Abstract
Staphylococcus epidermidis and Staphylococcus aureus are the leading causative agents of indwelling medical device infections because of their ability to form biofilms on artificial surfaces. Here we describe the antibiofilm activity of a class of small molecules, the aryl rhodanines, which specifically inhibit biofilm formation of S. aureus, S. epidermidis, Enterococcus faecalis, E. faecium, and E. gallinarum but not the gram-negative species Pseudomonas aeruginosa or Escherichia coli. The aryl rhodanines do not exhibit antibacterial activity against any of the bacterial strains tested and are not cytotoxic against HeLa cells. Preliminary mechanism-of-action studies revealed that the aryl rhodanines specifically inhibit the early stages of biofilm development by preventing attachment of the bacteria to surfaces.
Indwelling medical devices have become integral components of modern health care. The surfaces of these devices can be colonized by bacterial pathogens that are capable of forming biofilms. Resulting biofilms frequently compromise the function of the device or cause serious systemic infections. These device-related infections are often very difficult to eradicate with conventional antibiotics, as bacteria and fungi growing in the biofilm mode of growth are extremely resistant to antibiotics, biocides, and attack from the immune system (9). Consequently, device-related infections result in increased patient morbidity, mortality, and health care costs (7, 46).
Because device colonization precedes device-related infections, several approaches have been used to prevent biofilm colonization of indwelling devices, such as central venous catheters. These include the implementation of stringent infection control measures by health care workers during the insertion and maintenance of vascular access devices. Such measures have been shown to decrease the incidence of catheter-related bloodstream infections when there is strict compliance with established protocols (38). Alternatively, medical devices coated with antimicrobial compounds have been tested for prevention of bacterial colonization. Central venous catheters impregnated with a combination of antibacterial agents (rifampin [rifampicin] and minocycline) or coated with a combination of antiseptics (chlorhexidine and silver sulfadiazine) have been shown to be effective in reducing catheter colonization and catheter-related bloodstream infections in clinical studies (reviewed in reference 45). However, there are lingering concerns related to the use of antiseptics and antibiotics as coatings for medical devices. For example, it is possible that the use of antibiotic-impregnated catheters could lead to increases in the occurrence of strains resistant to antimicrobial agents. While increased resistance to antibiotics used as catheter coatings has not been detected in several in vitro and in vivo studies (14, 27, 32, 45), evidence suggests that rifampin-minocycline-impregnated catheters are more susceptible to colonization when challenged with a rifampin-resistant strain (49). Therefore, continuing concerns have limited the antibiotics used as coatings to those that are not currently in clinical use.
Because of the ongoing need for effective biofilm prophylaxis, several experimental approaches to preventing biofilm-related infections are currently being tested in laboratory studies. For example, several investigators are examining the antibiofilm activities of combining antibiotics or biocides with biofilm-specific agents that primarily target the extracellular matrix of biofilms, such as N-acetylcysteine (21), protamine sulfate (13), and dispersin B (12). In addition, the utility of coatings containing biological agents, such as bacteriophages, in preventing biofilm formation on medical devices has been investigated (11). Strategies that do not require antimicrobial agents for preventing bacterial colonization of medical devices are also under investigation. Polymer brush coatings are monolayer coatings of modified polymer chains that have been shown to prevent bacterial adhesion to coated surfaces (33-35). The application of low-intensity electrical currents to electrically conductive implants has also been shown to decrease bacterial colonization (16, 22, 52-55). Similarly, the application of low-energy surface acoustic waves to urinary catheters prevented bacterial colonization in an animal infection model (18). While several of these approaches have shown promising results in laboratory studies, their efficacy in preclinical and clinical studies has not been proven. Therefore, the development of additional approaches for the prevention of biofilm-related infections is necessary to ensure that there are several effective strategies available to the clinician.
Staphylococcus epidermidis and Staphylococcus aureus are the leading causative agents of indwelling medical device infections because of their ability to form biofilms on artificial surfaces (7, 37, 57). To address the unmet medical need of preventing device-related infections, we have taken a novel approach. We have discovered a class of small molecules, the aryl rhodanines, which specifically inhibit biofilm formation by gram-positive pathogens, such as S. aureus, S. epidermidis, Enterococcus faecalis, E. faecium, and E. gallinarum. Here, we report the antibiofilm activities of four members of this compound class and a preliminary study of the mechanism of action of a representative compound. The aryl rhodanines are potent inhibitors of biofilm formation in the staphylococci and enterococci, do not act on planktonic bacteria, and are not cytotoxic. The aryl rhodanines specifically affect the initial stage of biofilm formation and appear to inhibit the primary adhesion of bacterial cells to a surface. In principle, since these inhibitors lack antibacterial activity, they should prevent biofilm formation on medical devices without exerting strong selective pressure for resistance.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are described in Table 1. All of these strains are proficient in biofilm formation under the conditions described below.
TABLE 1.
Bacterial strains used in this studya
| Species and strain | Description | Reference or source |
|---|---|---|
| S. aureus | ||
| ATCC 35556 (SA113) | Laboratory strain, biofilm proficient | 23; ATCC |
| NCTC 8325 (NRS-77) | Laboratory strain | 36; NARSA |
| MW2 (NRS-123) | CA-MRSA, USA 400b | 1; NARSA |
| MSSA | Clinical isolate, osteomyelitis | 3 |
| MRSA-14234 | Clinical isolate, CA-MRSA | University of Massachusetts Medical Center, Worcesterc |
| MRSA-22733 | Clinical isolate, CA-MRSA | |
| MRSA-23542 | Clinical isolate, CA-MRSA, USA 300b | |
| MRSA-24102 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-24209 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-27687 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-33548 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-35392 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-38230 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-43349 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-44081 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-47263 | Clinical isolate, HA-MRSA, USA 100 | |
| MRSA-49404 | Clinical isolate, CA-MRSA, USA 300 | |
| MRSA-58336 | Clinical isolate, HA-MRSA, USA 100b | |
| MRSA-1094 | Clinical isolate, HA-MRSA, USA 100 | |
| ATCC 35556 (pAJ22) | pAJ22 constitutively expresses β-galactosidase encoded by lacZ | 39, this study |
| SA113 Δica::Tc | PNAG deficient | 10 |
| ATCC 35556 ΔatlA-atlR::erm | Deficient in major autolysin | This study |
| S. epidermidis | ||
| 18972 | Clinical isolate, endocarditis, MRSE | Oscient Pharmaceuticals, Waltham, MA |
| 1457 | 26 | |
| O-47 | 19 | |
| ATCC 35984 (RP62A) | 5; ATCC | |
| CoNS | ||
| WTBF-40 | Clinical isolate | University of Massachusetts Medical Center, Worcester |
| WTBF-41 | Clinical isolate | |
| WTBF-45 | Clinical isolate | |
| WTBF-46 | Clinical isolate | |
| WTBF-47 | Clinical isolate | |
| WTBF-48 | Clinical isolate | |
| E. faecalis | ||
| ATCC 51299 | Clinical isolate, VRE | ATCC |
| ATCC 51575 | ATCC | |
| F317 | Clinical isolate, VRE | Northwestern Memorial Hospital, Chicago, ILd |
| F177 | Clinical isolate, VRE | |
| F217 | Clinical isolate, VRE | |
| E. faecium | ||
| EF208 | Clinical isolate, VRE | |
| 1401 | Clinical isolate, VRE | |
| EF1509 | Clinical isolate, VRE | |
| F118 | Clinical isolate, VRE | |
| E. gallinarum 2392 | Clinical isolate, VRE | |
| E. coli ATCC 25922 | Reference strain, biofilm proficient | ATCC |
| P. aeruginosa PAO1 | Laboratory strain, biofilm proficient | 51 |
Abbreviations: NARSA, Network on Antibiotic Resistance in Staphylococcus aureus (Chantilly, VA); CA, community acquired; HA, hospital acquired; MRSE, methicillin-resistant S. epidermidis.
The USA 400, USA300, and USA100 designations of MRSA refer to distinct genetic lineages assigned on the basis of pulsed-field gel electrophoresis analyses (31).
Clinical strains of MRSA and CoNS strains were selected from the collection at the University of Massachusetts Medical Center, Worcester, and kindly provided by Richard Ellison.
Clinical strains of VRE were selected from the collection at Northwestern Memorial Hospital, Chicago, IL, and were provided by Jason Pryztwoski.
Screening assay for biofilm inhibitors.
