Abstract
Oritavancin is a novel glycopeptide antimicrobial agent with potent in vitro activity against a wide variety of gram-positive bacteria, including multidrug-resistant strains of staphylococci and enterococci. A population pharmacokinetic model was developed to describe the disposition of oritavancin with data from a pooled population of phase 1 healthy subjects and phase 2 and 3 patients with complicated skin and skin structure infections or Staphylococcus aureus bacteremia. In addition, the potential influence of factors such as the subject's age, gender, and clinical laboratory measures on oritavancin disposition was evaluated. Oritavancin was administered as both single- and multiple-dose intravenous (i.v.) infusions in fixed doses ranging from 100 to 800 mg or weight-based doses ranging from 0.02 to 10 mg/kg of body weight, with infusion durations ranging from 0.13 to 6.5 h across all studies. The most robust fit to the data (n = 6,290 oritavancin plasma concentrations from 560 subjects) was obtained using a three-compartment model with zero-order i.v. infusion and first-order elimination. The model was parameterized using total clearance (CL), volume of central compartment (Vc), distributional clearances from the central to both the first and second peripheral compartments, and volumes of distribution for both the first and second peripheral compartments. Weight and study phase (phase 1 versus phase 2/3) were identified as significant predictors of the interindividual variability in CL, while body surface area and age were significant for Vc. These results suggest that dose modification may be warranted in patients weighing >110 kg. However, the mild nature of the observed relationships for Vc suggest that dosing adjustments are not necessary for elderly patients.
Oritavancin (LY333328) is a novel, semisynthetic glycopeptide that has shown superior activity against gram-positive bacteria, including multidrug-resistant strains of staphylococci and enterococci (16). In vitro studies have demonstrated rapid, dose-dependent bactericidal activity for oritavancin against enterococci, staphylococci, and Streptococcus pneumoniae (1).
Oritavancin is currently being developed for the treatment of complicated skin and skin structure infections (cSSSI) caused by susceptible isolates of gram-positive microorganisms (10, 15). Numerous phase 1, 2, and 3 studies have been conducted evaluating oritavancin in both healthy subjects and infected patients with cSSSI or Staphylococcus aureus bacteremia (3, 8, 12). It has been administered as either single- or multiple-dose intravenous (i.v.) infusions in fixed doses ranging from 100 to 800 mg or as weight-based doses ranging from 0.02 to 10 mg/kg of body weight. Pharmacokinetic (PK) studies have shown that oritavancin displays linear PKs for weight-based or fixed dose ranges (3, 8, 12). Additionally, oritavancin was shown to be safe and well tolerated, with no clinically relevant changes in renal, hepatic, or hematological indices compared to the individual's baseline. Elimination of oritavancin from the body employs both renal and fecal routes, with renal as the major route with a very slow process (<5% of a dose is excreted in the urine over 14 days while <1% is eliminated in feces over that time), indicating a rapid tissue accumulation and prolonged retention (3). Furthermore, there is no evidence of metabolism of oritavancin, as no metabolites were detected in either urine or feces in humans (3) or any other species. In animal studies, the tissue distribution of oritavancin showed high retention and slow clearance in the reticuloendothelial systems of the liver, kidney, spleen, and lung, with 59 to 64%, 2.7%, 1.8%, and 1.7% accumulation of the administered dose, respectively (data on file at The Medicines Company [formerly Targanta Therapeutics]).
Based on a previous population PK analysis, plasma concentrations of oritavancin displayed a multiexponential decline and were best described using a three-compartment model (12). The long half-life estimates, >24 h for the β phase and >300 h for the γ phase (3, 8), suggest that drug accumulation can occur after the administration of multiple-dose regimens and that a once-daily dosing regimen is feasible. The goals of this analysis were the following: (i) to develop a population PK model that described the disposition of oritavancin using pooled data from 12 studies; (ii) to assess the impact of subject demographic and disease characteristics on intersubject variability on selected PK parameters; and (iii) to generate individual exposure measures for subsequent pharmacokinetic-pharmacodynamic (PK-PD) analyses for efficacy.
MATERIALS AND METHODS
Study design.
Data from phase 1, 2, and 3 studies from the clinical development of oritavancin were pooled to develop a population PK model. A total of 12 studies were included in this analysis. These included nine phase 1 studies evaluating healthy subjects, two phase 2 studies of patients with either cSSSI or S. aureus bacteremia, and one phase 3 study of patients with cSSSI. Briefly, the phase 1 studies were of the following designs: three were dose escalation, two assessed the effect of oritavancin on corrected QT (QTc) interval, one examined hepatic impairment, one investigated the potential for oritavancin to cause a drug-drug interaction with desipramine, and two assessed the accumulation in the skin or intrapulmonary fluids. Various dosing regimens were employed, ranging from single doses to multiple doses administered up to 14 days. Furthermore, the PK sampling strategies were designed to capture as much information on the disposition of oritavancin as possible. Thus, the majority of studies employed robust PK sampling strategies for extended periods of time (>200 h after the dose).
