Abstract
Serious Enterococcus faecalis infections usually require combination therapy to achieve a bactericidal effect. In orthopedic infections, the prognosis of enterococcal etiology is considered poor, and the use of aminoglycosides is questioned. The ampicillin-ceftriaxone combination has recently been accepted as alternative therapy for enterococcal endocarditis. After one of our patients with endocarditis and vertebral osteomyelitis was cured with ampicillin-ceftriaxone, we started a pilot study of orthopedic infections. Patients with infections due to E. faecalis (with two or more surgical samples or blood cultures) diagnosed during 2005 to 2008 were recruited. Polymicrobial infections with ampicillin- and ceftriaxone-resistant microorganisms were excluded. Patients received ampicillin (8 to 16 g/day)-ceftriaxone (2 to 4 g/day) and were followed up prospectively. Of 31 patients with E. faecalis infections, 10 received ampicillin-ceftriaxone. Including the first patient, 11 patients were treated with ampicillin-ceftriaxone: 3 with prosthetic joint infections, 3 with instrumented spine arthrodesis device infections, 2 with osteosynthesis device infections, 1 with foot osteomyelitis, and 2 with vertebral osteomyelitis and endocarditis. Six infections (55%) were polymicrobial. All cases except the vertebral osteomyelitis ones required surgery, with retention of foreign material in six cases. Ampicillin-ceftriaxone was given for 25 days (interquartile range, 15 to 34 days), followed by amoxicillin (amoxicilline) being given to seven patients (64%). One patient with endocarditis died within 2 weeks (hemorrhagic stroke) and was not evaluable. For one patient with prosthesis retention, the infection persisted; 9/10 patients (90%) were cured, but 1 patient was superinfected. Follow-up was for 21 months (interquartile range, 14 to 36 months). Ampicillin-ceftriaxone may be a reasonable synergistic combination to treat orthopedic infections due to E. faecalis. Our experience, though limited, shows good outcomes and tolerability and may provide a basis for further well-designed comparative studies.
Enterococcus faecalis is a low-virulence microorganism that colonizes the human gastrointestinal tract (23) and produces a variety of infections, especially under antimicrobial pressure or in nosocomial settings, including urinary tract and intra- abdominal infections, bacteremia, endocarditis, meningitis, and orthopedic and foreign-body-related infections (20). In orthopedic infections, enterococci are relatively common etiologic agents (26, 27); however, it is often difficult to distinguish infection from colonization, as the bacteria may be isolated in samples of doubtful significance or in combination with other microorganisms.
As is well known in clinical practice, some enterococcal infections are difficult to treat (21). Though susceptible at relatively low MICs, enterococci are characteristically resistant to the bactericidal effect of cell wall-active antibiotics (16). Most E. faecalis strains show the “paradoxical or Eagle effect,” in which penicillins are more bactericidal just above the MIC and less bactericidal as the drug concentration increases (6, 9). This phenomenon has been attributed by some authors to an intrinsic defect in the autolytic activity of the microorganism (14). As a result of these special features, in the absence of high-level aminoglycoside (AG) resistance, an ampicillin-AG combination is now the therapy of choice for deep-seated infections by E. faecalis where a bactericidal effect is desirable, such as for endocarditis or meningitis (20, 23).
In orthopedic and foreign-body infections, in which biofilm formation occurs, the bactericidal effect is sought in order to eradicate infection and avoid relapses. For serious enterococcal orthopedic infections, most authors recommend a combination therapy with AG (26, 37). However, the role of AG in the treatment of orthopedic infections has often been questioned, as the local conditions in infected bone may reduce their efficacy against susceptible microorganisms (17), and they have serious side effects that may limit their use.
The ampicillin-ceftriaxone (AMP-CRO) combination has recently been recommended (strength, IIbC) for endocarditis due to E. faecalis that is highly resistant to AG (2) after the studies of Gavaldà et al. (11-13). The basis for these reports was an in vitro study by Mainardi et al. which found a synergistic effect between amoxicillin (amoxicilline) and low levels of cefotaxime against several AG-susceptible and AG-resistant E. faecalis strains (18).
Pyogenic vertebral osteomyelitis may present as a complication of infective endocarditis (22, 25, 32). We undertook this pilot study after one of our patients with enterococcal endocarditis and vertebral osteomyelitis was treated with AMP-CRO and cured at both infection sites. To our knowledge, this is the first study to evaluate a double β-lactam combination for the treatment of orthopedic infections caused by E. faecalis.