A high-throughput assay for biofilm inhibitors was developed with flat-bottom, tissue culture-treated, 96-well assay plates (Costar 3595; Corning Life Sciences). In each assay plate, columns 1 and 12 contained untreated cultures, which served as negative controls (0% biofilm inhibition). Each of the assay wells in columns 2 to 11 contained a unique small molecule from the Microbiotix screening library (MSL) at a final concentration of 100 μM. Assay plates were inoculated with 200 μl/well of a culture of S. epidermidis 18972 in 0.5× tryptic soy broth (TSB; Becton Dickinson) in which the concentration of glucose was adjusted to 0.25% (wt/vol). The bacterial inocula were prepared by making a 1:100 dilution of an overnight culture grown in TSB in the medium used for the screening. After inoculation, assay plates were sealed with foil tape and incubated at 37°C for 18 to 20 h. The optical density at 600 nm (OD600) of each well was measured with a Victor2V multiplate reader (Perkin-Elmer, Waltham, MA) in order to quantify overall bacterial growth. The assay plates were processed to remove bacterial growth medium and nonbiofilm cells from the bottom of each assay well with a BioTek ELx405 plate washer (BioTek Instruments, Inc., Winooski, VT). Biofilm bacteria were fixed by the addition of 50 μl of 95% ethanol and incubation for 30 min. The ethanol was removed, and the fixed biofilm cultures were stained with 50 μl of 0.06% crystal violet (CV) for 60 min. Excess CV was removed by repeated washes with the BioTek ELx405 plate washer. The amount of CV bound to each assay well was quantified by measuring OD600 with a Victor2V plate reader (Perkin-Elmer). The percent inhibition of overall growth caused by each compound was calculated with the formula {1 − [OD600 (compound)/average OD600 (negative control)]} × 100. The percent inhibition of biofilm growth produced by each compound was calculated with the formula {1 − [CV OD600 (compound)/average CV OD600 (negative control)]} × 100. Compounds that produced ≥80% biofilm inhibition and ≤40% overall growth inhibition were scored as primary (positive) hits. Primary hits were retested in triplicate with the assay described above. Primary hits that produced an average biofilm inhibition of ≥80% and overall growth inhibition of ≤40% were scored as confirmed hits. Confirmed hits were evaluated in the antibiofilm activity assay (described below) against a panel of 10 staphylococcal strains. Compounds with minimal biofilm inhibitory concentrations (MBICs) of ≤12.5 μM against at least one strain and MICs of ≥100 μM were scored as validated hits. The aryl rhodanine compound class was identified as a validated hit series with low levels of mammalian cell cytotoxicity.
The entire MSL was screened for biofilm inhibitors with the assay described above. The MSL contains 87,250 unique compounds and is composed of commercially available screening collections purchased from several vendors. For a summary of the names of the screening collections that make up the MSL, the number of compounds in each collection, and the vendor names, along with the results of the screening for inhibitors of biofilm formation in terms of the numbers of primary, confirmed, and validated hits obtained for each screening collection, see Table S1 in the supplemental material.
Synthesis of aryl rhodanines.
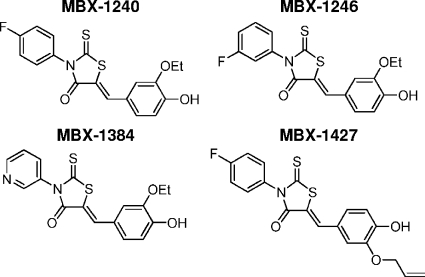
MBX-1240 [(Z)-3-(4-fluorophenyl)-5-(3-ethoxy-4-hydroxybenzylidene)-2-thioxothiazolidin-4-one], MBX-1246 [(Z)-3-(3-fluorophenyl)-5-(3-ethoxy-4-hydroxybenzylidene)-2-thioxothiazolidin-4-one], MBX-1384 [(Z)-3-(pyridin-3-yl)-5-(3-ethoxy-4-hydroxybenzylidene)-2-thioxothiazolidin-4-one], and MBX-1427 [(Z)-3-(4-fluorophenyl)-5-[3-(allyloxy)-4-hydroxybenzylidene-2-thioxothiazolidin-4-one] were synthesized according to literature precedent (28, 41). Briefly, the appropriate isothiocyanate (4-fluorophenyl, 3-fluorophenyl, or 3-pyridyl) was treated with ethyl thioglycolate and triethylamine to provide an intermediate N-arylrhodanine. This was condensed with an appropriately substituted benzaldehyde (3-ethoxy-4-hydroxybenzaldehyde or 3-allyloxy-4-hydroxybenzaldehyde) by heating the two components in the presence of ammonium acetate/acetic acid to complete the synthesis.
Antibiofilm and antibacterial activity assay.
To quantify the antibiofilm and antibacterial activities of experimental compounds, we have developed an assay that measures the MBIC. The MBIC is defined as the concentration of a compound in a serial twofold dilution series that inhibits biofilm formation by ≥80% compared to an untreated control. This assay is formatted in a manner that is similar to the broth microdilution assays described in CLSI document M7-A7 (8), with several modifications (described below). Briefly, 96-well assay plates (tissue culture-treated polystyrene; Costar 3595) containing 8 μl of a twofold dilution series of each compound dissolved in dimethyl sulfoxide (DMSO; final concentration range, 0.2 to 100 μM) and untreated controls were generated. The compound dilutions were prepared by serially diluting each compound 2-fold in DMSO at concentrations that were 50-fold greater than the final concentration. The final concentration of DMSO in each well was 2%. Each assay well was inoculated with 200 μl of bacterial culture. The bacterial inocula were prepared by diluting an overnight culture 1:100 in fresh medium, which corresponds to an inoculum of approximately 5 × 106 cells/well. The medium conditions have been optimized for biofilm growth in 96-well assay plates, such that the amount of biofilm formation is within the linear range of detection of the plate reader. The medium conditions used for each of the species analyzed in this study were as follows. S. aureus, S. epidermidis, E. faecalis, E. faecium, and E. gallinarum were grown in 0.5× TSB supplemented with 1% glucose. The assay plates were covered with adhesive foil lids (Costar 3904; Corning) and incubated for 18 h at 37°C. The OD600 of each well was measured with a Victor3V multiplate reader (Perkin-Elmer) to quantify overall bacterial growth. The liquid culture and nonadherent cells were removed by repeated washing with a BioTek ELx405 plate washer. Adherent cells (biofilms) were fixed by heat (60°C for 60 min) and stained with 50 μl of 0.06% CV as previously described (4, 6). The amount of CV bound in each well, which is proportional to the amount of biofilm, was quantified by measuring OD600 in a Victor3V multiplate reader (Perkin-Elmer). Biofilms of Pseudomonas aeruginosa and Escherichia coli were grown in polystyrene plates (Costar 3595) in 1× TSB (0.25% glucose) with breathable lids (AeraSeal; Excel Scientific) at 37°C for 18 h. Because these strains form biofilms at the air-liquid interface, they were stained and quantified essentially as described by O'Toole and Kolter (40), except that CV was eluted from stained biofilms with 30% acetic acid. The MBICs reported in Table 1 are the geometric means of the results of at least three independent experiments.
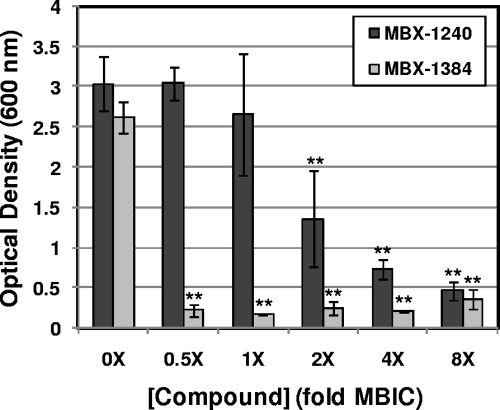
The effect of aryl rhodanines on biofilm formation on silicone disks was measured essentially as described by Shanks et al. (50). Briefly, disks were cut from sheets of nonreinforced medical grade silicone sheeting (Cardiovascular Instrument Corp., Wakefield, MA) with a no. 7 cork borer (1.3 cm in diameter, 0.03 in. thick) and sterilized with an autoclave. Disks were attached to the bottoms of 24-well assay plates (Costar 3526) with silicone sealant (Silicone II; GE Sealants and Adhesives, Huntersville, NC). Bacterial inocula were prepared as described above. MBX-1240 or MBX-1384 was added to each inoculum to achieve the desired concentration while maintaining the final concentration of DMSO at 2%, and 2 ml of each mixture was added to at least three wells. Assay plates were covered and incubated for approximately 18 h at 37°C. Media and nonadherent cells were removed by repeated washing with water. Adherent cells (biofilms) were fixed by heat (60°C for 60 min) and stained with 200 μl of 0.06% CV as previously described (50). Excess CV was removed by repeated washing, and bound CV was eluted with 1 ml 30% acetic acid. Eluted CV was transferred to 4 separate wells (200 μl/well) of a 96-well assay plate, and OD600 was measured with a Victor3V multilabel plate reader (Perkin-Elmer). The average and standard deviation of the OD600 values obtained for all three disks were determined. The assay was repeated at least three times; for a representative result, see Fig. 2.