Only those subjects who met eligibility requirements in these studies were included in this analysis. Oritavancin was administered as either single- or multiple-dose i.v. infusions in fixed doses ranging from 100 to 800 mg or as weight-based doses ranging from 0.02 to 10 mg/kg across all studies, with the planned duration of infusion ranging from 0.5 to 1.5 h. Whenever possible, the actual oritavancin dose amounts administered and the infusion start-stop dates and times were recorded for each study and used in this analysis. When these data were not available, dose times and/or infusion durations were imputed according to the protocol-specified dosing times and scheduled infusion duration.
PK sample and assay methods.
Blood samples were collected into Vacutainer tubes containing EDTA at protocol-specified times after the start of infusion in each of the studies. The actual dates and times for collected PK samples recorded on each case report form were used for this analysis.
Each blood sample was kept on ice and centrifuged at 3,000 × g for 15 min at 4°C within 30 min of collection. Plasma was separated and stored at −70°C until analysis. In only one phase 1 study, the plasma concentration of oritavancin was analyzed using a competitive radioimmunoassay with a lower limit of quantitation of 0.01 ng/ml. PK samples from all other studies were analyzed by a validated liquid chromatography with tandem mass spectrophotometry (LC/MS/MS) method with a lower limit of quantitation of 75 ng/ml. Inter- and intra-assay bias and precision were <10% and <15%, respectively.
Free-drug concentration was not measured for oritavancin in the studies described herein. However, other investigations have found that the protein binding for oritavancin ranges from 85.7% to 89.9% and appears to be concentration independent over a concentration range of 1 to 91 μg/ml (13a).
The potential loss of oritavancin to plastic and/or glass surfaces was recognized early in the development of this agent as an important process variable that could affect oritavancin assay results. This loss was also recognized to be concentration dependent, such that the surface binding and/or loss was greatest at low concentrations (i.e., below 1 μg/ml). However, oritavancin concentrations in the biological matrices under investigation were shown to be constant over time. In addition, extensive work was completed to define the rate and extent of this plastic/glass binding phenomenon, with the specific goal of attenuating this process. This work revealed that use of detergents or acidification of the medium to a pH of ≤3 reduced adsorption of oritavancin to these surfaces (data on file at The Medicines Company [formerly Targanta Therapeutics]). These process variables were incorporated into the radioimmunoassay and the high-performance LC-based techniques (LC-fluorescence or LC/MS/MS), which were used to assay oritavancin concentrations in plasma or urine collected from patients enrolled in phase 1, 2, and 3 clinical trials. These assays were performed using Good Laboratory Practice standards and, as such, included multiple quality control measures, including a demonstration of the linearity between concentration and signal strength which ensures that unforeseen losses during manipulation do not impact the determination of concentrations.
Subject demographic and disease characteristics.
Prior to the administration of the study drug, subject demographic and disease characteristics were evaluated. Demographic information included the following: age, weight, height, gender, and race. Ideal body weight (IBW), body mass index (BMI), and body surface area (BSA) were also calculated for each subject (9, 13). Renal function was assessed using creatinine clearance (CLcr), as calculated from baseline serum creatinine using the method described by Cockcroft and Gault (5). If the subject's actual weight was greater than their calculated IBW, IBW was replaced for actual weight in the CLcr equation. CLcr was further normalized according to the individual's BSA. Categorical variables were also generated including gender (male or female), race (Caucasian, Black, Asian, Hispanic, or other), and subject population (healthy, with cSSSI, or with S. aureus bacteremia). These subject demographics and disease characteristics were used to describe the analysis population and to evaluate their ability to explain the interindividual variability of selected PK parameters. All continuous covariates were evaluated as such during covariate model development.
Population PK analysis.
All data preparation, manipulation, and presentation across the 12 pooled studies were performed using SAS version 9.1.3 software (14). The PK analyses were conducted using Monte-Carlo parametric expectation maximization (MCPEM) as implemented in the open-source software program S-ADAPT 1.5.6. S-ADAPT (scriptable-ADAPT) is a version of ADAPT II that contains an augmented interface as well as additional parameter estimation, simulation, and optimization abilities (2, 6, 7).
