Abstract
We have evaluated the antifungal activity of micafungin in serum by using the disk diffusion method with serum-free and serum-added micafungin standard curves. Serum samples from micafungin-treated patients have been shown to exhibit adequate antifungal activity, which was in proportion to both the applied dose and the actual concentration of micafungin measured by high-performance liquid chromatography. The antifungal activity of micafungin in serum was also confirmed with the broth microdilution method.
Micafungin has been shown to bind to serum proteins at a level of 99.8% (13). If the unbound drug contributes to its pharmacological activity (the free-drug hypothesis), only 0.2% of total micafungin would be available to exert antifungal activity in the presence of serum, and the MIC for micafungin in vitro would increase 500-fold. However, several studies have shown that this ratio varies from 4- to 267-fold (6, 7, 11), indicating that the antifungal activities of micafungin in serum may not follow the free-drug hypothesis; instead, observed activities are mostly superior to those predicted. Furthermore, it remains unclear whether these results can be applied to micafungin in a patient's serum. To address this issue, we collected serum samples from micafungin-treated patients and examined the relationship between micafungin concentration and its in vitro antifungal activity in serum.
This study was approved by the institutional review board, and informed consent was obtained from each patient. Patients with hematologic malignancies, admitted into Osaka University Medical Hospital, were administered micafungin at a dose of 50 to 300 mg/body once daily. The efficacy of prophylaxis was defined as the absence of proven, probable (EORTC-IFICG/NIAID-MSG) (1), or suspected (unexplained persistent fever and clinical findings) (10) fungal infection, through the end of therapy. The efficacy of the drug for suspected fungal infections was indicated by improvement of persistent fever and clinical findings.
Blood samples were collected from patients just before (trough) and after (peak) micafungin infusion, at least 4 days after initiating treatment (steady state) (2). Micafungin concentration in serum was measured by high-performance liquid chromatography (HPLC) (9, 12). The disk diffusion method was performed according to National Committee for Clinical Laboratory Standards (NCCLS) M44-A guidelines (5). To obtain standard curves, we prepared two types of serial dilution disks impregnated with micafungin standard solution, one in RPMI 1640 (serum-free standard) and the other in heat-inactivated serum from volunteers (serum-added standard). Disks were applied to Sabouraud dextrose agar plates inoculated with Candida albicans FP633, a clinical isolate kindly provided by Astellas Pharma Inc., Tokyo, Japan. The diameter of the area of complete growth inhibition (inhibitory zone) was measured. Similarly, disks were impregnated with serum samples collected from patients, and the inhibitory zones were measured. The determination of antifungal activity of micafungin in a patient's serum was based on two standard curves, as described above. To determine the inhibitory titer in a patient's serum, we utilized the broth microdilution method based on the guidelines in NCCLS M27-A2 (4). Serum from a patient was serially diluted twofold with serum from a volunteer, supplemented with 20 mM HEPES, and inoculated with C. albicans FP633. MIC was defined as the lowest concentration where no visible growth was observed. Serum inhibitory titers were defined as the highest dilution of serum that completely inhibited fungal growth.
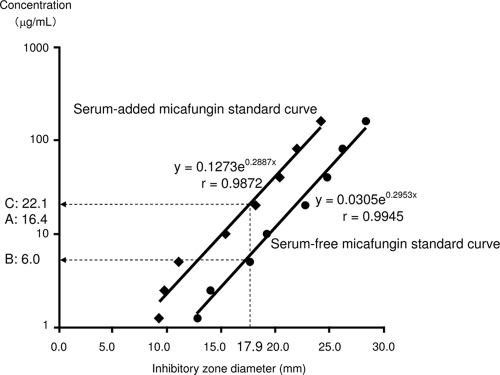
In all seven patients, micafungin was effective for prophylaxis or treatment against fungal infections (Table 1). Serum peak concentrations (Cmax) of micafungin (measured by HPLC) ranged from 5.59 to 37.1 μg/ml at a dose of 50 to 300 mg/body and closely correlated with both daily dose and dosage in terms of body weight (Table 2). Standard curves were prepared from both serum-free and serum-added micafungin standard disks (Fig. 1). The antifungal activity of micafungin remained intact in serum: 20 to 50% (by measured value) or 25 to 30% (by standard curve).
TABLE 1.
Patient background
| Patient no. | Age (yr) | Gendera | BWb (kg) | Diagnosisc | HSCTd | Antifungal treatment | Dose of micafungin (mg/body) | Duration of therapy (days) | Clinical efficacy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | M | 76 | ML | Auto-PBSCT | Preemptive therapy | 300 | 11 | Effective |
| 2 | 59 | F | 52 | ML | Auto-PBSCT | Preemptive therapy | 300 | 8 | Effective |
| 3 | 33 | M | 52 | MS | Allo-BMT | Empirical therapy | 75 | 55 | Effective |
| 4 | 51 | F | 47 | ML | Allo-BMT | Empirical therapy | 50 | 9 | |
| 150 | 16 | Effective | |||||||
| 225 | 20 | ||||||||
| 150 | 4 | ||||||||
| 5 | 47 | F | 58 | ML | Auto-PBSCT | Empirical therapy | 150 | 21 | Effective |
| 300 | 7 | ||||||||
| 6 | 22 | F | 45 | AML | Allo-BMT | Prophylaxis | 50 | 22 | Effective |
| 100 | 6 | ||||||||
| 7 | 46 | F | 43 | ALL | Allo-BMT | Prophylaxis | 50 | 22 | Effective |
| 100 | 9 |
M, male; F, female.