FIG. 2.
Aryl rhodanines inhibit biofilm formation on silicone disks. Biofilms of S. aureus ATCC 35556 were grown on silicone disks in the presence of various concentrations of MBX-1240 and MBX-1384 (0× to 8× the MBIC) for 18 h, at which time nonadherent cells were removed by repeated washing. Adherent cells were heat fixed and stained with CV. The OD600 of the eluted CV from each sample was measured, and the average values obtained from a total of three silicone disks is plotted as a function of the compound concentration. The Student t test was used to determine whether differences in biofilm formation between treated and untreated controls are significant. Significant differences are indicated by the asterisks (P < 0.001) above the bars. The error bars represent the standard deviation.
To determine whether the presence of human host proteins affects the antibiofilm activity of aryl rhodanines, assay plates were pretreated with 100 μl of fibronectin or fibrinogen solution with concentrations ranging from 0 to 20 μg protein/ml at 4°C for 24 h. After pretreatment, the protein solutions were removed, the assay wells were washed with sterile water, and the pretreated 96-well plates were used for an antibiofilm activity assay as described above.
Antibacterial activity assay.
Antibacterial assays to determine the MIC for each compound against planktonic bacteria were performed essentially as described by CLSI guideline M7-A7 (8), except that serial twofold dilutions of test compounds dissolved in DMSO were made in DMSO at a 50-fold final concentration and added to the assay plates prior to inoculation. The final concentration of DMSO in the MIC assay was 2%.
Mammalian cell cytotoxicity assay.
The 50% cytotoxic concentration (CC50) of the aryl rhodanines against a mammalian cell line (HeLa, ATCC CCL-2; American Type Culture Collection, Manassas, VA) was determined as previously described (2). Briefly, 96-well plates were seeded with HeLa cells at a density of 4 × 103 per well in the presence or absence of serial dilutions of a compound dissolved in DMSO. Following incubation for 3 days at 37°C in minimal essential medium (plus 10% calf serum), cell viability was measured with the vital tetrazolium salt stain 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (30) according to the manufacturer's instructions (Promega, Madison, WI). Cytotoxicity (CC50) was quantified as the compound concentration that inhibited the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide to formazan by 50% (30).
Time-of-addition assay.
Assay plates were inoculated with S. aureus ATCC 35556 as described above. At various times after inoculation, MBX-1240 was added to an entire column (eight wells) of the assay plate to a final concentration of 4× the MBIC (12.5 μM). After 22 h of incubation, biofilm formation was quantified as described above. In a parallel experiment, a biofilm growth curve experiment was carried out as follows. A series of 96-well assay plates were inoculated with S. aureus ATCC 35556 in six columns (48 wells) and incubated for various times that corresponded to the times of MBX-1240 additions. At these times, a single plate was removed from the incubator and biofilm formation was quantified as described above.
Autolysis assays.
The effects of various concentrations of MBX-1240 on autolysis were measured essentially as described previously (29). Briefly, cultures of S. aureus ATCC 35556 (wild type) and a ΔatlA::erm mutant were grown to mid-log phase in TSB medium, harvested by centrifugation (8,000 × g, 4°C, 10 min), washed twice with 50 ml of ice-cold 10 mM Tris-HCl (pH 7.5), and resuspended in 50 ml autolysis buffer (50 mM Tris-HCl [pH 7.5] containing 0.05% [vol/vol] Triton X-100). The wild-type cell suspension was transferred in 1-ml aliquots to the wells of a 24-well assay plate containing MBX-1240 dissolved in DMSO, resulting in final concentrations ranging from 0× to 8× the MBIC (the final concentration of DMSO was 2%). The ΔatlA::erm mutant strain was exposed to DMSO only. Assay plates were incubated at 37°C with shaking, and the OD600 of each well was monitored with a Victor V3 multilabel plate reader (Perkin-Elmer). Each condition was tested in at least three identical assay wells, the average OD600 and standard deviation were determined, and the fraction of the initial OD600 for each condition was calculated (OD600 − TX/OD600 − T0, where TX and T0 are the time of measurement and time zero, respectively). Alternatively, the effect of MBX-1240 on autolysis was measured with the β-galactosidase release assay described by Rice et al. (47). Briefly, culture flasks containing TSB with 6 μg chloramphenicol/ml and various concentrations of MBX-1240 were inoculated with a 1:100 dilution of an overnight culture of ATCC 35556 carrying pAJ22 (39). The resulting cultures were incubated at 37°C for 96 h with vigorous shaking (250 rpm). At various times, the OD600 of each culture was measured and culture supernatants were obtained by removing bacterial cells by centrifugation. The β-galactosidase activity in culture supernatants was measured with o-nitrophenyl-β-d-galactopyranoside as described previously (47) and is reported in Miller units.
Poly-N-acetylglucosamine (PNAG) assay.
Measurement of ica-dependent polysaccharide production by dot blot Western analysis was performed as described previously (50), except that the blots were probed with wheat germ agglutinin conjugated with horseradish peroxidase (WGA-HRP) by a previously published protocol (25).
Primary adhesion assays.
S. aureus ATCC 35556 was grown aerobically to mid-log phase (OD600 = 0.5) at 37°C in TSB medium. Bacterial cells were harvested by centrifugation, washed with phosphate-buffered saline (PBS), and resuspended in a volume of PBS equivalent to the volume of the original culture. Aliquots of the cell suspension were mixed with MBX-1240, MBX-1384, or chloramphenicol to achieve various compound concentrations (the final DMSO concentration was 2% in all samples), and 200 μl of the mixture was transferred to the wells of a tissue culture-treated 96-well polystyrene assay plate (Costar 3904) or a 96-well polyvinyl chloride (PVC) assay plate (BD Biosciences catalog no. 353912) and incubated at 37°C for 30 min. Each compound concentration was tested in a total of eight assay wells. The assay wells were washed with a BioTek ELx405 plate washer to remove nonadherent cells, 50 μl BacLight Live/Dead (Molecular Probes) stain was added to each well, and the plate was incubated for 30 min at room temperature. Fluorescence emission (excitation wavelength, 485 nm; emission wavelength, 530 nm) was measured with a Victor3V multilabel plate reader (Perkin-Elmer) to quantify adherent cells. The average fluorescence value and standard deviation of the eight samples tested were calculated, and the fraction of adherent cells, compared to untreated cells, was calculated. Alternatively, cell suspensions were incubated with various concentrations of MBX-1240 or MBX-1384 in tubes for 30 min at 37°C. The cells were harvested by centrifugation to remove free compound, and the cell pellet was resuspended with PBS at the original cell density. The treated cell suspension (200-μl aliquots) was transferred to the wells of a tissue culture-treated polystyrene assay plate, incubated at 37°C for an additional 30 min, and washed and stained as described above. To measure adhesion to medical grade silicone under static conditions, 2-ml aliquots of cell suspensions were added to the wells of a 24-well plate (Costar 3526) containing a silicone disk fixed to the bottom of the well with silicone sealant as described above, and each compound concentration was tested in a total of three assay wells. The assay plates were incubated at 37°C for 30 min, and each well was washed a total of five times with PBS to remove nonadherent cells. Adherent cells were stained with 200 μl BacLight Live/Dead (Molecular Probes) stain and quantified as described above. Each of these experiments was repeated at least three times, and representative results are presented.
To measure adhesion to silicone in the presence of shear forces, bacterial suspensions (2 ml) containing various concentrations of MBX-1240 (final DMSO concentration = 2%) were transferred to culture tubes containing a sterile medical grade silicone disk (∼1 cm in diameter, 0.03 in. thick). The disks were incubated with shaking (200 rpm) for 2 h at 37°C and washed five times with PBS to remove nonadherent bacteria. The disks were transferred to fresh glass culture tubes, and adherent bacteria were removed from the disk by adding 1 ml of a solution containing 0.5% Tween 80 and 10 mM EDTA as described by Polonio et al. (42), followed by 5 min of ultrasonic treatment (Bransonic Ultrasonic Cleaner 1510R-MT) at a 70-W output. Adherent bacteria were quantified by plating appropriate dilutions on Difco tryptic soy agar plates (BD, Sparks, MD) and counting colonies after incubation at 37°C for 18 h. A total of two disks per compound concentration were tested, and the average CFU count and standard deviation were calculated for each treatment. The experiment was repeated at least three times; for a representative experiment, see Fig. 4E.