An outlier was defined as an aberrant oritavancin plasma concentration that substantially deviated from the rest of the observations within an individual. Suspected outlier observations were tested and, if justified, excluded, based on the following procedure. Data for each subject were fit with and without the individual suspected outlier plasma concentrations. If the difference between the value of the fitted concentration and the observed concentration was at least 3 error standard deviations (SD), the trajectory of the PK profile was altered significantly, and an improvement was seen in the fit of the remaining samples for that subject without the suspected outlier observation, then the observation was declared a significant outlier. If the majority of the suspected outlier concentrations appeared to occur at roughly the same time since the last dose, additional attempts were made to modify the structural model to try to capture these observations. Additionally, if an entire intensively sampled individual PK profile failed to follow a reasonable pattern relative to the dosing history, the data from the entire subject were excluded to prevent introducing bias into the analysis.
Based on previous population PK analyses of oritavancin (4, 12), a three-compartment model was initially evaluated while other structural models were explored only if needed. The model was parameterized using total clearance from the central compartment (CL), volume of distribution of the central compartment (Vc), distributional clearances from the central compartment to both the first and second peripheral compartments (Q2 and Q3, respectively), and the volumes of distribution for both the first and second peripheral compartments (V2 and V3, respectively). Weighting of each oritavancin concentration was based on the reciprocal of the estimated observation SD for that observation, which was predicted as a function of the fitted concentration. A combined additive plus proportional error model was used to describe the residual variability (RV). The additive component (SDin) was fixed across the population, and the proportional component (SDsl) was estimated but not allowed to vary between subjects. Other RV models were also explored.
Covariate analysis.
Once an adequate base structural PK model was selected, individual predicted post-hoc parameter estimates were generated for each subject. The PK parameters along with subject demographic and disease characteristics were merged to assess the impact of these subject descriptors on explaining the intersubject variability for selected PK parameters (CL and Vc). Diagnostic plots of the individual predicted parameters versus each of the covariates were then generated to discern the functional form of the relationship between the PK parameter and the covariate. Linear, exponential, power, additive, or proportional shifts, as well as piece-wise combinations of these functional forms, were applied for covariate analysis. Only covariates that were statistically significant in a linear or multiple linear regression (α = 0.05) were formally tested in S-ADAPT. All covariate effects whose addition resulted in a significant reduction in the value of the objective function (OF) (a decrease of at least 3.84 [<0.005, 1 degree of freedom by likelihood ratio test]) were included in the full multivariable model. The resulting multivariable model was used to examine the appropriateness of the interindividual variability and RV models. A recursive backward-elimination procedure was then performed to further refine the model and eliminate correlated predictors. During each step of backward elimination, the covariate whose removal resulted in the smallest insignificant increase in the OF (a change of no more than 5.94 [<0.0001, 1 degree of freedom]) was removed from the model. This process was repeated until no further covariate effects could be removed from the model without a statistically significant difference in the minimum value of the OF. The final model was then assessed for the appropriateness of the interindividual variability and RV models.
Calculation of secondary PK parameters and exposure estimates.
Since a three-compartment model was employed, the three elimination half-lives (α, β, and γ) were calculated by solving the cubic function (17). The area under the plasma concentration-time curve from 0 to 24 h (AUC0-24) and the maximum (Cmax) and minimum (Cmin) plasma concentration values were generated by PK simulation using the final population PK model implemented in S-ADAPT. In this simulation, the subjects' dosing histories and post-hoc PK parameter estimates were used to generate a PK profile after each dose. The AUC0-24 was calculated by integrating that PK profile over time in very small time increments. Cmaxs and Cmins were obtained by simulating the concentrations immediately following the end of the infusion and immediately prior to the next infusion, respectively.
RESULTS
Data.
A total of 574 subjects and 6,432 oritavancin plasma concentrations were available from 12 clinical studies from phase 1, 2, and 3 from either healthy subjects or infected patients with cSSSI or S. aureus bacteremia. However, 14 subjects and 96 oritavancin plasma concentrations were removed from the PK analysis data set due to one of the following reasons: subjects had no oritavancin PK data, samples were collected prior to the first oritavancin dose, samples were reported as missing or not collected, or samples came from a dosing schedule that appeared to be switched. Additionally, 46 oritavancin plasma concentrations were excluded in the remaining data set since they were deemed to be significant outliers. Thus, the final data set, which contained 6,290 oritavancin concentrations from 560 subjects, was used to develop the population PK model.