(These data were partially reported at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2008 [8a].)
MATERIALS AND METHODS
Setting.
The study was performed at a 900-bed tertiary care teaching hospital in Barcelona, Spain. Patients were attended in the orthopedic infection ward, a multidisciplinary section where the orthopedic surgery and infectious disease departments collaborate.
Study design. (i) Inclusion and exclusion criteria.
After approval of the proposal by the hospital's ethical committee, patients diagnosed with orthopedic infections due to E. faecalis between January 2005 and January 2008 were sequentially recruited as eligible subjects for the study. Osteomyelitis and foreign-body infection were diagnosed on a clinical basis (local pain and purulent draining), with complementary laboratory parameters (acute-phase reactant elevation) and compatible imaging (plain radiographs, computed tomography scans, or magnetic resonance). Endocarditis was diagnosed using Duke's criteria. Spontaneous vertebral osteomyelitis was diagnosed by the presence of clinical symptoms (spinal pain and localized tenderness or limited range of motion) and using characteristic image findings (in plain radiographs and computed tomography scans or using magnetic resonance), as described elsewhere (8). Prosthetic joint infection was diagnosed and classified according to the work of Tsukayama et al. (31).
The following parameters were considered exclusion criteria: infection due to an AMP-resistant E. faecalis strain, polymicrobial infection with microorganisms resistant to the AMP-CRO combination, and allergy to penicillin.
(ii) Microbiological diagnosis.
The diagnosis of osteomyelitis due to E. faecalis required the isolation of E. faecalis in two or more surgical samples or blood cultures. E. faecalis identification was performed using the automatic Dade Behring MicroScan system (Sacramento, CA). MICs were determined according to the Clinical and Laboratory Standards Institute guidelines (5) with a microdilution method using Sensititre Emiza panels (STAENC1F; Trek Diagnostic Systems, Ltd., United Kingdom).
(iii) Antibiotic therapy.
Cases treated with the AMP-CRO combination for at least 1 week within the global treatment schedule were considered evaluable. Antibiotic dosing varied depending on the site of infection, but a minimum of AMP (8 g/day)-CRO (2 g/day) was considered appropriate (37).
(iv) Follow-up and outcome definitions.
The follow-up period was calculated from the end of antibiotic therapy until February 2009. During follow-up, patients were evaluated periodically for persistent or new clinical signs of infection. The patient was considered cured if the enterococcal infection was eradicated by the time of the last control. Persistence of the enterococcal infection was considered to occur when clinical symptoms persisted and cultures were still positive for E. faecalis. Relapse was defined as a temporary remission of the symptoms and later reappearance of local inflammatory signs, pain, or purulent draining, with the isolation of E. faecalis as in the previous episode. Superinfection was diagnosed when a temporary remission of the symptoms was followed by reappearance of local inflammatory signs and the isolation of a different microorganism.
(v) In vitro time-kill curves.
Twenty-four-hour time-kill curves were made in exponential growth and stationary phases with two of the clinical isolates. Exponential-growth-phase studies were performed using a tube macrodilution method in Mueller-Hinton broth, with 107 CFU/ml inocula and multiple antibiotic concentrations (AMP, ranging from 0.12 to 64 mg/liter; CRO, 5 and 10 mg/liter). Quantitative bacterial counts were determined as log CFU/ml at 6 and 24 h of incubation at 37°C. To avoid the in vitro carryover effect, the sample was allowed to be absorbed by the agar until the plate surface appeared dry and then was spread over the plate. The stationary-phase studies were performed using bacteria at 108 CFU/ml, which were recovered from an overnight culture in Trypticase soy broth, centrifuged, and resuspended in a nutrient-restricted medium (phosphate-buffered saline, 1% glucose, and 4% Mueller-Hinton broth), thus ensuring that bacteria remained stable for up to 24 h under these conditions. Quantitative cultures were performed as described above. Bactericidal activity was defined as a >3 log10 decrease from the initial number of CFU/ml of inoculum after 24 h.
The results of using the combination were compared with the most active single drug results; synergy, indifference, and antagonism were then defined as a ≥2 log increase in killing, a <1 log change (increase or decrease) in killing, and a ≥2 log decrease in killing, respectively.
AMP (Normon, Madrid, Spain) and CRO (Roche Farma, Madrid, Spain) purified powders were resuspended by following the laboratory's recommendations.
