virus susceptibility data that indicate V-073 has specific, potent, and broad-spectrum antipoliovirus activity. Together with its other pharmacological attributes (to be reported elsewhere), V-073 represents a promising poliovirus antiviral drug candidate.
Antipoliovirus activity of V-073.
To assess the spectrum of antipoliovirus activity of V-073, a panel of 45 polioviruses was assembled and evaluated in a cell culture cytopathic effect assay. The panel consisted of viruses from all three poliovirus serotypes and included wild reference strains (poliovirus type 1 [PV1]-Brunhilde, PV1-Mahoney, PV2-Lansing, PV2-MEF-1, PV2-P712, PV3-Leon, and PV3-Saukett), the three Sabin vaccine strains, and representative circulating vaccine-derived poliovirus (cVDPV) isolates (12 PV1-cVDPV and 9 PV2-cVDPV) and vaccine-derived polioviruses from immunodeficient chronic excretors (iVDPV; eight PV1-iVDPV, four PV2-iVDPV, two PV3-iVDPV) collected from numerous geographic regions of the world over a period of time between 1981 and 2007. PV1-LSc/2ab, PV2-P712, and PV3-Leon are the parental strains from which the Sabin oral vaccine strains were derived, while PV1-Mahoney, PV2-MEF-1, and PV3-Saukett are the strains used to produce the inactivated polio vaccine. The assay for drug susceptibility measured protection by the drug of an LLC-MK2 cell monolayer from the virus replication-induced cytopathic effect and has been previously described (6).
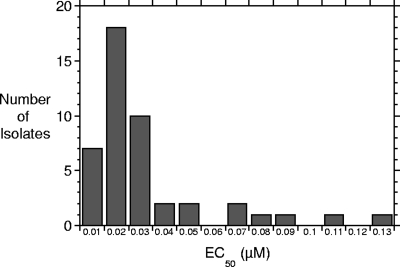
The 50% effective concentration (EC50) values for the 45 individual viruses, as determined from seven-point concentration curves using a four-parameter curve-fitting program (SoftMax Pro; Molecular Devices), are provided in Table 1. V-073 was active against all viruses in this panel, with EC50s ranging from 0.003 μM to 0.126 μM. The distribution of drug susceptibilities among these 45 polioviruses is depicted in Fig. 2. Ninety percent of all polioviruses tested were inhibited by V-073 at EC50s of ≤0.076 μM (MIC90 = 76 nM; 32 ng/ml). In Table 2, the mean EC50s for the various categories of polioviruses are provided. There appears to be no bias in the activity of V-073 among these virus groupings.
TABLE 1.
Activity of V-073 against individual polioviruses
| Strain | Country/Year collected | Serotype | Origin | EC50 ± SDc |
|---|---|---|---|---|
| 10235 | Dominican Republic/2000 | PV1 | cVDPV | 0.018 ± 0.003 |
| 10236 | Dominican Republic/2000 | PV1 | cVDPV | 0.029 ± 0.021 |
| 10238 | Dominican Republic/2000 | PV1 | cVDPV | 0.014 ± 0.006 |
| 10237 | Haiti/2000 | PV1 | cVDPV | 0.011 ± 0.005 |
| 10239 | Haiti/2001 | PV1 | cVDPV | 0.014 ± 0.003 |
| 10240 | Haiti/2001 | PV1 | cVDPV | 0.015 ± 0.007 |
| 10241 | Haiti/2001 | PV1 | cVDPV | 0.083 ± 0.055 |
| 10242 | Haiti/2001 | PV1 | cVDPV | 0.017 ± 0.013 |
| 10690 | Philippines/2001 | PV1 | cVDPV | 0.029 ± 0.006 |
| 10691 | Philippines/2001 | PV1 | cVDPV | 0.018 ± 0.012 |