The robust data set included patients with a wide range of ages, body sizes, and renal function. The mean (percent coefficient of variation [%CV]) age was 47.9 (33%) years and ranged from a minimum of 19 years to a maximum of 90 years. The mean (%CV) body weight was 79.4 (30%) kg and ranged from 39.3 to 227 kg. Of the 46 subjects with body weights above 110 kg, the majority (n = 36; 78%) were below a weight of 150 kg, and only 3 subjects had a body weight above 200 kg. The mean (%CV) CLcr was 85.0 (45%) ml/min/1.73 m2 and ranged from 6.70 to 326 ml/min/1.73 m2. The population was predominantly male (65.5%) and Caucasian (59.5%). Of the 560 subjects in the data set, 200 (35.7%) had been enrolled in a phase 1 study; the remainder had been enrolled in phase 2 or 3 studies, with the majority of these patients (n = 360) having cSSSI as a primary diagnosis (n = 274; 76.1%).
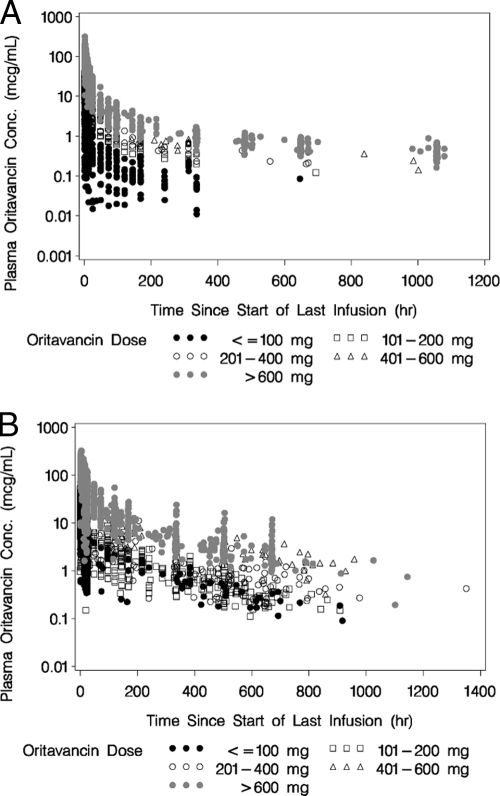
In the 12 pooled clinical studies, a wide range of oritavancin doses from 1 to 1,220 mg was administered and included in the population PK analysis. The actual duration of infusion ranged from 0.13 to 6.5 h across all subjects, with 68.5% of subjects having received oritavancin as a one-hour infusion. PK sampling for phase 1 healthy subjects was more intensive, while that for patients enrolled in phase 2 and 3 studies was more sparse. Nevertheless, more than 90% of the subjects contributed at least six samples to the population PK analysis. Semilog scatterplots of oritavancin concentration versus time since the start of the last infusion, stratified by dose range, following either a single dose or multiple doses of oritavancin can be seen in Fig. 1A and 1B, respectively. Note that PK samples were collected over a period of several days after either a single- or multiple-dose regimen.
FIG. 1.
(A) Semilog scatterplot of oritavancin concentrations versus time since start of last infusion, stratified by dose range, following a single dose of oritavancin. (B) Semilog scatterplot of oritavancin concentrations versus time since start of last infusion, stratified by dose range, following multiple doses of oritavancin.
Population PK analysis.
A robust fit to the data was obtained using a three-compartment model with zero-order i.v. infusion and first-order, linear elimination, utilizing a combined additive and proportional RV model. The population PK parameter estimates and associated standard errors for the base structural model are provided in Table 1. Most of the mean PK parameters had a moderate magnitude of interindividual variability and high precision (percent standard error of the mean [%SEM], <5%), with the exception of Q3. This high precision of interindividual variability parameters can be explained by the relatively intensive sampling strategy in the majority of subjects and the large number of subjects in the data set.
TABLE 1.