BW, body weight.
ML, malignant lymphoma; MS, myelodysplastic syndrome; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia.
HSCT, hematopoietic stem cell transplantation; PBSCT, peripheral blood stem cell transplantation; BMT, bone marrow transplantation.
TABLE 2.
Antifungal activities and inhibitory titers of serum samples from patients administered micafungin
| Patient no. | Dose of micafungin
|
Collection point
|
Antifungal activity of serum samples (μg/ml) measured using:
|
Ratio (%) of antifungal activities measured by:
|
Serum inhibitory titer | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg | mg/kg | Day | Time | HPLC | Disk diffusion methodc
|
Serum-free standard curve/HPLCa | Serum-added standard curve/HPLCb | |||
| Serum-free standard curve | Serum-added standard curve | |||||||||
| 1 | 300 | 3.9 | 10 | Peak | 34.2 | ND | ND | 32 | ||
| 2 | 300 | 5.8 | 8 | Peak | 33.6 | ND | ND | 32 | ||
| 3 | 75 | 1.4 | 45 | Peak | 6.7 | 2.5 | 4.2 | 38 | 63 | 4 |
| 4 | 225 | 4.8 | 43 | Peak | 37.1 | 14.1 | 34.8 | 38 | 94 | 32 |
| 5 | 150 | 2.6 | 12 | Peak | 16.4 | 6.0 | 22.1 | 37 | 135 | 16 |
| 6 | 50 | 1.1 | 8 | Trough | 2.7 | 1.1 | 2.1 | 42 | 78 | 2 |
| 8 | Peak | 8.4 | 3.8 | 8.1 | 42 | 96 | 8 | |||
| 15 | Trough | 3.0 | 1.0 | 1.7 | 32 | 58 | 4 | |||
| 15 | Peak | 5.6 | 2.5 | 5.2 | 45 | 92 | 8 | |||
| 7 | 50 | 1.2 | 15 | Trough | 2.3 | 0.9 | 1.6 | 40 | 71 | 2 |
| 15 | Peak | 6.4 | 3.3 | 7.0 | 52 | 109 | 4 | |||
| 17 | Trough | 2.0 | ND | ND | 2 | |||||
| 17 | Peak | 6.5 | 2.9 | 5.9 | 44 | 91 | 8 | |||
Mean ± standard deviation is 41% ± 6%.
Mean ± standard deviation is 89% ± 23%.
These serum concentrations were estimated using the two standard curves. ND, not determined.
FIG. 1.
Estimation of micafungin concentration in serum samples from patient no. 5, using the disk diffusion method. (A) Concentration measured using HPLC, 16.4 μg/ml. (B) Concentration estimated from the serum-free micafungin standard curve, 6.0 μg/ml. (C) Concentration estimated from the serum-added micafungin standard curve, 22.1 μg/ml. Ratio of concentration B to concentration A (%) = 6.0/16.4 × 100 = 37. Ratio of concentration C to concentration A (%) = 22.1/16.4 = 134.8.
Results for all seven successfully treated patients are summarized in Table 2, as are the micafungin concentrations in serum samples measured by HPLC. The antifungal activity of micafungin in serum samples from these patients was 41% ± 6% (mean value ± standard deviation, ranging from 37% to 52%) of the actual micafungin serum concentration (the ratio of antifungal activity estimated by the disk diffusion method based on the serum-free standard curve to that measured by HPLC). Representative results for patient no. 5 are shown in Fig. 1. Meanwhile, the antifungal activity of micafungin calculated with the serum-added standard curve was almost equal to the actual micafungin serum concentration (the ratio of antifungal activity estimated by the disk diffusion method based on the serum-free standard curve to that measured by HPLC was 89% ± 23% [mean ± standard deviation, ranging from 58% to 135%]) (Table 2).
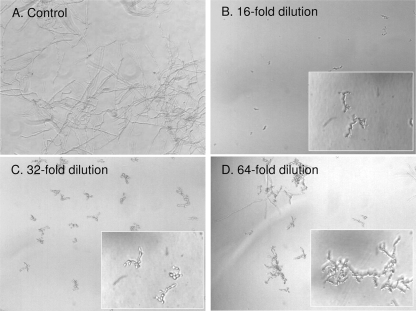
MIC for micafungin against C. albicans FP633 in heat-inactivated serum from a volunteer was 1 μg/ml, which was consistent with previously reported data using the same strain (3). At this concentration, micafungin induced swelling and subsequent burst of mycelia. Inhibitory titers for serum samples from all patients are summarized in Table 2. Representative results from patient no. 5 are shown in Fig. 2. These titers were in excellent agreement with both micafungin concentrations in serum samples by HPLC and those estimated from the serum-added standard curve (Table 2).