| 10692 | Philippines/2001 | PV1 | cVDPV | 0.029 ± 0.015 |
| 10693 | Philippines/2001 | PV1 | cVDPV | 0.008 ± 0.003 |
| 10694 | USA/2005 | PV1 | iVDPV | 0.014 ± 0.002 |
| 10695 | USA/2005 | PV1 | iVDPV | 0.008 ± 0.005 |
| 10668 | Taiwan/2002 | PV1 | iVDPV | 0.021 ± 0.015 |
| 10689 | Taiwan/2002 | PV1 | iVDPV | 0.042 ± 0.029 |
| 10223 | USA/1982 | PV1 | iVDPV | 0.010 ± 0.008 |
| 10222 | USA/1981 | PV1 | iVDPV | 0.015 ± 0.009 |
| 10225 | USA/1990 | PV1 | iVDPV | 0.017 ± 0.010 |
| 10224 | USA/1987 | PV1 | iVDPV | 0.069 ± 0.064 |
| Brunhilde | USA/1939 | PV1 | Wild | 0.018 ± 0.009 |
| Mahoney | USA/1942b | PV1 | Wild | 0.076 ± 0.052 |
| Sabin 1 OPVa | PV1 | Vaccine | 0.017 ± 0.000 | |
| 10229 | Egypt/1993 | PV2 | cVDPV | 0.020 ± 0.022 |
| 10230 | Egypt/1998 | PV2 | cVDPV | 0.036 ± 0.019 |
| 10231 | Egypt/1998 | PV2 | cVDPV | 0.028 ± 0.013 |
| 10232 | Egypt/1999 | PV2 | cVDPV | 0.019 ± 0.013 |
| 10233 | Egypt/1999 | PV2 | cVDPV | 0.024 ± 0.014 |
| 10234 | Egypt/1999 | PV2 | cVDPV | 0.009 ± 0.001 |
| 10219 | Madagascar/2002 | PV2 | cVDPV | 0.023 ± 0.014 |
| 10220 | Madagascar/2002 | PV2 | cVDPV | 0.010 ± 0.007 |
| 10243 | Nigeria/2002 | PV2 | cVDPV | 0.038 ± 0.008 |
| 10221 | USA/1999 | PV2 | iVDPV | 0.027 ± 0.008 |
| 10228 | USA/1992 | PV2 | iVDPV | 0.126 ± 0.063 |
| 10226 | USA/1991 | PV2 | iVDPV | 0.019 ± 0.001 |
| 10227 | USA/1991 | PV2 | iVDPV | 0.019 ± 0.019 |
| Lansing | USA/1937 | PV2 | Wild | 0.007 ± 0.000 |
| MEF-1 | Egypt/1942b | PV2 | Wild | 0.026 ± 0.025 |
| P712 | USA/1954 | PV2 | Wild | 0.003 ± 0.000 |
| Sabin 2 OPVa | PV2 | Vaccine | 0.016 ± 0.012 | |
| 10804 | Egypt/2007 | PV3 | iVDPV | 0.042 ± 0.026 |
| 10805 | Iran/2007 | PV3 | iVDPV | 0.029 ± 0.005 |
| Saukett | USA/1962b | PV3 | Wild | 0.062 ± 0.008 |
| Leon | USA/1937 | PV3 | Wild | 0.109 ± 0.119 |
| Sabin 3 OPVa | PV3 | Vaccine | 0.020 ± 0.007 |
The Sabin type 1, 2, and 3 oral polio vaccine (OPV) strains are PV1-LSc/2ab, PV2-P712, and PV3-Leon, respectively.
PV1-Mahoney, PV2-MEF-1, and PV3-Saukett are seed strains for inactivated polio vaccine.
All values are in μM and based on at least two independent determinations.
FIG. 2.
Distribution of V-073 EC50s against 45 polioviruses.
TABLE 2.
Susceptibility of various categories of polioviruses to V-073
| Serotype category | Susceptibilitya
|
|||
|---|---|---|---|---|
| PV1 | PV2 | PV3 | Overall | |
| Wild | 0.047 ± 0.041 (2) | 0.012 ± 0.012 (3) | 0.086 ± 0.033 (2) | 0.043 ± 0.040 (7) |
| Vaccine | 0.017 ± 0.000 (1) | 0.016 ± 0.012 (1) | 0.020 ± 0.007 (1) | 0.026 ± 0.013 (3) |
| cVDPV | 0.024 ± 0.020 (12) | 0.023 ± 0.010 (9) | 0.023 ± 0.016 (21) | |
| iVDPV | 0.025 ± 0.021 (8) | 0.048 ± 0.052 (4) | 0.029 ± 0.005 (2) | 0.033 ± 0.031 (14) |
| Overall | 0.026 ± 0.022 (23) | 0.027 ± 0.027 (17) | 0.054 ± 0.035 (5) | 0.029 ± 0.026 (45) |
Mean EC50s ± standard deviations (in μM). The number in parentheses indicates the number of viruses included in the category.