Base structural and final population PK model for oritavancin
| Parameter | Base structural population PK modela
|
Final population PK modelb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Population mean
|
Magnitude of interindividual variability (%CV)
|
Population mean
|
Magnitude of interindividual variability (%CV)
|
|||||
| Final estimate | %SEM | Final estimate | %SEM | Final estimate | %SEM | Final estimate | %SEM | |
| CL (liter/h) | 0.453 | 1.83 | 42.2 | 0.0000413 | 0.456 | 33.7 | 8.71 | |
| Vc (liter) | 5.89 | 1.72 | 37.1 | 0.0000805 | 5.88 | 35.2 | 8.96 | |
| Q2 (liter/h) | 0.846 | 2.64 | 57.5 | 0.0601 | 0.872 | 3.68 | 60.2 | 11.3 |
| V2 (liter) | 14.2 | 1.45 | 31.7 | 0.0970 | 14.8 | 5.63 | 86.9 | 11.3 |
| Q3 (liter/h) | 0.318 | 3.51 | 80.2 | 0.000109 | 0.259 | 4.78 | 76.5 | 8.86 |
| V3 (liter) | 99.7 | 2.52 | 57.8 | 0.0153 | 90.0 | 5.61 | 79.5 | 10.7 |
| CL intercept (liter/h) | 0.0374 | 2.04 | ||||||
| CL WTKG slope (liter/h/kg) | 0.00164 | 26.9 | ||||||
| CL shift, phase 2/3 patients | 1.30 | 10.0 | ||||||
| Vc intercept (liter) | 5.56 | 2.07 | ||||||
| Vc BSA slope (liter/m2) | 1.85 | 16.6 | ||||||
| Vc age slope (liter/yr) | −0.0354 | 16.6 | ||||||
| SDin | 0.220 | |||||||
| SDsl | 0.250 | 1.11 | ||||||
| SDin, phase 1 subjects | 0.150 | |||||||
| SDsl, phase 1 subjects | 0.146 | 1.59 | ||||||
| SDin, phase 2/3 patients | 0.220 | |||||||
| SDsl, phase 2/3 patients | 0.304 | 1.80 | ||||||
Minimum value of the objective function, 11,501.
Minimum value of the objective function, 10,568. Final population mean CL = {0.374 + [0.00164·(WTKG − 80)·(1 − WTKGc)]·1.30(1 − phase 1)}. WTKGc is an indicator variable set to 1 if WTKG is ≤80 and set to 0 if WTKG is >80, and phase 1 is an indicator variable set to 1 for phase 1 subjects and set to 0 for phase 2 or 3 patients. Final population mean Vc = 5.56 + [1.85·(BSA − 1.73)] − [0.0354·(age − 48)].
A total of 560 records in the data set was used for the covariate analysis along with the patient demographics, disease characteristics, and PK parameter estimates. Using univariable r2 values, BSA was chosen as the one body size covariate (among total body weight, BSA, IBW, and BMI) which appeared to explain the largest amount of interindividual variability in both CL and Vc. Both linear and nonlinear functions were attempted, and based on the fit of the relationships, a linear relationship between CL or Vc and BSA was used throughout the initial covariate selection process (i.e., prior to RV model refinement). Using multivariable linear regression, the other covariates showing small, yet significant, apparent relationships with CL were age, CLcr, and gender. Perhaps due to the high degree of correlation between Vc and CL, very similar results were found when evaluating relationships between these covariates and Vc. The main difference seen was that the slope of the body size relationships appeared much stronger than it had for CL, despite the fact that r2 values were not significantly higher (data not shown). Using multivariable linear regression, the covariates selected for inclusion in the covariate selection process for Vc in S-ADAPT were BSA, age, gender, and CLcr.
During the forward selection process in S-ADAPT, the only covariates that proved to be significant and were included in the full multivariable model were the effect of BSA and CLcr on CL and the effect of BSA and age on Vc. Although the addition of these covariates did not result in impressive changes in the interindividual variability in Vc or CL, they all contributed to significant reductions in the OF. Specifically, the first covariate added to the model was BSA on Vc (OF dropped 259 units), followed by age on Vc (drop of 29 units), BSA on CL (drop of 9 units), and CLcr on CL (drop of 17 units).
Using the full multivariable model described above, the adequacy of the residual variability model was assessed. At that time, it was apparent that there was a trend for a small degree of bias in the higher concentrations drawn from the phase 2 or 3 patients compared to those of the phase 1 subjects. Thus, separate residual variability models were employed for the two populations; incorporating this refinement resulted in a significant drop in the OF (390 units). The impact of assay methodology on the fit of the model was also assessed at this point. Based on the adequacy of the fit, regardless of assay methodology, no changes to the RV model were necessary to account for those concentrations obtained by radioimmunoassay.
After the change in the RV model, examination of the screening plots suggested that the covariate relationships for CL should also be reevaluated. As a result, three changes to the covariate model for CL were necessary: (i) since the relationship between CL and CLcr was no longer significant, it was removed from the model; (ii) a piece-wise linear relationship between weight in kilograms (WTKG) and CL became more significant than a direct linear relationship between CL and BSA, and the former was added to the model (OF dropped 45 units); and (iii) a categorical shift upward in CL for phase 2 and 3 patients relative to phase 1 subjects was significant and added to the model (OF dropped 34 units).
