Abstract
Vertilmicin is a novel aminoglycoside antibiotic with potent activity against gram-negative and -positive bacteria in vitro. In this study, we further evaluated the efficacy of vertilmicin in vivo in systemic and local infection animal models. We demonstrated that vertilmicin had relatively high and broad-spectrum activities against mouse systemic infections caused by Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, and Enterococcus faecalis. The 50% effective doses of subcutaneously administered vertilmicin were 0.63 to 0.82 mg/kg, 0.18 to 0.29 mg/kg, 0.25 to 0.99 mg/kg, and 4.35 to 7.11 mg/kg against E. coli, K. pneumoniae, S. aureus, and E. faecalis infections, respectively. The therapeutic efficacy of vertilmicin was generally similar to that of netimicin, better than that of gentamicin in all the isolates tested, and better than that of verdamicin against E. coli 9612 and E. faecalis HH22 infections. The therapeutic efficacy of vertilmicin was further confirmed in local infection models of rabbit skin burn infection and mouse ascending urinary tract infection.
Aminoglycosides are a group of highly potent, broad-spectrum bactericidal antibiotics (8). Their history began with the discovery of streptomycin (12), followed by kanamycin, gentamicin, tobramycin, and a series of semisynthetic aminoglycosides (dibekacin, amikacin, and netilmicin) for the treatment of resistant organisms (8). The mechanisms of aminoglycoside resistance involved (i) modifying enzymes (the most common mechanism), (ii) mutations of the ribosomal binding site (causes resistance to streptomycin), and (iii) reduced drug uptake (mostly seen in Pseudomonas spp.) (2, 13). The semisynthetic aminoglycosides are mainly designed for the treatment of organisms that have developed resistance by producing aminoglycoside-modifying enzymes, i.e., N-acetyltransferase, O-nucleotidyltransferase, and O-phosphotransferases (8).
Vertilmicin is a novel semisynthetic aminoglycoside derived from verdamicin. Our earlier study showed that it had broad in vitro antimicrobial activity which is similar to that of netilmicin and has the advantage of lower susceptibility to N-acetyltransferase 6′-Ie modification (5). In this study, we further investigated the in vivo antibacterial activities of this agent in a systemic infection model, as well as local infection models, to fill the gap between in vitro characterization and clinical evaluation. All of our animals studies were approved by the Animal Research Committee of the Institute of Medicinal Biotechnology.
Mouse systemic infection model.
The in vivo efficacy of vertilmicin against mouse systemic infections versus those of netilmicin, verdamicin, and gentamicin was determined with three strains of Escherichia coli, two strains of Klebsiella pneumoniae, three strains of Staphylococcus aureus, and two strains of Enterococcus faecalis (Table 1). The experiment was carried out by a method modified from the literature (11). CD-1 ICR mice (18 to 21 g) were randomly distributed into 21 groups with 5 groups for each compound and 1 control group (10 mice per group, 5 males and 5 females). The mice were intraperitoneally infected with 0.5 ml of a bacterial suspension in 5% mucin (100 times the median lethal dose). Different doses of vertilmicin and the reference compounds (saline for the control group) were administered subcutaneously 15 min and 6 h after infection, respectively. The dose ranges of vertilmicin were 0.32 to 1.59 mg/kg for E. coli infections, 0.1 to 0.5 mg/kg for K. pneumoniae infections, 0.16 to 2.5 mg/kg for S. aureus infections, and 2.5 to 12.6 mg/kg for E. faecalis infections, respectively. Deaths in each group were recorded daily for 7 days, and the 50% effective dose (ED50) and 95% confidence limits were determined by Probit analysis (6).
TABLE 1.