FIG. 4.
Aryl rhodanines inhibit primary adhesion. (A) Aryl rhodanines inhibit attachment of S. aureus ATCC 35556 to tissue culture-treated polystyrene in a static assay. A mid-log-phase culture was harvested, resuspended in PBS to an OD600 of 0.5, and aliquoted. Aryl rhodanines were added to aliquots to achieve various concentrations (0× to 8× the MBIC), which were then transferred to 96-well tissue culture-treated polystyrene assay plates and incubated at 37°C for 30 min. After repeated washing, adherent cells were stained with BacLight Live/Dead stain (Molecular Probes) and fluorescence emission (excitation wavelength, 485 nm; emission wavelength, 530 nm) was measured to quantify adherent cells. Each bar represents the average of at least eight assay wells, and the error bars represent the standard deviation. The asterisks indicate a statistically significant difference between the untreated control and a treated sample determined with a two-tailed Student t test (*, P ≤ 0.05; **, P ≤ 0.001). (B) Pretreatment of S. aureus ATCC 35556 with aryl rhodanines inhibits primary attachment. A cell suspension was prepared as described above and pretreated with aryl rhodanines for 30 min. Cells were harvested to remove free compound, resuspended in PBS, and used in an adhesion assay as described above. (C) Aryl rhodanines inhibit attachment to PVC in a static assay. The assay described in panel A was used to measure adhesion to PVC. (D) Aryl rhodanines inhibit attachment to silicone disks in a static assay. The assay described in panel A was used to measure adhesion to medical grade silicone disks. Each bar represents the average of three silicone disks, and the error bars represent the standard deviation. (E) MBX-1240 inhibits attachment to silicone disks in the presence of shear force. Cell suspensions containing various concentrations of MBX-1240 were prepared as described above, transferred to a culture tube containing a silicone disk, and incubated at 37°C with moderate shaking. After repeated washes, the disk was transferred to a fresh tube and adherent bacteria were removed by sonication. The numbers of adherent bacteria were determined by plating appropriate dilutions and counting colonies. Each bar represents the average value obtained from two silicone disks. Light and dark bars represent log10 CFU/disk and fraction of control, respectively.
RESULTS
Identification of inhibitors of staphylococcal biofilm formation.
High-throughput screening was performed to identify compounds that specifically inhibit the ability of S. epidermidis to form biofilms but do not inhibit planktonic bacterial growth. A compound library consisting of approximately 88,000 compounds was screened with a cell-based phenotypic assay in 96-well assay plates that measured the effect of each compound on both planktonic and biofilm growth. The high-throughput screening assay is described in detail in Materials and Methods. Compounds that inhibited biofilm growth by ≥80% and planktonic growth by ≤40% were scored as primary screening hits and were confirmed with the screening assay. For a summary of the results of the primary screening, see Table S1 in the supplemental material. A total of 1,055 confirmed hits were identified, which corresponds to a hit rate of 1.2%. The confirmed hits were evaluated in dose-response assays for antibiofilm activity (MBIC) and antibacterial activity (MIC) against a panel of biofilm-forming strains of S. aureus, S. epidermidis, and E. faecalis and for cytotoxicity against a mammalian (HeLa) cell line (CC50). These experiments resulted in the identification of 23 aryl rhodanines, which are potent inhibitors of biofilm growth of the majority of the staphylococcal strains tested (MBIC range, 3.1 to 50 μM), as well as E. faecalis. Significantly, the aryl rhodanines did not measurably affect planktonic growth of these strains (MIC, ≥100 μM) and did not exhibit mammalian cell cytotoxicity (CC50, ≥100 μM). To optimize the antibiofilm activity of the aryl rhodanines, several analogs of this series were synthesized and evaluated in biological assays. These analyses revealed clear trends in the structure-activity relationships of this compound series and are ongoing, and the relationships will be described in detail in a subsequent report. The biological activities of four compounds that are representative of this series (shown in Fig. 1) were analyzed in detail.
FIG. 1.
Structures of the aryl rhodanines used in this study.
Antibiofilm activities of the aryl rhodanines.
The antibiofilm and antibacterial activities of the four aryl rhodanines shown in Fig. 1 were determined with an assay that measures the MBIC and MIC, respectively, of each compound against a panel of gram-positive pathogens. This assay is conceptually the same as the commonly used broth microdilution assay (8), except that the growth conditions have been optimized for biofilm growth (described in Materials and Methods). The results of this assay, shown in Table 2, demonstrate that the aryl rhodanines have potent antibiofilm activity against a broad range of gram-positive bacteria, including S. aureus (methicillin-susceptible and -resistant S. aureus [MSSA and MRSA, respectively] strains), S. epidermidis, and coagulase-negative staphylococci (CoNS). While the aryl rhodanines were initially identified and developed for antibiofilm activity against the staphylococci, they also exhibit potent antibiofilm activity against several vancomycin-resistant strains of E. faecalis, E. faecium, and E. gallinarum. Only one gram-positive strain in the panel, E. faecium F118, was resistant to all of the aryl rhodanines tested. All of the compounds were most active against S. aureus ATCC 35556, a laboratory strain. While MBX-1384 was less active against this strain than were MBX-1240, MBX-1246, and MBX-1427, it was more active against a broader spectrum of strains. MBX-1384 was active (MBIC, ≤50 μM) against 95% of the gram-positive strains tested, compared to MBX-1240 (79%), MBX-1246 (69%), and MBX-1427 (67%). This is reflected in the MBIC90s (concentrations necessary to inhibit 90% of the strains tested) shown in Table 3, in which MBX-1384 was more potent than the other compounds against S. aureus, CoNS, and the enterococci. However, none of the aryl rhodanines were active against the gram-negative pathogen P. aeruginosa PAO1 or E. coli ATCC 25922 in inhibiting biofilm formation. Importantly, the aryl rhodanines did not exhibit detectable antibacterial activity (MIC, ≥100 μM) against any of the strains tested (data not shown), and they were not measurably cytotoxic against HeLa cells. The CC50s (in μM) of the aryl rhodanine compounds MBX-1240, MBX-1246, MBX-1384, and MBX-1427 against HeLa cells were ≥100, ≥100, ≥100, and 76.4, respectively. Taken together, these results indicate that the aryl rhodanines exhibit a broad spectrum of antibiofilm activity against gram-positive pathogens but do not display significant antibacterial activity.
TABLE 2.