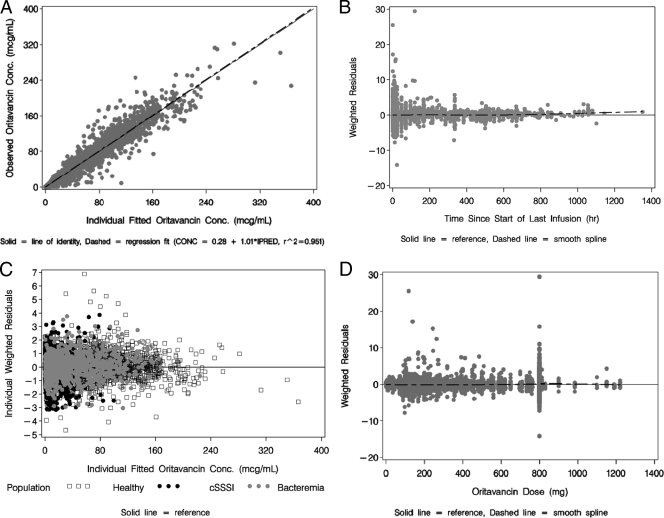
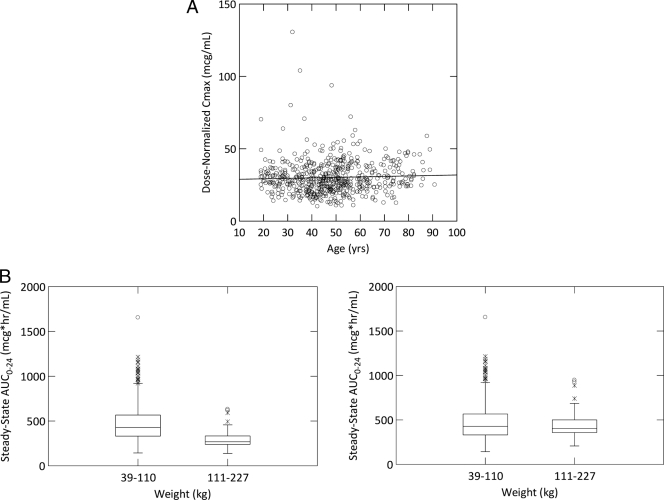
Thus, the final population PK model for oritavancin was a three-compartment model with linear elimination which incorporated a relationship between CL and both weight in kilograms and study phase population and a relationship between Vc and both BSA and age. The final parameter estimates and associated standard errors are provided in Table 1. The magnitude of the interindividual variability was moderate for CL and Vc and somewhat higher for Q2, Q3, V2, and V3. Compared to the precision of the base structural model, the precision of the final population PK parameter estimates still remained high (%SEM, <15%). The precision of the parameters that defined the covariate relationships was also high (<30%), with the exception of the parameter that defined the proportional shift in CL for phase 2 and 3 patients. Regarding the RV model, the proportional component of the model for phase 1 subjects was 15% compared with 31% for phase 2 and 3 patients, which was most likely due to the less-controlled environment of the latter trials, thus contributing to inaccuracies in both dose and sampling times. The summary statistics for selected PK parameter and exposure estimates, stratified by study phase, are provided in Table 2. Overall, excellent fits to the data were obtained. The plot of observed versus individual fitted (equivalent to the Bayesian post-hoc) concentrations using the final population PK model is provided in Fig. 2A, with an overall r2 of 0.951. Figure 2B shows the weighted residuals versus time since the start of the last infusion and demonstrates a consistent fit across the range of times after administration of a dose, indicating a lack of model misspecification. The scatterplot of individual weighted residuals versus individual fitted concentrations, stratified by study population, is provided in Fig. 2C, which demonstrates appropriate scatter around the zero reference line with no trend for bias in the fit. The scatterplot of individual weighted residuals versus oritavancin dose can be seen in Fig. 2D and demonstrates appropriate scatter around the zero reference line with no trend for bias in the fit, indicating that the assumption of linear PKs is justified. Figure 3 illustrates the impacts of age (Fig. 3A) and body weight (Fig. 3B) on the PKs of oritavancin.
TABLE 2.
Summary statistics of key PK parameter estimates, stratified by study phase
| Variable | Phase 1 (n = 200)
|
Phase 2/3 (n = 360)
|
||
|---|---|---|---|---|
| Mean (SD) | Median (min-max) | Mean (SD) | Median (min-max) | |
| CL (liter/h) | 0.351 (0.109) | 0.350 (0.121-0.702) | 0.601 (0.204) | 0.584 (0.172-1.45) |
| Vc (liter) | 5.19 (1.27) | 5.04 (2.37-13.8) | 7.10 (2.46) | 6.79 (1.17-18.3) |
| t1/2α (h) | 2.56 (0.653) | 2.48 (1.23-4.78) | 2.04 (0.440) | 2.04 (0.910-4.08) |
| t1/2β (h) | 27.0 (11.5) | 25.4 (9.38-99.6) | 31.2 (11.4) | 29.2 (8.37-86.3) |
| t1/2γ (h) | 318 (59.1) | 314 (191-584) | 393 (73.5) | 394 (142-602) |
| AUC0-24 (μg·h/ml)a | 252 (78.6) | 240 (104-614) | 146 (63.7) | 133 (42.2-618) |
| Cmax (μg/ml)a | 35.7 (9.09) | 34.5 (20.4-80.0) | 28.5 (12.2) | 25.9 (10.9-131) |
| Cmin (μg/ml)a | 4.11 (1.80) | 3.65 (1.23-10.2) | 1.99 (1.10) | 1.74 (0.540-9.81) |
AUC0-24, Cmax, and Cmin have been normalized to a dose of 200 mg for those subjects of ≤110 kg and to a dose of 300 mg for those subjects of >110 kg.