In vivo antibacterial activities in mouse systemic infection model
| Strain (challenge dose [CFU/mouse]) and antibiotic | MICa (μg/ml) | ED50 (mg/kg) (95% confidence limit) | P value vs vertilmicin |
|---|---|---|---|
| E. coli ATCC 25922 (1.0 × 105) | |||
| Vertilmicin | 0.5 | 0.82 (0.69-0.95) | |
| Verdamicin | 0.5 | 1.09 (0.82-1.46) | >0.05 |
| Netilmicin | 0.5 | 0.77 (0.64-0.93) | >0.05 |
| Gentamicin | 0.5 | 1.50 (1.28-1.76) | <0.01 |
| E. coli 9612 (3.7 × 104) | |||
| Vertilmicin | 0.5 | 0.67 (0.58-0.77) | |
| Verdamicin | 4 | 2.66 (1.87-3.80) | <0.01 |
| Netilmicin | 0.5 | 0.63 (0.56-0.72) | >0.05 |
| Gentamicin | 8 | 7.88 (6.31-9.83) | <0.01 |
| E. coli 1515 (2.4 × 106) | |||
| Vertilmicin | 0.5 | 0.63 (0.50-0.78) | |
| Verdamicin | 0.5 | 0.49 (0.43-0.57) | >0.05 |
| Netilmicin | 0.5 | 0.46 (0.39-0.55) | >0.05 |
| Gentamicin | 0.5 | 1.11 (0.89-1.37) | <0.01 |
| K. pneumoniae 935 (3.0 × 105) | |||
| Vertilmicin | 0.5 | 0.29 (0.20-0.41) | |
| Verdamicin | 0.5 | 0.29 (0.23-0.38) | >0.05 |
| Netilmicin | 0.5 | 0.33 (0.27-0.40) | >0.05 |
| Gentamicin | 0.5 | 0.68 (0.56-0.82) | <0.01 |
| K. pneumoniae 967 (2.6 × 105) | |||
| Vertilmicin | 0.25 | 0.18 (0.14-0.23) | |
| Verdamicin | 0.25 | 0.16 (0.14-0.19) | >0.05 |
| Netilmicin | 0.5 | 0.17 (0.10-0.29) | >0.05 |
| Gentamicin | 0.5 | 0.32 (0.24-0.42) | <0.05 |
| S. aureus ATCC 29213b (1.5 × 105) | |||
| Vertilmicin | 0.125 | 0.25 (0.21-0.30) | |
| Verdamicin | 0.125 | 0.23 (0.17-0.31) | >0.05 |
| Netilmicin | 0.125 | 0.26 (0.22-0.32) | >0.05 |
| Gentamicin | 0.125 | 0.60 (0.48-0.75) | <0.01 |
| S. aureus 9344c (2.5 × 105) | |||
| Vertilmicin | 0.25 | 0.99 (0.65-1.50) | |
| Verdamicin | 0.25 | 1.33 (0.86-2.06) | >0.05 |
| Netilmicin | 0.25 | 0.88 (0.43-1.80) | >0.05 |
| Gentamicin | 0.25 | 2.84 (2.17-3.74) | <0.01 |
| S. aureus 15b (2.5 × 105) | |||
| Vertilmicin | 0.125 | 0.70 (0.57-0.86) | |
| Verdamicin | 0.125 | 0.66 (0.51-0.86) | >0.05 |
| Netilmicin | 0.125 | 0.49 (0.39-0.61) | >0.05 |
| Gentamicin | 0.125 | 0.61 (0.45-0.81) | >0.05 |
| E. faecalis ATCC 29212 (2.8 × 107) | |||
| Vertilmicin | 8 | 4.35 (3.64-5.21) | |
| Verdamicin | 8 | 5.24 (4.41-6.24) | >0.05 |
| Netilmicin | 8 | 3.44 (3.03-3.91) | >0.05 |
| Gentamicin | 8 | 17.52 (14.92-20.57) | <0.01 |
| E. faecalis HH22 (1.8 × 108) | |||
| Vertilmicin | 8 | 7.11 (5.55-9.12) | |
| Verdamicin | >128 | >100 | <0.01 |
| Netilmicin | 8 | 6.56 (5.27-8.17) | >0.05 |
| Gentamicin | >128 | >100 | <0.01 |
MICs were determined by the agar dilution method according to CLSI recommendations (10).