Antibiofilm activities of representative aryl rhodanine compounds against a panel of biofilm-forming staphylococcal and enterococcal strainsa
| Organism | MBIC (μM [μg/ml]) of:
|
|||
|---|---|---|---|---|
| MBX-1240 | MBX-1246 | MBX-1384 | MBX-1427 | |
| S. aureus | ||||
| ATCC 35556 | 2.6 (1) | 4.4 (1.7) | 10.5 (3.8) | 2.6 (1) |
| MRSA-47263 | 8.8 (3.3) | 8.8 (3.3) | 12.5 (4.5) | 8.8 (3.4) |
| MRSA-58336 | 10.5 (3.9) | 10.5 (3.9) | 14.9 (5.3) | 10.5 (4.1) |
| MSSA | 14.9 (5.6) | 14.9 (5.6) | 12.5 (4.5) | 10.5 (4.1) |
| MRSA-27687 | 17.7 (6.6) | 25 (9.4) | 17.7 (6.3) | 50 (19.4) |
| NCTC 8325 | 25 (9.4) | 25 (9.4) | 12.5 (4.5) | 14.9 (5.8) |
| MRSA-14234 | 25 (9.4) | 17.7 (6.6) | 17.7 (6.3) | 25 (9.7) |
| MRSA-24102 | 25 (9.4) | 31.5 (11.8) | 19.8 (7.1) | 100 (38.7) |
| MRSA-33548 | 25 (9.4) | 35.4 (13.3) | 17.7 (6.3) | 31.5 (12.2) |
| MRSA-35392 | 25 (9.4) | 25 (9.4) | 25 (9) | 39.7 (15.4) |
| MRSA-23542 | 31.5 (11.8) | 19.8 (7.4) | 25 (9) | 25 (9.7) |
| MRSA-43349 | 35.4 (13.3) | 25 (9.4) | 12.5 (4.5) | 70.7 (27.4) |
| MW2 | 42 (15.8) | 31.5 (11.8) | 21 (7.5) | 25 (9.7) |
| MRSA-22733 | 50 (18.8) | 70.7 (26.5) | 17.7 (6.3) | 100 (38.7) |
| MRSA-38230 | 50 (18.8) | 25 (9.4) | 19.8 (7.1) | 35.4 (13.7) |
| MRSA-49404 | 63 (23.7) | 25 (9.4) | 31.5 (11.3) | 15.7 (6.1) |
| MRSA-44081 | 79.4 (29.8) | 25 (9.4) | 25 (9) | 39.7 (15.4) |
| MRSA-24209 | 100 (37.5) | 100 (37.5) | 25 (9) | 100 (38.7) |
| MRSA-1094 | 100 (37.5) | 100 (37.5) | 25 (9) | 100 (38.7) |
| S. epidermidis | ||||
| ATCC 35984 | 17.7 (6.6) | 25 (9.4) | 12.5 (4.5) | 17.7 (6.8) |
| GTC 18972 | 21 (7.9) | 50 (18.8) | 12.5 (4.5) | 17.7 (6.8) |
| 1457 | 42 (15.8) | 50 (18.8) | 12.5 (4.5) | 29.7 (11.5) |
| O-47 | 50 (18.8) | 100 (37.5) | 59.5 (21.3) | 59.5 (23) |
| CoNS | ||||
| WTBF-41 | 14.9 (5.6) | 17.7 (6.6) | 17.7 (6.3) | 17.7 (6.8) |
| WTBF-45 | 25 (9.4) | 50 (18.8) | 17.7 (6.3) | 25 (9.7) |
| WTBF-48 | 25 (9.4) | 50 (18.8) | 17.7 (6.3) | 12.5 (4.8) |
| WTBF-47 | 29.7 (11.2) | 42 (15.8) | 12.5 (4.5) | 29.7 (11.5) |
| WTBF-40 | 50 (18.8) | 59.5 (22.3) | 35.4 (12.7) | 50 (19.4) |
| WTBF-46 | 50 (18.8) | 59.5 (22.3) | 25 (9) | 70.7 (27.4) |
| E. faecalis | ||||
| ATCC 51299 (VRE) | 12.5 (4.7) | 25 (9.4) | 8.8 (3.2) | 14.9 (5.8) |
| F217 | 12.5 (4.7) | 29.7 (11.2) | 12.5 (4.5) | 14.9 (5.8) |
| F317 | 25 (9.4) | 100 (37.5) | 15.7 (5.6) | 100 (38.7) |
| ATCC 51575 | 29.7 (11.2) | 63 (23.7) | 21 (7.5) | 35.4 (13.7) |
| F177 | ≥100 (≥37.5) | ≥100 (≥37.5) | 19.8 (7.1) | 70.7 (27.4) |
| E. faecium | ||||
| EF208 | 31.5 (11.8) | 50 (18.8) | 15.7 (5.6) | 35.4 (13.7) |
| 1401 | 100 (37.5) | ≥100 (≥37.5) | 19.8 (7.1) | 79.4 (30.8) |
| EF1509 | ≥100 (≥37.5) | ≥100 (≥37.5) | 21 (7.5) | 59.5 (23) |
| F1181 | ≥100 (≥37.5) | ≥100 (≥37.5) | ≥100 (≥35.8) | ≥100 (≥38.7) |
| E. gallinarum 2392 | 84.1 (31.6) | 50 (18.8) | 17.7 (6.3) | ≥100 (≥38.7) |
| E. coli ATCC 25922 | ≥100 (≥37.5) | ≥100 (≥37.5) | ≥100 (≥35.8) | ≥100 (≥38.7) |
| P. aeruginosa PAO1 | ≥100 (≥37.5) | ≥100 (≥37.5) | ≥100 (≥35.8) | ≥100 (≥38.7) |
Antibiofilm activity is expressed as the MBIC, which is the lowest concentration of a compound in a twofold dilution series that inhibits biofilm formation by at least 80%. The values presented are the geometric mean MBICs obtained from at least three independent experiments.
TABLE 3.
MBIC90s of the aryl rhodanines obtained from the data presented in Table 1
Aryl rhodanines inhibit biofilm formation on medical grade silicone.
To determine whether the aryl rhodanines prevent biofilm formation on medically relevant material, the abilities of these compounds to inhibit biofilm formation by S. aureus ATCC 35556 on medical-grade silicone disks was determined. The results of these experiments are shown in Fig. 2. While MBX-1240 produced a dose-dependent decrease in biofilm formation on silicone, this compound exhibited significantly decreased antibiofilm activity on silicone compared to that on a polystyrene surface. The lowest concentration of MBX-1240 that produced ≥80% inhibition of biofilm formation on silicone was equivalent to 8× the MBIC for tissue culture-treated polystyrene (Table 2). In contrast, the antibiofilm potency of MBX-1384 on silicone was slightly increased compared to that on tissue culture-treated polystyrene. The lowest MBX-1384 concentration that produced ≥80% inhibition of biofilm formation was equivalent to 0.5× the MBIC for a polystyrene surface (Table 2). These results demonstrate the antibiofilm activity of the aryl rhodanines when medical grade silicone is used as the substrate.
Aryl rhodanines specifically inhibit the early phase of biofilm formation.
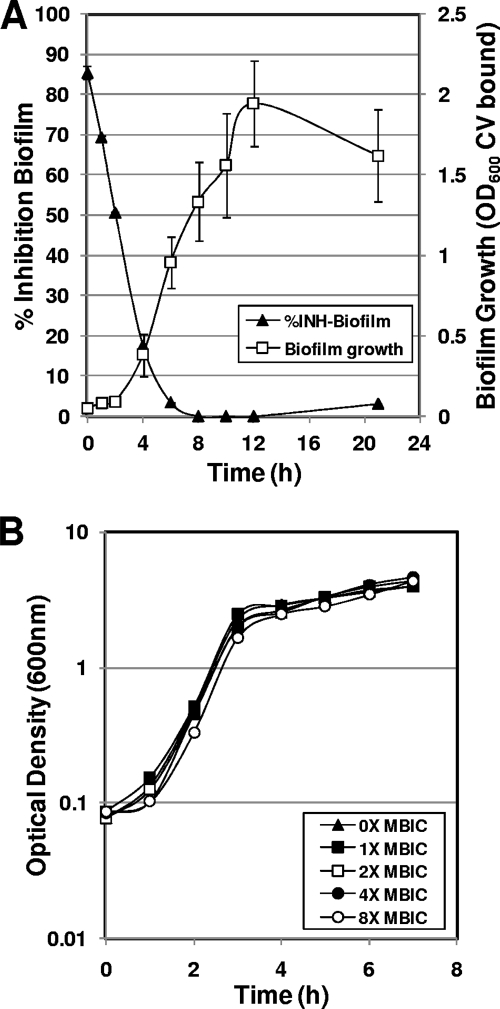
To verify that the aryl rhodanines specifically inhibit biofilm growth, the effect of MBX-1240 on planktonic growth was evaluated with a standard planktonic growth curve assay (Fig. 3B). The results of this assay demonstrate that MBX-1240 does not inhibit planktonic growth at concentrations up to eightfold greater than the MBIC, indicating that the aryl rhodanines specifically inhibit biofilm growth.
FIG. 3.
Aryl rhodanines specifically inhibit the early phase of biofilm growth in S. aureus ATCC 35556. (A) MBX-1240 specifically inhibits the early phases of staphylococcal biofilm formation. MBX-1240 (4× the MBIC) was added to biofilm cultures at various times during growth, and the effect on biofilm formation after a total of 22 h of growth was quantified by staining with CV and measuring OD600. The percent inhibition of biofilm growth compared to that of an untreated control was calculated and plotted on the primary y axis as a function of the time of addition (% Inhibition Biofilm; closed triangles). A biofilm growth curve was carried out in parallel in which biofilm growth was quantified at various times after inoculation as described above and was plotted on the secondary y axis (Biofilm Growth [OD600 CV bound]; open squares). (B) MBX-1240 does not inhibit planktonic growth at concentrations of up to 8× the MBIC.
In order to further characterize the antibiofilm activity of the aryl rhodanines, MBX-1240 (fourfold MBIC) was added to biofilm cultures of S. aureus ATCC 35556 at various times during biofilm growth and the effect on biofilm formation was measured after a total of 22 h of incubation. A biofilm growth curve was measured in parallel in the absence of any inhibitor addition in order to correlate sensitivity to MBX-1240 with a phase of normal biofilm growth. The results of this experiment are shown in Fig. 3A. Addition of MBX-1240 immediately after inoculation (0 h) resulted in 90% inhibition of biofilm formation. The antibiofilm activity of MBX-1240 decreased significantly during the subsequent 4 h of biofilm growth. After 6 h of growth, the biofilm culture was completely resistant to MBX-1240, which corresponds to the growth phase characterized by a rapid increase in the biomass of the biofilm (biofilm growth curve, Fig. 3A). These results indicate that the aryl rhodanine MBX-1240 specifically inhibits an early phase of biofilm formation.