FIG. 2.
(A) Scatterplot of observed versus individual fitted oritavancin concentrations for the final population PK model. (B) Scatterplot of population weighted residuals versus time since the start of the last infusion for the final population PK model. (C) Scatterplot of individual weighted residuals versus individual fitted oritavancin concentrations for the final population PK model, stratified by population. (D) Scatterplot of the population weighted residuals versus dose for the final population PK model.
FIG. 3.
(A) Scatterplot of dose-normalized Cmax versus age (line represents a linear regression fit of the data). (B) Boxplots of predicted, steady-state AUC0-24 values versus weight categories for two different dosing regimens. The left panel presents the predicted AUC0-24 when subjects are given 200 mg daily, regardless of body weight. The right panel presents predicted AUC0-24 when subjects are given 200 mg daily when body weight is less than or equal to 110 kg and 300 mg daily when body weight is greater than 110 kg.
DISCUSSION
A population PK model using pooled data from phase 1 healthy subjects and phase 2 and 3 patients with cSSSI or S. aureus bacteremia was developed to characterize the disposition of oritavancin. A robust data set with 6,290 oritavancin PK samples obtained from 560 subjects was created along with various subject descriptors and disease characteristics to assess its influence. Overall, excellent fits to the data were obtained using a three-compartment model with linear elimination and separate RV models for phase 1 subjects and phase 2 and 3 patients. Exploration of subject factors that explain the interindividual variability in oritavancin CL and Vc demonstrated relationships between both body weight and study phase and CL and both BSA and age and Vc. No other relationships were identified between CL and Vc and gender, age, or renal and hepatic dysfunction.
For CL, the most statistically significant relationship was observed in subjects of >80 kg of total body weight, with CL increasing in a linear fashion above 80 kg. The magnitude of this relationship was such that the population predicted CL would be expected to rise by approximately 53% over a total body weight range of 80 to 200 kg. There was no relationship between total body weight and CL in subjects of ≤80 kg. For Vc, the most statistically significant body size measure was BSA. The magnitude of this relationship was such that, assuming age is constant, the population predicted Vc would be expected to rise by approximately 85% over the BSA range of 1.3 to 3.5 m2. Vc was also found to decrease in a linear fashion as age increased. The magnitude of this relationship was such that, assuming BSA is constant, the population predicted Vc would fall by approximately 35% over the age range of 20 to 85 years.
The relationship between CL and study phase is notable given that, for many drugs, CL decreases in sick patients due to compromised organ function. However, our results show CL to be increased in phase 2 and 3 patients by an average of 30% after accounting for differences in body weight. The exact mechanism for this observation is not clear but may be related to differences in oritavancin tissue distribution between subjects and patients. However, the clinical impact of higher CL in healthy subjects is somewhat irrelevant, as the drug will be used only in infected patients, and the other covariate relationships were similar in patients and subjects.
Although the covariate relationships for CL and Vc were statistically significant, they were not necessarily clinically significant, as evidenced by the small amount of the interindividual variability explained by the covariates retained in the final model. The interindividual variability in CL decreased from 40.7% using the base structural model to 33.7% using the final covariate model; these same values were 36.2% and 35.2% for Vc, respectively. The clinical insignificance of the relationship between Vc and age is clearly illustrated in Fig. 3A, given that dose-normalized Cmax is not increased in elderly subjects. In contrast, those subjects with very high total body weight (>110 kg) had clearances high enough that they would be expected to have oritavancin AUC0-24 values lower than those for subjects of ≤110 kg for fixed 200-mg daily doses (Fig. 3B, left panel). Thus, a dosage adjustment for patients of >110 kg may be warranted to maintain similar steady-state AUC0-24 values. As shown in Fig. 3B, right panel, when administering a 50% increased dose to these patients (i.e., 300 mg daily instead of 200 mg daily), the distribution of AUC0-24 values for these patients is predicted to fall closer to the center of the distribution for patients with a total body weight of ≤110 kg.