Statistical analysis.
A descriptive analysis of data was performed with SPSS software version 15.0 (SPSS Inc., Chicago, IL). Continuous variables are expressed with the median and range or interquartile range (IQR).
RESULTS
Clinical results.
In the study period, 31 orthopedic infections due to E. faecalis were diagnosed. None of the E. faecalis strains was AMP resistant. Twenty-five patients (80%) had polymicrobial infections. The following were excluded: 16 cases due to polymicrobial infection including an AMP-CRO-resistant microorganism (mostly staphylococci and Pseudomonas aeruginosa strains); 1 case due to a penicillin allergy; and 4 cases in which the physician decided to give penicillin monotherapy. Finally, 10 of the patients were treated with AMP-CRO.
Including the first case with endocarditis and vertebral osteomyelitis treated before 2005, 11 patients received AMP-CRO. The clinical data are summarized in Table 1. Five patients (45%) were male, and the median age was 69 years (range, 24 to 83 years). Six patients (55%) had polymicrobial infections: four with Escherichia coli, one with Proteus mirabilis, and one with Lactobacillus spp.
TABLE 1.
Summary of patients treated with the AMP-CRO combinationa
| Patient no. | Infection site(s) | Bacteremia | Etiology | Surgery(ies) | Length of IV therapy (days) | Duration of AMP-CRO treatment (days) | Duration of sequential oral therapy (drug, days of treatment) | Follow-up time (mo.) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Endocarditis and vertebral OM | + | E. faecalis | None | 47 | 29 | None | 50 | Cured |
| 2 | Knee PJI (early) | − | E. faecalis, Lactobacillus sp. | Debridement | 43 | 25 | AMX, 61 | 26 | Cured |
| 3 | Hip PJI (late) | − | E. faecalis | Partial exchange (1 stage) | 46 | 42 | AMX, 16 | 14 | Cured |
| 4 | Knee PJI (late) | − | E. faecalis | Debridement, partial removal | 58 | 16 | AMX ST | ST | Persistence |
| 5 | Open fracture of tibia, OS | − | E. faecalis, E. coli | Debridement, OS removal | 44 | 22 | None | 21 | Cured/superinfection |
| 6 | Femur OS | − | E. faecalis | Exchange (2 stages) | 30 | 30 | AMX, 25 | 36 | Cured |
| 7 | Spine OS, meningitis | + | E. faecalis, E. coli | Debridement | 40 | 34 | None | 13 | Cured |
| 8 | Spine OS | − | E. faecalis, P. mirabilis | Debridement | 7 | 7 | AMX-CIP, 32 | 12 | Cured |
| 9 | Spine OS, meningitis | − | E. faecalis, E. coli | Debridement | 35 | 15 | ACL, 20 | 40 | Cured |
| 10 | Chronic OM of the foot | − | E. faecalis, E. coli | Debridement | 38 | 35 | AMX-CXM, 20 | 19 | Cured |
| 11 | Endocarditis and vertebral OM | + | E. faecalis | None | 11 | 7 | None | Death (hemorrhagic stroke) |
Abbreviations: ACL, amoxicillin-clavulanic acid; AMX, amoxicillin; CXM, cefuroxime-axetil; CIP, ciprofloxacin; IV, intravenous; OM, osteomyelitis; OS, osteosynthesis; PJI, prosthetic joint infection; ST, suppressive therapy.
The sites of infection were as follows: three prosthetic joint infections (27%) (one located in the hip, and two located in the knee); three instrumented spine arthrodesis device infections (27%), with two of them also having meningitis due to a postsurgical cerebrospinal fluid leak; two osteosynthesis device infections (18%); one osteomyelitis of the foot (9%); and two vertebral osteomyelitis infections with endocarditis (18%). With the exception of the patients with spontaneous vertebral osteomyelitis, all underwent surgery (median, one surgery; range, one to three surgeries). Among the patients with meningitis, patient 7 required the placement of a lumbar catheter for 7 days in order to allow a cerebrospinal fluid fistula to heal, and patient 9 was cured with debridement and antibiotics only.