Effect of aryl rhodanines on early-phase processes essential for biofilm formation.
To gain insight into the mechanism of action of the aryl rhodanines, their activity against several processes required during initial phases of staphylococcal biofilm development was examined. These processes include: (i) primary adhesion and attachment of bacteria to a surface, the initial step in biofilm formation; (ii) autolysis, which is required to release extracellular DNA, an essential component of the extracellular matrix during the initial stages of biofilm development (24, 43, 47); and (iii) synthesis of PNAG, the major component of the extracellular matrix of mature biofilms (10).
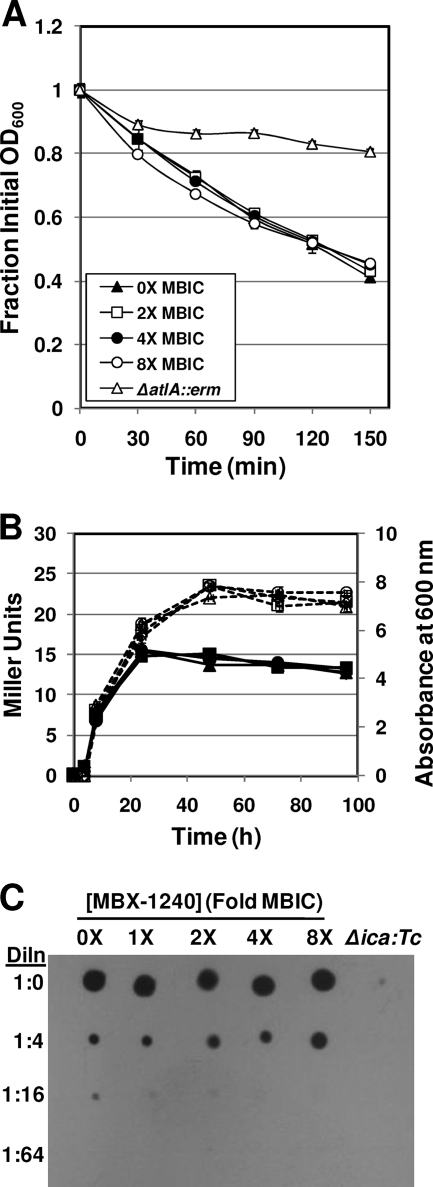
As shown in Fig. 4A, MBX-1240 and MBX-1384 inhibit primary attachment of S. aureus ATCC 35556 to a polystyrene surface under static conditions at a concentration equivalent to 1× and 0.5× the MBIC, respectively. These compounds also inhibit primary attachment to surfaces composed of PVC (Fig. 4C) and to surfaces composed of medical grade silicone (Fig. 4D) under static conditions at concentrations equivalent to 1× and 4× the MBIC, respectively. In addition, MBX-1240 inhibits attachment to silicone disks under conditions in which shear force is applied to the disk by shaking the assay tubes (Fig. 4E). MBX-1240 produced a significant decrease in the numbers of cells that were able to attach to the silicone disks at concentrations equivalent to 4× the MBIC. Taken together, these results indicate that the aryl rhodanines inhibit primary attachment to a variety of surfaces. In contrast, MBX-1240 did not affect autolysis or PNAG synthesis of S. aureus ATCC 35556 at concentrations as high as 8× the MBIC, as shown in Fig. 5A to C.
FIG. 5.
Aryl rhodanines do not affect autolysis or PNAG synthesis. (A) MBX-1240 does not inhibit Triton X-100-induced autolysis of S. aureus ATCC 35556 at concentrations of up to 8× the MBIC. Triton X-100-induced autolysis of cultures grown to mid-log phase and resuspended in buffer containing 0.05% Triton X-100 was monitored as the change in OD600 over time. Key: 0× the MBIC, closed triangles; 2× the MBIC, open squares; 4× the MBIC, open circles; 8× the MBIC, closed circles; autolysin-defective ΔatlA::erm mutant, open triangles. (B) MBX-1240 does not inhibit autolysis of stationary-phase cultures. Autolysis of ATCC 35556(pAJ22) was monitored as the levels of β-galactosidase activity (Miller units) in the culture supernatant that were release during stationary phase with the Miller assay. Key: 0× the MBIC, squares; 1× the MBIC, triangles; 4× the MBIC, diamonds; 8× the MBIC, circles; β-galactosidase activity in Miller units, open symbols and dashed lines; OD600, closed symbols and solid lines. (C) MBX-1240 does not inhibit PNAG synthesis by S. aureus ATCC 35556 at concentrations of up to 8× the MBIC. PNAG extracts were prepared from S. aureus ATCC 35556 cultures grown in the presence of various concentrations of MBX-1240 (0× to 8× the MBIC), serially diluted 1:4, spotted onto nitrocellulose, and probed with WGA-HRP. The amount of WGA-HRP bound was detected by adding a chemiluminescent substrate and exposing the blot to film. The specificity of the assay is demonstrated by the lack of reaction in the extract prepared from a PNAG-defective (Δica::Tc) strain. Dln, dilution.
To determine whether the inhibition of attachment by the aryl rhodanines is caused by an interaction with a cellular target or with the plastic surface, a suspension of S. aureus ATCC 35556 was treated with various concentrations of MBX-1240 and MBX-1384 for 30 min (equivalent to the time of the adhesion assay). Bacterial cells were then harvested by centrifugation to remove free compound, and the resulting pellet was resuspended in PBS to the original cell density. The pretreated cell suspension was subjected to the static adhesion assay with a polystyrene assay plate. The results of this assay are shown in Fig. 4B. The initial attachment to polystyrene by S. aureus ATCC 35556 pretreated with MBX-1240 and MBX-1384 was decreased by ≥80% at concentrations equivalent to 4× and 1× the MBIC, respectively, suggesting that the aryl rhodanines inhibit attachment through an interaction with a cellular target. To determine whether de novo protein synthesis is required for initial attachment to polystyrene, the adhesion assays with S. aureus ATCC 35556 were performed in the presence of 15 and 30 μg chloramphenicol/ml (MIC = 1 μg/ml). The results of these experiments demonstrated that protein synthesis is not required for the initial attachment of S. aureus to polystyrene (data not shown).
Antibiofilm activity of the aryl rhodanines is not affected by host factors.
Staphylococci and enterococci produce several distinct adhesins that are covalently linked to the cell wall, where they can bind to specific host proteins. For example, S. aureus produces several proteins, such as FnBPA, FnBPB, ClfA, and SdrG, that bind to fibronectin, as well as the fibrinogen-binding proteins ClfA and ClfB (15, 48). Since medical devices inserted into the human body are rapidly coated with these host factors (56), it is possible that bacteria could bypass the adhesion defect produced by aryl rhodanines by binding to host factors. To determine whether the antibiofilm activity of the aryl rhodanines is affected by the presence of host factors, the wells of an assay plate were preconditioned with solutions containing various concentrations of the host factors fibrinogen and fibronectin, and the antibiofilm activity (MBIC) of an aryl rhodanine was determined. The results, shown in Table 4, demonstrate that the presence of fibronectin or fibrinogen at concentrations of up to 20 μg/ml did not significantly affect the MBICs. These results suggest that the presence of host factors will not interfere with the antibiofilm activity of the aryl rhodanines in clinical use.
TABLE 4.
The presence of fibrinogen or fibronectin does not affect antibiofilm activity (MBIC) of aryl rhodanines against S. aureus ATCC 35556a
| Pretreatment and concn (μg/ml) | MBIC (μM) of:
|
|||
|---|---|---|---|---|
| MBX-1240 | MBX-1246 | MBX-1384 | MBX-1427 | |
| Fibronectin | ||||
| 0 | 3.125 | 6.25 | 12.5 | 3.125 |
| 1 | 3.125 | 6.25 | 12.5 | 3.125 |
| 2 | 3.125 | 6.25 | 12.5 | 3.125 |
| 5 | 3.125 | 6.25 | 12.5 | 3.125 |
| 10 | 6.25 | 6.25 | 12.5 | 3.125 |
| 20 | 6.25 | 6.25 | 12.5 | 3.125 |
| Fibrinogen | ||||
| 0 | 3.125 | 6.25 | 12.5 | 3.125 |
| 1 | 3.125 | 6.25 | 12.5 | 3.125 |
| 2 | 3.125 | 6.25 | 12.5 | 3.125 |
| 5 | 3.125 | 6.25 | 12.5 | 3.125 |
| 10 | 3.125 | 6.25 | 12.5 | 3.125 |
| 20 | 6.25 | 6.25 | 12.5 | 3.125 |
Assay wells were pretreated with solutions of fibronectin or fibrinogen at concentrations ranging from 0 to 20 μg protein/ml at 4°C for 24 h. After pretreatment, the protein solutions were removed, the assay wells were washed with sterile water, and the pretreated 96-well plates were used for an antibiofilm activity assay as described in Materials and Methods. This assay was repeated twice, and identical results were obtained.