MSSA.
MRSA.
The MICs, ED50s, and 95% confidence limits of vertilmicin and the reference compounds are listed in Table 1. Vertilmicin showed relatively potent and broad-spectrum in vivo activity against both gram-negative (E. coli and K. pneumoniae) and gram-positive (S. aureus and E. faecalis) bacteria. The ED50s of vertilmicin against E. coli ATCC 25922, E. coli 9612, and E. coli 1515 systemic infections were 0.82, 0.67, and 0.63 mg/kg, respectively, which were similar to those of netilmicin but significantly lower than those of gentamicin (P < 0.01). Vertilmicin showed therapeutic efficacy similar to that of verdamicin against E. coli ATCC 25922 and E. coli 1515 systemic infections but significantly better efficacy against E. coli 9612 infection (P < 0.01). The ED50s of vertilmicin against K. pneumoniae 935 and K. pneumoniae 967 systemic infections were 0.29 and 0.18 mg/kg, respectively, which were significantly lower than those of gentamicin (P < 0.01 or 0.05). However, vertilmicin was not significantly different from verdamicin and netilmicin. Vertilmicin showed ED50s against S. aureus ATCC 29213 (methicillin [meticillin]-susceptible S. aureus [MSSA] strain), S. aureus 9344 (methicillin-resistant S. aureus [MRSA] strain), and S. aureus 15 (MSSA) infections (0.25, 0.99, and 0.70 mg/kg, respectively) similar to those of verdamicin and netilmicin. However, vertilmicin had significantly lower ED50s against S. aureus ATCC 29213 and S. aureus 9344 infections than did gentamicin (P < 0.01). In S. aureus 9344 (MRSA) infections, the ED50s of the semisynthetic aminoglycosides (vertilmicin and netilmicin) were lower than those of the natural compounds (verdamicin and gentamicin), suggesting the possible superiority of the semisynthetic aminoglycosides against MRSA infections. The ED50s of vertilmicin against E. faecalis ATCC 29212 and E. faecalis HH22 infections were 4.35 and 7.11 mg/kg, respectively, which were higher than the ED50s of vertilmicin against other isolates, suggesting that aminoglycosides have relatively lower efficacy against E. faecalis infections. The ED50s of vertilmicin against both of the strains were significantly lower (P < 0.01) than those of gentamicin. In addition, the ED50 of vertilmicin was also significantly lower (P < 0.01) than that of verdamicin against infection caused by E. faecalis HH22, a clinical isolate highly resistant (MIC, >2,000 μg/ml) to gentamicin, tobramycin, amikacin, streptomycin, and kanamycin (7, 9).
Interestingly, there is a correlation between gentamicin MICs and ED50s. For example, all of the agents tested had lower ED50s against strains with lower gentamicin MICs (0.125 to 0.5 μg/ml). On the other hand, for the isolates with higher gentamicin MICs (≥8 μg/ml; e.g., E. coli 9612, E. faecalis HH22, and E. faecalis ATCC 29212), the semisynthetic antibiotics demonstrated superior efficacies against the first two isolates, but not against E. faecalis ATCC 29212. These findings may be related to the fact that E. coli 9612 and E. faecalis HH22 produce aminoglycoside-modifying enzymes. We recently demonstrated that the ant(2")-Ia, ant(3")-Ia, aph(4)-Ia, and aph(3′)-IIIa genes were detected in E. coli 9612 and the aac(6′)-aph(2") gene was carried on pBEM10 in E. faecalis HH22 (3). In comparison, E. faecalis ATCC 29212 did not contain any aminoglycoside-modifying enzyme; the high MIC of gentamicin was due to the intrinsic resistance of enterococci to aminoglycosides (1). Therefore, no superiority of the semisynthetic compounds against this strain could be demonstrated.