In eight cases (72%), a foreign body was involved, which was retained in six cases and removed in two. Among those with prosthetic joint infections (patients 2, 3, and 4), two patients had late chronic infections, and the other one (patient 2) had an early postoperative infection with stability of the implant. The latter could be managed with debridement only. In patient 3, the acetabular component was exchanged in one stage, and the femoral component was retained, with a successful outcome. Though the complete removal of the knee prosthesis was indicated for patient 4, the procedure proved to be impossible to perform due to technical problems. As a consequence, the intramedullary component of the prosthesis, which was involved in the infection, had to be retained. This patient was given suppressive antibiotic therapy (long-term oral antibiotic therapy given only to control clinical symptoms, not to eradicate infection [27, 37]).
The AMP dose ranged between 8 and 16 g/day, and the CRO dose ranged between 2 and 4g/day. The median duration of AMP-CRO treatment was 25 days (IQR, 15 to 34 days). Sequential therapy with amoxicillin was given in seven cases (64%) (as suppressive therapy in one case). None of the patients developed any side effects with the protocol treatment.
The median follow-up time was 21 months (IQR, 14 to 36 months). Patient 11 (with endocarditis) died within 2 weeks due to a hemorrhagic stroke and was not evaluable for the outcome of the osteomyelitis. In 9/10 patients (90%), enterococcal infection was eradicated, but 1 patient was superinfected due to Staphylococcus aureus. Only in the case of patient 4 did the infection persist; finally, this patient required the complete removal of the foreign material by a supracondylar amputation to eradicate the infection.
In vitro results.
E. faecalis strains from patients 3 and 4 were recovered for further in vitro studies. The MICs of the strain obtained from patient 3 (cured) for AMP and CRO were 1 and >128 mg/liter, respectively, and the MICs of the strain obtained from patient 4 (with persistence) were 0.5 and >128 mg/liter, respectively.
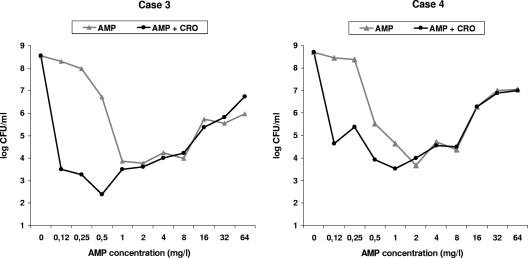
Exponential-phase time-kill curves obtained for the strains from patients 3 and 4 showed bactericidal effect, with AMP alone ranging between 1 and 8 mg/liter and 0.5 and 8 mg/liter, respectively. In both cases, concentrations higher than 8 mg/liter were less active (the Eagle effect). For both strains, the AMP-CRO combination using 5 and 10 mg/liter CRO achieved a synergistic effect, and the bactericidal window was extended to AMP concentrations between 0.12 and 8 mg/liter (Fig. 1).
FIG. 1.
Twenty-four-hour time-kill curves, using AMP alone or in combination with CRO against clinical strains of E. faecalis in exponential growth phase. The AMP-CRO combination results are shown using 5 mg/liter CRO. Case 3: AMP alone was bactericidal using concentrations of 1 to 8 mg/liter; higher concentrations were less active. The AMP-CRO combination was synergistic for AMP concentrations using 0.12 to 0.5 mg/liter, extending the bactericidal effect to AMP concentrations of between 0.12 and 8 mg/liter. Case 4: AMP alone was bactericidal using concentrations of 0.5 to 8 mg/liter. The AMP-CRO combination was synergistic for AMP concentrations of 0.12 to 0.25 mg/liter, extending the bactericidal effect to AMP concentrations of between 0.12 and 8 mg/liter.
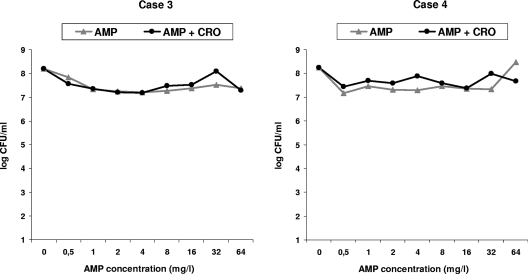
Stationary-phase studies did not achieve any bactericidal activity, either with AMP alone or with the AMP-CRO combination at any concentration, and no synergy was observed for either of the strains tested (Fig. 2).
FIG. 2.
Twenty-four-hour time-kill curves, using AMP alone or in combination with CRO against clinical strains of E. faecalis in stationary phase. The AMP-CRO combination results are represented using 5 mg/liter CRO. Cases 3 and 4: no bactericidal effect or synergy was observed.
DISCUSSION
This pilot study presents promising results regarding the clinical efficacy and bactericidal activity of the AMP-CRO combination in the treatment of osteoarticular infections.