DISCUSSION
In this report, we have described a novel approach to the prevention of biofilm-related infections, namely, the development of small molecules that specifically inhibit biofilm formation but do not affect overall growth or survival. The strategies currently employed for preventing biofilm formation on medical devices involve antimicrobial coatings, such as rifampin/minocycline (44) or chlorhexidine/silver sulfadiazine (27), to inhibit bacterial growth on catheter surfaces. In contrast, we have identified a class of compounds, the aryl rhodanines, that specifically inhibit the ability of S. aureus, S. epidermidis, E. faecalis, and E. faecium to attach to a surface and form biofilms. These compounds do not exhibit antibacterial activity in the standard broth microdilution assay, and they do not affect bacterial growth rates in liquid cultures. Therefore, the aryl rhodanines are apparently specific antibiofilm compounds and might be used as antibiofilm coatings on medical devices to prevent infections. Preliminary mechanism-of-action studies indicate that the aryl rhodanines act specifically on a process required during the initial phase of biofilm development, which prevents the formation of a fully developed biofilm.
The antibiofilm activity of the aryl rhodanines was evaluated with an assay that is conceptually similar to the broth microdilution assay for antibacterial activity described by the CLSI (8). The antibiofilm assay yields the MBIC of a given compound under conditions optimized for biofilm growth. Therefore, this assay provides a stringent measurement of antibiofilm activity under in vitro conditions. While this assay has not been clinically validated, it has proven to be an extremely useful tool for identifying and evaluating compounds with antibiofilm activity. In addition, the growth and assay conditions can be optimized for many different bacterial species, making it possible to evaluate antibiofilm activity against a broad spectrum of biofilm pathogens.
The spectrum of bacteria used to determine the antibiofilm activity of the aryl rhodanines represents the bacterial species that are the most prevalent causative agents of biofilm-related infections, such as catheter-related bloodstream infections (7, 37, 57). While all four aryl rhodanines shown in Fig. 1 exhibited antibiofilm activity against the majority of the strains tested, MBX-1384 had higher activity against all of the strains tested, as demonstrated by the MBIC90s (Table 3). The preliminary MBIC90s of MBX-1384 against S. aureus, CoNS, and the enterococci ranged from 19.8 to 35.4 μM, corresponding to 7.1 to 12.7 μg/ml. Only one strain in the panel, E. faecium F118 (a vancomycin resistant enterococcus [VRE]), was completely resistant to MBX-1384. These data indicate that MBX-1384 has broad-spectrum antibiofilm activity against gram-positive biofilm-forming pathogens, suggesting that this compound could be used as a device coating or a catheter lock solution to prevent colonization by the most prevalent group of biofilm pathogens (staphylococci and enterococci). Initial cytotoxicity studies with a mammalian cell line indicate that MBX-1384 is not toxic (see Results), suggesting that relatively high concentrations of this compound can be tolerated.
Our initial mechanism-of-action studies indicated that the aryl rhodanines inhibit the primary adhesion of bacterial cells to a surface, the initial step of staphylococcal biofilm formation (17). One possible mechanism of action involves a physical interaction between the aryl rhodanines and one or more of the adhesins located on the bacterial cell surface, which could prevent the initial interaction between the bacteria cell and the surface. For example, S. aureus expresses several adhesins that are located on the cell surface, including AtlA, which is required for cellular attachment to polystyrene (20), and the surface-attached adhesins that bind to fibronectin (FnBPA, FnBPB, ClfA, and SdrG) and fibrinogen (ClfA and ClfB) (15, 48). Our finding that pretreatment of S. aureus with an aryl rhodanine, followed by removal of free compound, inhibited primary attachment to polystyrene (Fig. 4B) is consistent with an interaction between the aryl rhodanines and a cellular target. However, if the aryl rhodanines prevent adhesion through the inhibition of a single adhesin, it should be possible to rescue biofilm formation if another substrate is provided. Our finding that precoating plates with fibrinogen or fibronectin does not alter the MBICs of the aryl rhodanines tested suggests that the aryl rhodanines do not inhibit a specific adhesin. Alternatively, the aryl rhodanines may inhibit one or more processes required for strengthening the interaction between the bacterial cell and the surface. Potential targets include (but are not limited to) one or more of the putative signal transduction pathways that may be required to initiate biofilm development. However, our finding that de novo protein synthesis is not required for primary attachment suggests that the aryl rhodanines do not inhibit changes in gene expression required for attachment. Because of the spectrum of antibiofilm activity of the aryl rhodanines, it is likely that the molecular target of these compounds is present in both the staphylococci and the enterococci but is not present or is not accessible in gram-negative species, such as E. coli and P. aeruginosa. Further experiments are required to elucidate the molecular target(s) of the aryl rhodanines.
The unique biofilm-specific activity, spectrum of activity, and mechanism of action of the aryl rhodanines indicate that they could be developed as antibiofilm coatings for indwelling medical devices to prevent colonization by gram-positive pathogens. The absence of antibacterial activity decreases the likelihood that resistance will develop, as selective pressure against biofilm formation is lower than that exerted by inhibition of growth. The promising in vitro activities of the aryl rhodanines merit further evaluation in in vitro and in vivo experimental systems.
Supplementary Material
Acknowledgments
Funding for this work was provided by the National Institutes of Health in the form of an SBIR grant (1 R43 AI074161-01).
We thank Richard Ellison (University of Massachusetts Medical School) for generously providing many of the clinical isolates used in this study and Mark Shirtliff (University of Maryland—Baltimore), Jason Pryztwoski (Northwestern Memorial Hospital, Chicago, IL), Michael Otto (National Institute of Allergy and Infectious Diseases, Bethesda, MD), Kenneth Bayles (University of Nebraska Medical Center, Omaha), and Steven Lory (Harvard Medical School, Boston, MA) for providing additional bacterial strains used in this study.