In general, vertilmicin showed broad-spectrum in vivo activity in the mouse systemic infection model; it had greater efficacy than gentamicin against most of the study isolates and better activity than verdamicin against isolates producing aminoglycoside-modifying enzymes (E. coli 9612 and E. faecalis HH22). However, vertilmicin showed ED50s similar to those of netilmicin against all of the isolates tested, which reflected their similar in vitro activities (5).
The ED50s of vertilmicin, netilmicin, and verdamicin track their MICs, but that of gentamicin does not (Table 1). The most likely reason for this is that the pharmacokinetic (PK) profile of gentamicin is slightly different from those of the other three compounds, considering the relatively similar structures of vertilmicin, netilmicin, and verdamicin in comparison to that of gentamicin, which is a mixture of gentamicin C1, C1a, C2, etc.
Rabbit skin burn infection model.
The skin burn infection study was carried out with six male New Zealand White rabbits (body weight, 2.5 to 3.0 kg) infected with E. coli 9612. Three doses of vertilmicin (0.5, 1, and 2 mg/ml) and one dose of netilmicin, verdamicin, and gentamicin (1 mg/ml) were tested in this model.
One day before infection, the back hair of the animals was clipped and then removed by applying a paste of barium sulfide-zinc oxide-starch (2:3:3, wt/wt/wt). On the day of infection, the rabbits were anesthetized by intravenous injection of pentobarbital sodium (30 mg/kg of body weight) and the back skin of the animals was sterilized with 75% ethanol. Seven deep second-degree burn wounds (six for administering compounds and one for a control) were then created on the back of each animal by applying a brass probe (diameter of 1.8 cm, heated to 100°C) for 10 s (14, 16). Fifteen minutes later, 0.1 ml (challenge dose, 1 × 107 CFU/burn wound) of an E. coli 9612 suspension in saline was intracutaneously injected into each wound and a homemade cap was used to protect each wound. One hour after the burning, 0.4 ml of different compound solutions or saline (for the control) was administered to the corresponding wounds by loading it onto a sterile gauze patch of the same size as the burn wound. Sterile petrolatum-containing gauze was used on top of the compound-containing gauze to keep the wounds humid. The rabbits were sacrificed 24 h after administration of the compounds. Full-thickness skin biopsy samples were then taken from the center of the burns sterilely and homogenized in saline. The homogenates were serially 10-fold diluted, and 0.1-ml aliquots were spread onto nutrient agar plates. The plates were incubated at 35°C for 48 h, and the numbers of viable organisms in the burn wounds (CFU per gram) were determined.
Vertilmicin significantly reduced the viable colony counts in comparison to that of the control group in a dose-dependent manner (P < 0.05; Table 2). The netilmicin (1 mg/ml) group also showed a significantly lower viable colony count than the untreated control (P < 0.05). However, the reductions of viable cell counts in the verdamicin (1 mg/ml) and gentamicin (1 mg/ml) groups were not significant in comparison to that of the control group. In addition, vertilmicin at 1 mg/ml produced significantly lower bacterial counts than did verdamicin (1 mg/ml) and gentamicin (1 mg/ml) (P < 0.01). The efficacies of the compounds tested were in the order vertilmicin ≥ netilmicin > verdamicin > gentamicin, which was similar to the results of the systemic infections (Table 1) and the MICs. We also studied the diffusion of vertilmicin, netilmicin, verdamicin, and gentamicin from the sites of placement by determining the concentrations of the compounds in skin tissues by liquid chromatography-tandem mass spectrometry. The results showed that these compounds diffused from the sites of placement similarly (data not shown), which supports the finding that the efficacies of the compounds in the rabbit skin burn infection model track the MICs.
TABLE 2.