The significance of the presence of E. faecalis in clinical samples of orthopedic infections and whether the prognosis in these cases is good or bad remain controversial issues. Nevertheless, as the process of eradicating E. faecalis from a foreign body or from a focus of osteomyelitis is complex, it has been described as a difficult-to-treat organism (37), probably due to the difficulty of achieving the bactericidal effect with the antimicrobial agents available.
Classically, the bactericidal effect has been hypothetically provided by a penicillin-AG combination, the current therapy of choice, as a consequence of the extended experience in the treatment of enterococcal endocarditis. However, it is well known that the synergistic bactericidal activity of AG may be highly compromised in the setting of orthopedic infections with local purulence, with acidic pH, and under anaerobic conditions. Also, as in the case of the treatment of enterococcal endocarditis, the serious side effects of AG discourage their use in prolonged treatments or fragile patients; thus, many cases of orthopedic infection due to E. faecalis are finally treated with therapies that are known to be bacteriostatic only. A recent retrospective study of prosthetic joint infections found a higher rate of adverse side effects in the group of patients treated with the AG combination compared with those on β-lactam monotherapy; moreover, the group that received combination therapy did not have a better outcome (7). In this study, patients were managed mainly with prosthesis removal, and the authors concluded that bacteriostatic therapies might be enough to treat those cases, in combination with aggressive surgery, surely meaning prosthesis removal. However, aggressive surgery is not always advisable or possible. For prosthetic joint infections classified as early postoperative or hematogenous according to Tsukayama et al. (31), most authors agree in recommending debridement and prosthesis retention (conservative surgery) when the duration of symptoms is short and the implant is stable (37). In addition, the infections affecting osteosyntheses that stabilize fractures may require intense antibiotic therapy until the consolidation of the fracture allows for implant removal. In these situations managed with conservative surgery, it is natural to think that antimicrobial therapies with a bactericidal effect might improve the evolution of enterococcal infection compared to bacteriostatic treatments, in agreement with classical recommendations.
Our patients correspond mainly to this group of cases managed medically or with conservative surgery, including two cases with vertebral osteomyelitis and six out of eight cases with retention of the foreign body. The isolation of E. faecalis in repeated samples highlighted the clinical role of this microorganism in these cases, even in those with polymicrobial isolates. The frequency of polymicrobial etiology in enterococcal osteoarticular infections has been noted previously (26). Therefore, it is not surprising that half of the patients on the protocol treatment and many of those excluded had polymicrobial infections. Many of the excluded cases were those having infections due to S. aureus and/or P. aeruginosa, very frequent pathogens in nosocomial infections that cannot be treated with the AMP-CRO combination.
In recent years, the double β-lactam combination has opened up a new possibility for the treatment of endocarditis due to E. faecalis. Mainardi et al. described that low concentrations of amoxicillin and cefotaxime were synergistic against AG-susceptible and AG-resistant E. faecalis strains compared with amoxicillin alone and tentatively attributed this synergy to the fact that each drug had different penicillin-binding proteins (PBPs) as targets (amoxicillin, partially saturated PBP 4 and 5; cefotaxime, PBP 2 and 3), thus improving the bactericidal effect (18). In further studies, Gavaldà et al. reported that the AMP-CRO combination was synergistic in vitro and also presented good results in vivo with experimental endocarditis due to E. faecalis in rabbits (12, 13). These studies gave rise to a multicenter, prospective, uncontrolled study in a cohort of patients with endocarditis due to E. faecalis strains that were highly resistant to AG and patients with endocarditis due to AG-susceptible strains but at risk of nephrotoxicity related to AG use (11). The AMP-CRO combination achieved promising results, being curative in 71.4% of the cases in the group with strains highly resistant to AG and in 63.6% of the cases in the AG-susceptible group.