Selectivity and specificity of V-073.
The selectivity index obtained for V-073 was 75, as calculated from a 50% cytotoxic concentration value for V-073 of approximately 6 μM [as determined by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (5) after 72 h of exposure (data not shown)] and the MIC90 value. An earlier reported 50% cytotoxic concentration value of >50 μg/ml (>118 μM) was based on 18 to 24 h of exposure (1).
Testing the compound against a variety of viruses, as well as bacteria and fungi, revealed the specificity of V-073. Among the human picornaviruses, V-073 is modestly active against some nonpolio enteroviruses and poorly active or inactive against rhinoviruses and hepatitis A virus (1). Table 3 summarizes drug susceptibility data for members of the Enterovirus genus, including the 45 poliovirus isolates, and a sampling of echoviruses, coxsackieviruses, and other enteroviruses. It is clear that V-073 is most potent against polioviruses and is substantially less active against the other enteroviruses.
TABLE 3.
Susceptibility of various enterovirus species to V-073a
| Virus (no. of viruses tested) | EC50 range | Mean EC50 | MIC90 |
|---|---|---|---|
| Poliovirus (45)b | 0.003-0.126 | 0.029 | 0.076 |
| Echovirus (64)c | 0.009-7.08 | 0.86 | 2.12 |
| Coxsackievirus (100)d | 0.007->14f | 3.79 | >14 |
| Enterovirus (9)e | 0.236->14 | 3.47 | 1.42 |
All values are in μM.
Viruses used in Table 1.
Of the 64 viruses tested, 56 were clinical isolates, and 8 were laboratory strains comprising serotypes 3, 4, 5, 6, 7, 9, 11, 24, and 30 (1).
All viruses tested were clinical isolates comprising serotypes A9, B1, B2, B3, B4, and B5 (1).
All viruses tested were clinical isolates comprising serotypes 68, 70, and 71.
The solubility limit in cell culture medium is approximately 14 μM.
V-073 had no activity against nonpicornaviruses, including adenovirus type 5, herpes simplex virus types 1 and 2, human immunodeficiency virus type 1, influenza A virus, measles virus, Punta Toro virus, respiratory syncytial virus, rotavirus 1, Semliki Forest virus, simian virus 40, and vaccinia virus (1). Finally, V-073 has no activity against a battery of gram-negative and gram-positive bacterial strains or fungus strains (data not shown).
Based on these in vitro antiviral results, the efficacy of V-073 in a mouse poliovirus challenge model (1), and the pharmacological attributes of the compound (our unpublished data), further development of V-073 is warranted. V-073 and compounds like it represent new tools that may play important roles in the posteradication management of poliovirus incidents. Such antiviral drugs could be used alone or as adjuncts to inactivated virus vaccines and may represent a key weapon in defending a polio-free world.
Acknowledgments
We thank the Task Force for Global Health, in particular Walter Dowdle, and the WHO for support. D.C.P. is a consultant to ViroDefense Inc.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Buontempo, P. J., S. Cox, J. Wright-Minogue, J. L. DeMartino, A. M. Skelton, E. Ferrari, R. Albin, E. J. Rozhon, V. Girijavallabhan, J. F. Modlin, and J. F. O'Connell. 1997. SCH 48973: a potent, broad-spectrum, antienterovirus compound. Antimicrob. Agents Chemother. 41:1220-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collett, M. S., J. Neyts, and J. F. Modlin. 2008. A case for developing antiviral drugs against polio. Antivir. Res. 79:179-187. [DOI] [PubMed] [Google Scholar]
- 3.Committee on Development of a Polio Antiviral and Its Potential Role in Global Poliomyelitis Eradication, National Research Council. 2006. Exploring the role of antiviral drugs in the eradication of polio. The National Academies Press, Washington, DC.
- 4.Diana, G. D., D. C. Pevear, M. J. Otto, M. A. McKinlay, M. G. Rossmann, T. Smith, and J. Badger. 1989. Inhibitors of viral uncoating. Pharmacol. Ther. 42:289-305. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 6.Pevear, D. C., T. M. Tull, M. E. Seipel, and J. M. Groarke. 1999. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 43:2109-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]