FIG. 2.
Determining the inhibitory titer values for serum from patient no. 5 using the broth microdilution method. MIC was defined as the lowest concentration at which no visible growth was observed (magnification of ×40). Serum inhibitory titers were defined as the highest dilution of serum that completely inhibited fungal growth. Insets show C. albicans morphologies (magnification of ×400).
These results indicate that serum proteins certainly bind to micafungin and reduce its antifungal activity, but this binding may be reversible and weak. These data are inconsistent with the free-drug hypothesis. One or more of the following reasons could explain this discrepancy. First, micafungin binds to serum proteins at 99.8% in situations without any other competitors, such as in ultrafiltration, the method measuring the equilibrium binding (13). If fungi susceptible to micafungin are present, however, micafungin may be easily released from the protein-bound form in a rapid equilibrium, bind to target pathogens, and exert its antifungal activity. In this case, increased MIC of micafungin in serum may depend on the fungal strains being tested (6, 7). Furthermore, although albumin is supposed to bind mainly to micafungin, several other proteins in serum, such as alpha and gamma globulins, might influence the interactions among micafungin, serum proteins, and target pathogens (8).
In conclusion, it seems to be unsuitable to apply the free-drug hypothesis to the pharmacodynamics of micafungin, because this may underestimate its antifungal activity. We have shown, using the disk diffusion and broth dilution methods, that serum samples from micafungin-treated patients exhibited adequate antifungal activity. Our data will be useful for understanding the pharmacodynamics of micafungin and for improving the clinical outcome of micafungin treatment.
Acknowledgments
This study was supported in part by a grant from Astellas Pharma Inc.
Footnotes
Published ahead of print on 17 August 2009.
REFERENCES
- 1.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh, the Invasive Fungal Infections Cooperating Group of the European Organization for Research and Treatment of Cancer, and Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Azuma, J., K. Nakahara, A. Kagayama, T. Kajiho, A. Kawamura, H. Suematsu, and T. Mukai. 2002. Phase I study of micafungin. Jpn. J. Chemother. 50(Suppl. 1):104-147. [Google Scholar]
- 3.Maki, K., S. Matsumoto, E. Watabe, Y. Iguchi, M. Tomishima, H. Ohki, A. Yamada, F. Ikeda, S. Tawara, and S. Mutoh. 2008. Use of a serum-based antifungal susceptibility assay to predict the in vivo efficacy of novel echinocandin compounds. Microbiol. Immunol. 52:383-391. [DOI] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A2. NCCLS, Wayne, PA.
- 5.National Committee for Clinical Laboratory Standards. 2004. Method for antifungal disc diffusion susceptibility testing of yeasts: proposed guideline M44-A. NCCLS, Wayne, PA.
- 6.Odabasi, Z., V. Paetznick, J. H. Rex, and L. Ostrosky-Zeichner. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paderu, P., G. Garcia-Effron, S. Balashov, G. Delmas, S. Park, and S. Perlin. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schäfer-Korting, M., H. C. Korting, W. Rittler, and W. Obermüller. 1995. Influence of serum protein binding on the in vitro activity of antifungal agents. Infection 23:292-297. [DOI] [PubMed] [Google Scholar]
- 9.Tabata, K., M. Katashima, A. Kawamura, Y. Tanigawara, and K. Sunagawa. 2006. Linear pharmacokinetics of micafungin and its active metabolites in Japanese pediatric patients with fungal infections. Biol. Pharm. Bull. 29:1706-1711. [DOI] [PubMed] [Google Scholar]
- 10.Tamura, K., A. Urabe, M. Yoshida, A. Kanamaru, Y. Kodera, S. Okamoto, S. Maesaki, and T. Masaoka. 2008. Efficacy and safety of micafungin, an echinocandin antifungal agent, on invasive fungal infections in patients with hematological disorders. Leuk. Lymphoma 50:92-100. [DOI] [PubMed] [Google Scholar]
- 11.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamato, Y., H. Kaneko, K. Tanimoto, M. Katashimaet, K. Ishibashi, A. Kamamura, M. Terakawa, and A. Kagayama. 2002. Simultaneous determination of antifungal drug, micafungin, and its two active metabolites in human plasma using high-performance liquid chromatography with fluorescence detection. Jpn. J. Chemother. 50(Suppl. 1):68-73. [Google Scholar]
- 13.Yamato, Y., H. Kaneko, T. Hashimoto, M. Katashima, K. Ishibashi, A. Kawamura, M. Terakawa, and A. Kagayama. 2002. Pharmacokinetics of the antifungal drug micafungin in mice, rats and dogs, and its in vitro protein binding and distribution to blood cells. Jpn. J. Chemother. 50(Suppl. 1):74-79. [Google Scholar]