In our in vitro studies, the exponential-growth-phase time-kill curves corroborated previous reports (13, 18) that the AMP-CRO combination was synergistic. An extension of the bactericidal window was found in the range of sub-MIC AMP concentrations using the AMP-CRO combination, compared with using AMP alone. This was confirmed in both of the strains recovered from two representative patients in our series, one who was cured with retention of some foreign material (patient 3) and one in whom the infection persisted (patient 4). Therefore, the clinical failure in this patient could not be attributed to a lack of synergy against the strain. Though the clinical relevance of this in vitro synergy is difficult to determine, it is logical to think that prolonging the time of bactericidal antibiotic concentration at the infection site may improve bactericidal activity. In contrast, in our stationary-phase experiments, neither AMP monotherapy (at all concentrations tested) nor the combination was bactericidal, and no synergy was observed with the addition of CRO. There is no mention of stationary bacteria in previous studies; to our knowledge, the effect of β-lactams on E. faecalis in the stationary phase has not been reported previously. This is an important point, because the ratio between the area under the concentration-time curve and the minimal bactericidal concentration of stationary or adherent bacteria has been reported to be the most reliable marker of antibiotic efficacy in foreign-body infections (1, 34, 36). Endocarditis and orthopedic infections are characterized by the presence of bacterial biofilms (3), where bacteria express variable phenotypic tolerance to antimicrobials (4). However, this loss of killing activity against biofilm-forming bacteria is not homogeneous among the different microorganisms and antimicrobials. Though the efficacy of β-lactams and AG in biofilms is strongly reduced (28), streptococcal and enterococcal osteoarticular infections have traditionally been managed with these antibiotics, which are considered the treatment of choice. Today, the exact in vitro-in vivo correlation in the treatment of these infections is difficult to determine.
Literature on enterococcal osteoarticular infection is heterogeneous and provides variable outcome data. The cure rates reported with cell wall-active antibiotic monotherapies range between 33 and 88% and, with AG combinations, between 67 and 100% (7, 26, 30, 32). In our small clinical series, the AMP-CRO combination was well tolerated, and the cure rate achieved at the last follow-up was 90%, despite the fact that most patients were managed with retention of the foreign material. The only case that failed was a patient with a late chronic infection of a knee arthroplasty for whom the treatment of choice (complete prosthesis removal) could not be performed for technical reasons.
Regarding the AMP-CRO doses used in this study, it is worth noting some pharmacokinetic (PK) and pharmacodynamic considerations. In the case of β-lactams, it is generally recognized that time above the MIC is the best predictor of efficacy, but no special attention has been paid to the minimum free drug time above the MIC required for efficacy, perhaps because of the low levels of protein binding of these antibiotics and the safety of using high doses. In fact, this parameter has not been clearly defined in animal models of enterococcal infection, and the possible negative consequences of using too high doses of AMP due to the Eagle effect in vivo are not known. In any case, considering a protein binding level of about 17% (19), 2 g of AMP should provide free serum concentrations above the MIC of E. faecalis (mean MIC, 1 mg/liter [range, 0.5 to 4 mg/liter]) (19, 23) for most of the 6-hour interval (10).
In the case of CRO, which is inactive individually against E. faecalis (MIC range, 1 to >128) (15, 33), the high-level and concentration-dependent protein binding contributes to its unique PK properties (24, 29). Nonetheless, the previous experimental studies by Gavaldà et al. (12, 13), the only ones published about AMP-CRO combination for enterococcal infection, did not refer to free drug concentrations; therefore, it is difficult to establish a relationship to the reports focused specifically on human PK of CRO (35). After considering all available data, we think that a daily dose of 2g of CRO may be sufficient to maintain concentrations of 5 to 10 mg/liter for a long interval at the site of infection, the required minimum concentrations that provide synergistic effect with sub-MIC AMP concentrations.
Based on the literature on enterococcal endocarditis, we believe that the AMP-CRO combination may be a reasonable synergistic alternative to the classical AMP-AG combination for the treatment of osteomyelitis and foreign body infections due to E. faecalis. The synergy of the double β-lactam combination against E. faecalis is reproducible in vitro in experiments representing planktonic bacteria, though no benefit against stationary bacteria has been demonstrated so far. The favorable outcome and good tolerability observed in our small series may provide a basis for further well-designed comparative studies.
Acknowledgments
None of the authors have any conflicts of interests.
We thank Michael Maudsley for revising the English in the manuscript.
G.E. was supported by a grant from the Instituto de Salud Carlos III (FI05/00438). This study was supported by the Spanish Network for the Research in Infectious Diseases (grants REIPI C03/14 and REIPI RD06/0008).
Footnotes
Published ahead of print on 10 August 2009.