Footnotes
Published ahead of print on 3 August 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 2.Butler, M. M., W. A. Lamarr, K. A. Foster, M. H. Barnes, D. J. Skow, P. T. Lyden, L. M. Kustigian, C. Zhi, N. C. Brown, G. E. Wright, and T. L. Bowlin. 2007. Antibacterial activity and mechanism of action of a novel anilinouracil-fluoroquinolone hybrid compound. Antimicrob. Agents Chemother. 51:119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoun, J. H., and J. T. Mader. 1997. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin. Orthop. Relat. Res. 341:206-214. [PubMed] [Google Scholar]
- 4.Christensen, G. D., L. Baldassarri, and W. A. Simpson. 1995. Methods for studying microbial colonization of plastics. Methods Enzymol. 253:477-500. [DOI] [PubMed] [Google Scholar]
- 5.Christensen, G. D., A. L. Bisno, J. T. Parisi, B. McLaughlin, M. G. Hester, and R. W. Luther. 1982. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann. Intern. Med. 96:1-10. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, V. H., D. R. Crosslin, J. Y. Friedman, S. D. Reed, C. H. Cabell, R. I. Griffiths, L. E. Masselink, K. S. Kaye, G. R. Corey, L. B. Reller, M. E. Stryjewski, K. A. Schulman, and V. G. Fowler, Jr. 2005. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am. J. Med. 118:1416. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-seventh edition, vol. M7-A7 vol. 26 (2). Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtin, J. J., and R. M. Donlan. 2006. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 50:1268-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darouiche, R. O., M. D. Mansouri, P. V. Gawande, and S. Madhyastha. 2009. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J. Antimicrob. Chemother. 64:88-93. [DOI] [PubMed] [Google Scholar]
- 13.Darouiche, R. O., M. D. Mansouri, P. V. Gawande, and S. Madhyastha. 2008. Efficacy of combination of chlorhexidine and protamine sulphate against device-associated pathogens. J. Antimicrob. Chemother. 61:651-657. [DOI] [PubMed] [Google Scholar]
- 14.Darouiche, R. O., I. I. Raad, S. O. Heard, J. I. Thornby, O. C. Wenker, A. Gabrielli, J. Berg, N. Khardori, H. Hanna, R. Hachem, R. L. Harris, and G. Mayhall. 1999. A comparison of two antimicrobial-impregnated central venous catheters. N. Engl. J. Med. 340:1-8. [DOI] [PubMed] [Google Scholar]
- 15.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Suárez, C., J. Pasma, A. J. van der Borden, J. Wingender, H. C. Flemming, H. J. Busscher, and H. C. van der Mei. 2002. Influence of extracellular polymeric substances on deposition and redeposition of Pseudomonas aeruginosa to surfaces. Microbiology 148:1161-1169. [DOI] [PubMed] [Google Scholar]
- 17.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 18.Hazan, Z., J. Zumeris, H. Jacob, H. Raskin, G. Kratysh, M. Vishnia, N. Dror, T. Barliya, M. Mandel, and G. Lavie. 2006. Effective prevention of microbial biofilm formation on medical devices by low-energy surface acoustic waves. Antimicrob. Agents Chemother. 50:4144-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Gotz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heilmann, C., M. Hussain, G. Peters, and F. Gotz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez, M. D., M. D. Mansouri, S. Aslam, B. Zeluff, and R. O. Darouiche. 2009. Efficacy of combination of N-acetylcysteine, gentamicin, and amphotericin B for prevention of microbial colonization of ventricular assist devices. Infect. Control Hosp. Epidemiol. 30:190-192. [DOI] [PubMed] [Google Scholar]
- 22.Hong, S. H., J. Jeong, S. Shim, H. Kang, S. Kwon, K. H. Ahn, and J. Yoon. 2008. Effect of electric currents on bacterial detachment and inactivation. Biotechnol. Bioeng. 100:379-386. [DOI] [PubMed] [Google Scholar]
- 23.Iordanescu, S., and M. Surdeanu. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96:277-281. [DOI] [PubMed] [Google Scholar]
- 24.Izano, E. A., M. A. Amarante, W. B. Kher, and J. B. Kaplan. 2008. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 74:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jefferson, K. K., and N. Cerca. 2006. Bacterial-bacterial cell interactions in biofilms: detection of polysaccharide intercellular adhesins by blotting and confocal microscopy. Methods Mol. Biol. 341:119-126. [DOI] [PubMed] [Google Scholar]
- 26.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maki, D. G., S. M. Stolz, S. Wheeler, and L. A. Mermel. 1997. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann. Intern. Med. 127:257-266. [DOI] [PubMed] [Google Scholar]
- 28.Mallick, S. K., A. R. Martin, and R. G. Lingard. 1971. Synthesis and antimicrobial evaluation of some 5-(5-nitrofurylidene)rhodanines, 5-(5-nitrofurylidene)thiazolidine-2,4-diones, and their vinylogs. J. Med. Chem. 14:528-532. [DOI] [PubMed] [Google Scholar]
- 29.Mani, N., P. Tobin, and R. K. Jayaswal. 1993. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J. Bacteriol. 175:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall, N. J., C. J. Goodwin, and S. J. Holt. 1995. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 5:69-84. [PubMed] [Google Scholar]
- 31.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munson, E. L., S. O. Heard, and G. V. Doern. 2004. In vitro exposure of bacteria to antimicrobial impregnated-central venous catheters does not directly lead to the emergence of antimicrobial resistance. Chest 126:1628-1635. [DOI] [PubMed] [Google Scholar]
- 33.Nejadnik, M. R., A. F. Engelsman, I. C. Saldarriaga Fernandez, H. J. Busscher, W. Norde, and H. C. van der Mei. 2008. Bacterial colonization of polymer brush-coated and pristine silicone rubber implanted in infected pockets in mice. J. Antimicrob. Chemother. 62:1323-1325. [DOI] [PubMed] [Google Scholar]
- 34.Nejadnik, M. R., H. C. van der Mei, H. J. Busscher, and W. Norde. 2008. Determination of the shear force at the balance between bacterial attachment and detachment in weak-adherence systems, using a flow displacement chamber. Appl. Environ. Microbiol. 74:916-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nejadnik, M. R., H. C. van der Mei, W. Norde, and H. J. Busscher. 2008. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials 29:4117-4121. [DOI] [PubMed] [Google Scholar]
- 36.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 37.O'Gara, J. P., and H. Humphreys. 2001. Staphylococcus epidermidis biofilms: importance and implications. J. Med. Microbiol. 50:582-587. [DOI] [PubMed] [Google Scholar]
- 38.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, I. I. Raad, A. Randolph, and R. A. Weinstein. 2002. Guidelines for the prevention of intravascular catheter-related infections. Am. J. Infect. Control 30:476-489. [DOI] [PubMed] [Google Scholar]
- 39.O'Neill, A. J., K. Miller, B. Oliva, and I. Chopra. 2004. Comparison of assays for detection of agents causing membrane damage in Staphylococcus aureus. J. Antimicrob. Chemother. 54:1127-1129. [DOI] [PubMed] [Google Scholar]
- 40.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 41.Panetta, J. A. 25 November 1997. Aryl-substituted rhodanine derivatives. U.S. patent 5,691,367.
- 42.Polonio, R. E., L. A. Mermel, G. E. Paquette, and J. F. Sperry. 2001. Eradication of biofilm-forming Staphylococcus epidermidis (RP62A) by a combination of sodium salicylate and vancomycin. Antimicrob. Agents Chemother. 45:3262-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin, Z., Y. Ou, L. Yang, Y. Zhu, T. Tolker-Nielsen, S. Molin, and D. Qu. 2007. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153:2083-2092. [DOI] [PubMed] [Google Scholar]
- 44.Raad, I., R. Darouiche, J. Dupuis, D. Abi-Said, A. Gabrielli, R. Hachem, M. Wall, R. Harris, J. Jones, A. Buzaid, C. Robertson, S. Shenaq, P. Curling, T. Burke, and C. Ericsson. 1997. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. Ann. Intern. Med. 127:267-274. [DOI] [PubMed] [Google Scholar]
- 45.Raad, I., H. Hanna, and D. Maki. 2007. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect. Dis. 7:645-657. [DOI] [PubMed] [Google Scholar]
- 46.Ramanathan, V., E. J. Chiu, J. T. Thomas, A. Khan, G. M. Dolson, and R. O. Darouiche. 2007. Healthcare costs associated with hemodialysis catheter-related infections: a single-center experience. Infect. Control Hosp. Epidemiol. 28:606-609. [DOI] [PubMed] [Google Scholar]
- 47.Rice, K. C., E. E. Mann, J. L. Endres, E. C. Weiss, J. E. Cassat, M. S. Smeltzer, and K. W. Bayles. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 104:8113-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivera, J., G. Vannakambadi, M. Hook, and P. Speziale. 2007. Fibrinogen-binding proteins of gram-positive bacteria. Thromb. Haemost. 98:503-511. [PubMed] [Google Scholar]
- 49.Sampath, L. A., S. M. Tambe, and S. M. Modak. 2001. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infect. Control Hosp. Epidemiol. 22:640-646. [DOI] [PubMed] [Google Scholar]
- 50.Shanks, R. M., N. P. Donegan, M. L. Graber, S. E. Buckingham, M. E. Zegans, A. L. Cheung, and G. A. O'Toole. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73:4596-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 52.van der Borden, A. J., P. G. Maathuis, E. Engels, G. Rakhorst, H. C. van der Mei, H. J. Busscher, and P. K. Sharma. 2007. Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials 28:2122-2126. [DOI] [PubMed] [Google Scholar]
- 53.van der Borden, A. J., H. C. van der Mei, and H. J. Busscher. 2004. Electric-current-induced detachment of Staphylococcus epidermidis strains from surgical stainless steel. J. Biomed. Mater. Res. B 68:160-164. [DOI] [PubMed] [Google Scholar]
- 54.van der Borden, A. J., H. C. van der Mei, and H. J. Busscher. 2005. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials 26:6731-6735. [DOI] [PubMed] [Google Scholar]
- 55.van der Borden, A. J., H. van der Werf, H. C. van der Mei, and H. J. Busscher. 2004. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl. Environ. Microbiol. 70:6871-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaudaux, P., P. François, D. P. Lew, and F. A. Waldvogel. 2000. Host factors predisposing to and influencing therapy of foreign body infections, p. 1-26. In F. A. Waldvogel and A. L. Bisno (ed.), Infections associated with indwelling medical devices, 3rd ed. ASM Press, Washington, DC.
- 57.von Eiff, C., G. Peters, and C. Heilmann. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677-685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.