In vivo antibacterial activities of vertilmicin and reference compounds in rabbit skin burn infections caused by E. coli 9612
| Antibiotic, dose (mg/ml) | Log CFU/g (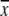 ± SD, n = 6) ± SD, n = 6) |
P value vs:
|
|
|---|---|---|---|
| Control | 1-mg/ml vertilmicin group | ||
| Vertilmicin | |||
| 0.5 | 6.33 ± 1.65 | <0.05 | |
| 1 | 5.09 ± 1.65 | <0.01 | |
| 2 | 5.00 ± 1.62 | <0.01 | |
| Netilmicin, 1 | 6.06 ± 1.97 | <0.05 | >0.05 |
| Verdamicin, 1 | 8.07 ± 0.63 | >0.05 | <0.01 |
| Gentamicin, 1 | 8.31 ± 0.54 | >0.05 | <0.01 |
| Control | 8.57 ± 0.35 | ||
Mouse ascending urinary tract infection model.
The therapeutic efficacies of vertilmicin and gentamicin in mouse ascending urinary tract infections caused by E. coli 9612 was evaluated with female CD-1 ICR mice (body weight, 20 to 22 g, 10 mice/group) as previously described with modifications (4, 15). Five different doses of vertilmicin or gentamicin were used in these experiments. Gentamicin doses were twofold higher than the corresponding doses of vertilmicin, considering the relatively lower efficacy of gentamicin shown previously (Tables 1 and 2). The mice were subjected to water restriction for 24 h prior to and after infection, respectively. Under pentobarbital anesthesia, a round-point needle was inserted transurethrally for injection of 0.05 ml of the bacterial suspension in 5% mucin (challenge dose, 2.2 × 109 CFU/mouse) into the bladder. The urethral needle was removed immediately after inoculation, and the external urethral meatus was clamped for 1 h. The compounds (saline for the control group) were administered subcutaneously at 6 h, 24 h, 30 h, 48 h, and 54 h postinfection. The mice were sacrificed 72 h after infection. The kidneys were then removed and homogenized in saline, the homogenates were serially 10-fold diluted, and 0.1-ml aliquots were spread onto nutrient agar plates. The plates were incubated at 35°C for 48 h, and the numbers of viable organisms in the kidneys (CFU per gram) were determined.
Vertilmicin exhibited a high degree of dose-dependent efficacy against E. coli 9612; all groups showed a significant decrease in kidney viable colony counts (P < 0.01) in comparison to that of the control group (Table 3). Gentamicin also showed dose-dependent antibacterial efficacy, with significantly decreased kidney viable colony counts (P < 0.01, except for the lowest-dose group). Vertilmicin had better efficacy than gentamicin at a similar dose. For example, vertilmicin at 20 mg/kg caused a decrease in the colony count of 2.09 log10 CFU/g, while a gentamicin dose of 40 mg/kg, which was twofold higher than that of vertilmicin, only caused a decrease of 1.67 log10 CFU/g. However, gentamicin seems to work better on a per-dose basis with respect to MICs (8 μg/ml of gentamicin versus 0.5 μg/ml of vertilmicin). One possible explanation is the difference of the PK profiles of the two compounds in the urinary tract.
TABLE 3.
In vivo antibacterial activities of vertilmicin and reference compound in mouse ascending urinary tract infections caused by E. coli 9612
| Antibiotic and dose (mg/kg) | Log CFU/g (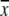 ± SD, n = 10) ± SD, n = 10) |
P value vs control |
|---|---|---|
| Vertilmicin | ||
| 20 | 2.36 ± 0.41 | <0.01 |
| 12 | 3.11 ± 0.34 | <0.01 |
| 7.2 | 3.07 ± 0.37 | <0.01 |
| 4.32 | 3.22 ± 0.48 | <0.01 |
| 2.59 | 3.32 ± 0.33 | <0.01 |
| Gentamicin | ||
| 40 | 2.78 ± 0.80 | <0.01 |
| 24 | 3.24 ± 0.48 | <0.01 |
| 14.4 | 3.34 ± 0.57 | <0.01 |
| 8.64 | 3.44 ± 0.63 | <0.01 |
| 5.18 | 3.71 ± 0.23 | >0.05 |
| Control | 4.45 ± 0.37 |
In conclusion, our systemic and local infection studies demonstrated that vertilmicin had in vivo antimicrobial activity comparable to that of netilmicin and better efficacy than verdamicin and gentamicin (especially against isolates producing aminoglycoside-modifying enzymes). This in vivo antimicrobial activity study confirmed that vertilmicin has high activity and deserves further investigation.