REFERENCES
- 1.Ariza, J., G. Euba, and O. Murillo. 2008. Orthopedic device-related infections. Enferm. Infecc. Microbiol. Clin. 26:380-390. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 2.Baddour, L. M., W. R. Wilson, A. S. Bayer, V. G. Fowler, Jr., A. F. Bolger, M. E. Levison, P. Ferrieri, M. A. Gerber, L. Y. Tani, M. H. Gewitz, D. C. Tong, J. M. Steckelberg, R. S. Baltimore, S. T. Shulman, J. C. Burns, D. A. Falace, J. W. Newburger, T. J. Pallasch, M. Takahashi, K. A. Taubert, et al. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394-e434. [DOI] [PubMed] [Google Scholar]
- 3.Brady, R. A., J. G. Leid, J. H. Calhoun, J. W. Costerton, and M. E. Shirtliff. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 52:13-22. [DOI] [PubMed] [Google Scholar]
- 4.Chuard, C., J. C. Lucet, P. Rohner, M. Herrmann, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1991. Resistance of Staphylococcus aureus recovered from infected foreign body in vivo to killing by antimicrobials. J. Infect. Dis. 163:1369-1373. [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Eagle, H., and A. D. Musselman. 1948. The rate of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J. Exp. Med. 88:99-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Helou, O. C., E. F. Berbari, C. E. Marculescu, W. I. El Atrouni, R. R. Razonable, J. M. Steckelberg, A. D. Hanssen, and D. R. Osmon. 2008. Outcome of enterococcal prosthetic joint infection: is combination systemic therapy superior to monotherapy? Clin. Infect. Dis. 47:903-909. [DOI] [PubMed] [Google Scholar]
- 8.Euba, G., J. A. Narvaez, J. M. Nolla, O. Murillo, J. Narvaez, C. Gomez-Vaquero, and J. Ariza. 2008. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin. Arthritis Rheum. 38:28-40. [DOI] [PubMed] [Google Scholar]
- 8a.Euba, G., J. Lora-Tamayo, O. Murillo, S. Pedrero, J. Cabo, R. Verdaguer, and J. Ariza. 2008. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. K-546. [DOI] [PMC free article] [PubMed]
- 9.Fontana, R., P. Canepari, M. M. Lleo, and G. Satta. 1990. Mechanisms of resistance of enterococci to beta-lactam antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 9:103-105. [DOI] [PubMed] [Google Scholar]
- 10.Gavaldà, J., P. J. Cardona, B. Almirante, J. A. Capdevila, M. Laguarda, L. Pou, E. Crespo, C. Pigrau, and A. Pahissa. 1996. Treatment of experimental endocarditis due to Enterococcus faecalis using once-daily dosing regimen of gentamicin plus simulated profiles of ampicillin in human serum. Antimicrob. Agents Chemother. 40:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavaldà, J., O. Len, J. M. Miro, P. Munoz, M. Montejo, A. Alarcon, J. de la Torre-Cisneros, C. Pena, X. Martinez-Lacasa, C. Sarria, G. Bou, J. M. Aguado, E. Navas, J. Romeu, F. Marco, C. Torres, P. Tornos, A. Planes, V. Falco, B. Almirante, and A. Pahissa. 2007. Brief communication: treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann. Intern. Med. 146:574-579. [DOI] [PubMed] [Google Scholar]
- 12.Gavaldà, J., P. L. Onrubia, M. T. Gomez, X. Gomis, J. L. Ramirez, O. Len, D. Rodriguez, M. Crespo, I. Ruiz, and A. Pahissa. 2003. Efficacy of ampicillin combined with ceftriaxone and gentamicin in the treatment of experimental endocarditis due to Enterococcus faecalis with no high-level resistance to aminoglycosides. J. Antimicrob. Chemother. 52:514-517. [DOI] [PubMed] [Google Scholar]
- 13.Gavaldà, J., C. Torres, C. Tenorio, P. Lopez, M. Zaragoza, J. A. Capdevila, B. Almirante, F. Ruiz, N. Borrell, X. Gomis, C. Pigrau, F. Baquero, and A. Pahissa. 1999. Efficacy of ampicillin plus ceftriaxone in treatment of experimental endocarditis due to Enterococcus faecalis strains highly resistant to aminoglycosides. Antimicrob. Agents Chemother. 43:639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handwerger, S., and A. Tomasz. 1985. Antibiotic tolerance among clinical isolates of bacteria. Rev. Infect. Dis. 7:368-386. [DOI] [PubMed] [Google Scholar]
- 15.Kim, M. Y., J. I. Oh, K. S. Paek, Y. Z. Kim, I. C. Kim, and J. H. Kwak. 1996. In vitro and in vivo activities of LB10522, a new catecholic cephalosporin. Antimicrob. Agents Chemother. 40:1825-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krogstad, D. J., and A. R. Pargwette. 1980. Defective killing of enterococci: a common property of antimicrobial agents acting on the cell wall. Antimicrob. Agents Chemother. 17:965-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mader, J. T., M. E. Shirtliff, S. C. Bergquist, and J. Calhoun. 1999. Antimicrobial treatment of chronic osteomyelitis. Clin. Orthop. Relat. Res. 47-65. [DOI] [PubMed]
- 18.Mainardi, J. L., L. Gutmann, J. F. Acar, and F. W. Goldstein. 1995. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob. Agents Chemother. 39:1984-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandell, G. L., R. G. Douglas, J. E. Bennett, and R. Dolin. 2005. Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 6th ed. Elsevier/Churchill Livingstone, New York, NY.
- 20.Moellering, R. C., Jr. 1992. Emergence of Enterococcus as a significant pathogen. Clin. Infect. Dis. 14:1173-1176. [DOI] [PubMed] [Google Scholar]
- 21.Moellering, R. C., Jr. 1991. The Garrod Lecture. The enterococcus: a classic example of the impact of antimicrobial resistance on therapeutic options. J. Antimicrob. Chemother. 28:1-12. [DOI] [PubMed] [Google Scholar]
- 22.Mund, D. J. 1980. Pyogenic vertebral osteomyelitis; manifestation of bacterial endocarditis. N. Y. State J. Med. 80:980-982. [PubMed] [Google Scholar]
- 23.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry, T. R., and J. J. Schentag. 2001. Clinical use of ceftriaxone: a pharmacokinetic-pharmacodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin. Pharmacokinet. 40:685-694. [DOI] [PubMed] [Google Scholar]
- 25.Pigrau, C., B. Almirante, X. Flores, V. Falco, D. Rodriguez, I. Gasser, C. Villanueva, and A. Pahissa. 2005. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am. J. Med. 118:1287. [DOI] [PubMed] [Google Scholar]
- 26.Raymond, N. J., J. Henry, and K. A. Workowski. 1995. Enterococcal arthritis: case report and review. Clin. Infect. Dis. 21:516-522. [DOI] [PubMed] [Google Scholar]
- 27.Steckelberg, J. M., and D. R. Osmon (ed.). 2000. Prosthetic joint infections, 3rd ed. American Society for Microbiology, Washington, DC.
- 28.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 29.Stoeckel, K., P. J. McNamara, R. Brandt, H. Plozza-Nottebrock, and W. H. Ziegler. 1981. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin. Pharmacol. Ther. 29:650-657. [DOI] [PubMed] [Google Scholar]
- 30.Tarr, P. E., G. Sakoulas, A. Ganesan, M. A. Smith, and D. R. Lucey. 2004. Hematogenous enterococcal vertebral osteomyelitis: report of 2 cases and review of the literature. J. Infect. 48:354-362. [DOI] [PubMed] [Google Scholar]
- 31.Tsukayama, D. T., R. Estrada, and R. B. Gustilo. 1996. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Joint Surg. Am. 78:512-523. [DOI] [PubMed] [Google Scholar]
- 32.Vlahakis, N. E., Z. Temesgen, E. F. Berbari, and J. M. Steckelberg. 2003. Osteoarticular infection complicating enterococcal endocarditis. Mayo Clin. Proc. 78:623-628. [DOI] [PubMed] [Google Scholar]
- 33.Washington, J. A., R. N. Jones, S. D. Allen, E. H. Gerlach, F. P. Koontz, P. R. Murray, M. A. Pfaller, and M. E. Erwin. 1991. In vitro comparison of GR69153, a novel catechol-substituted cephalosporin, with ceftazidime and ceftriaxone against 5,203 recent clinical isolates. Antimicrob. Agents Chemother. 35:1508-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widmer, A. F., R. Frei, Z. Rajacic, and W. Zimmerli. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96-102. [DOI] [PubMed] [Google Scholar]
- 35.Yuk, J. H., C. H. Nightingale, and R. Quintiliani. 1989. Clinical pharmacokinetics of ceftriaxone. Clin. Pharmacokinet. 17:223-235. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerli, W., R. Frei, A. F. Widmer, and Z. Rajacic. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33:959-967. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645-1654. [DOI] [PubMed] [Google Scholar]