Acknowledgments
We thank Yan-Qiong Xiong, Los Angeles Biomedical Research Institute at Harbor-UCLA, for her review of the manuscript. We also thank Barbara E. Murray, University of Texas Health Science Center, for her kindness in donating E. faecalis HH22.
The project was supported by the National Natural Science Foundation of China (30472058 and 30672502), by the Beijing Natural Science Foundation (7062064), and by the 11th Five-Year Plan of the Ministry of Sciences and Technology (2009ZX09303-005 and 2008ZX09305-001), People's Republic of China.
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Aslangul, E., L. Massias, A. Meulemans, F. Chau, A. Andremont, P. Courvalin, B. Fantin, and R. Ruimy. 2006. Acquired gentamicin resistance by permeability impairment in Enterococcus faecalis. Antimicrob. Agents Chemother. 50:3615-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies, J., and G. D. Wright. 1997. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 5:234-240. [DOI] [PubMed] [Google Scholar]
- 3.Hodel-Christian, S. L., and B. E. Murray. 1991. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob. Agents Chemother. 35:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hvidberg, H., C. Struve, K. A. Krogfelt, N. Christensen, S. N. Rasmussen, and N. Frimodt-Moller. 2000. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 44:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, C. R., X. Y. Yang, R. H. Lou, W. X. Zhang, Y. M. Wang, M. Yuan, Y. Li, H. Z. Chen, B. Hong, C. H. Sun, L. X. Zhao, Z. R. Li, J. D. Jiang, and X. F. You. 2008. In vitro antibacterial activity of vertilmicin and its susceptibility to modifications by the recombinant AAC6′-APH2" enzyme. Antimicrob. Agents Chemother. 52:3875-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litchfield, J. T., Jr., and F. Wilcoxon. 1949. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96:99-113. [PubMed] [Google Scholar]
- 7.Mederski-Samoraj, B. D., and B. E. Murray. 1983. High-level resistance to gentamicin in clinical isolates of enterococci. J. Infect. Dis. 147:751-757. [DOI] [PubMed] [Google Scholar]
- 8.Mingeot-Leclercq, M. P., Y. Glupczynski, and P. M. Tulkens. 1999. Aminoglycosides: activity and resistance. Antimicrob. Agents Chemother. 43:727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray, B. E., and B. Mederski-Samaroj. 1983. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J. Clin. Investig. 72:1168-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed.; approved standard. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 11.O'Reilly, T., D. A. Andes, C. Østergaard, and N. Frimodt-Møller. 2005. Evaluation of antimicrobials in experimental animal infections, p. 654-718. In V. Lorian (ed.), Antibiotics in laboratory medicine. Lippincott Williams & Wilkins, Philadelphia, PA.
- 12.Schatz, A., E. Bugie, and S. A. Waksman. 2005. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. 1944. Clin. Orthop. Relat. Res. 437:3-6. [DOI] [PubMed] [Google Scholar]
- 13.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer, A. J., L. Berruti, H. C. Thode, Jr., and S. A. McClain. 2000. Standardized burn model using a multiparametric histologic analysis of burn depth. Acad. Emerg. Med. 7:1-6. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji, M., M. Takema, H. Miwa, J. Shimada, and S. Kuwahara. 2003. In vivo antibacterial activity of S-3578, a new broad-spectrum cephalosporin: methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa experimental infection models. Antimicrob. Agents Chemother. 47:2507-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White, J., D. L. Maass, B. Giroir, and J. W. Horton. 2001. Development of an acute burn model in adult mice for studies of cardiac function and cardiomyocyte cellular function. Shock 16:122-129. [DOI] [PubMed] [Google Scholar]