It is important to note that the dosage recommendation described above is based solely on maintaining a consistent distribution of AUC0-24 values for a fixed 200-mg daily dosing regimen, regardless of body size. This is reasonable given that the PK-PD measure most closely associated with efficacy for oritavancin is the ratio of the AUC0-24 to the MIC of oritavancin for the microorganism (AUC0-24:MIC ratio) (11). Based on the goodness of fit of the population PK model and the richness of the sampling scheme in the phase 2 and 3 studies, confidence for the model-derived individual PK estimates of oritavancin clearance is high. As CL is the sole determinant of AUC0-24, individual estimates of AUC0-24 among patients with cSSSI or S. aureus bacteremia should be accurate and precise. Thus, future PK-PD analyses may allow for the opportunity to identify critical threshold AUC0-24:MIC ratios associated with efficacy and will also help to further refine the importance of the above-described dosing recommendation in patients with a total body weight of >110 kg.
The results of this analysis illustrate the value of pooling data across the continuum of clinical drug development to confirm key PK concepts regarding a drug. Through this process, we were able to confirm that oritavancin exhibits linear PKs and that the only demographic factor of clinical importance in relation to dose adjustment may be body weight. Through these analyses, we were also able to evaluate appropriate dosage regimens for heavier patients in order to achieve exposures similar to those in lighter patients.
Acknowledgments
We acknowledge the assistance of Charlemagne T. Lacza in the preparation of the manuscript.
This work was supported by a grant from Targanta Therapeutics.
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Allen, N. E., and T. I. Nicas. 2003. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol. Rev. 26:511-532. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, R. J. 2006. S-ADAPT/MCPEM user's guide: software for pharmacokinetic, pharmacodynamic and population data analysis. http://bmsr.usc.edu/software/ADAPT/SADAPTsoftware.html.
- 3.Bhavnani, S. M., J. S. Owen, J. S. Loutit, S. B. Porter, and P. G. Ambrose. 2004. Pharmacokinetics, safety, and tolerability of ascending single intravenous doses of oritavancin administered to healthy human subjects. Diagn. Microbiol. Infect. Dis. 50:95-102. [DOI] [PubMed] [Google Scholar]
- 4.Bhavnani, S. M., C. M. Rubino, A. Forrest, D. Lehoux, O. O. Okusanya, G. L. Drusano, K. A. Rodvold, W. A. Craig, P. G. Ambrose, and T. R. Parr. 2007. Use of PK-PD principles to guide clinical drug development for oritavancin, abstr. A-51. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 5.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio, D. Z., and A. Schumitzky. 1979. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput. Programs Biomed. 9:115-134. [DOI] [PubMed] [Google Scholar]
- 7.D'Argenio, D. Z., A. Schumitzky, and W. Wolf. 1988. Simulation of linear compartment models with application to nuclear medicine kinetic modeling. Comput. Methods Programs Biomed. 27:47-54. [DOI] [PubMed] [Google Scholar]
- 8.Fetterly, G. J., C. M. Ong, S. M. Bhavnani, J. S. Loutit, S. B. Porter, L. G. Morello, P. G. Ambrose, and D. P. Nicolau. 2005. Pharmacokinetics of oritavancin in plasma and skin blister fluid following administration of a 200-milligram dose for 3 days or a single 800-milligram dose. Antimicrob. Agents Chemother. 49:148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gehan, E. A., and S. L. George. 1970. Estimation of human body surface area from height and weight. Cancer Chemother. Rep. 54:225-235. [PubMed] [Google Scholar]
- 10.Korbila, I. P., and M. E. Falagas. 2008. Investigational antimicrobial drugs for bloodstream infections. Curr. Opin. Investig. Drugs 9:871-878. [PubMed] [Google Scholar]
- 11.Lehoux, D., O. O. Okusanya, V. Ostiguy, A. Forrest, K. Laquerre, S. M. Bhavnani, I. Fadhil, M. Malouin, F. F. Arhin, I. Sarmiento, O. Belanger, A. Rafaifar, G. Moeck, T. R. Parr, and P. G. Ambrose. 2007. PK-PD of oritavancin against S. pneumoniae in a murine-pneumonia infection model, abstr. A-49. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 12.Owen, J. S., S. M. Bhavnani, J. Fiedler-Kelly, J. S. Loutit, S. B. Porter, and L. Phillips. 2004. Population pharmacokinetics of oritavancin, abstr. A-20. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 13.Pai, M. P., and F. P. Paloucek. 2000. The origin of the “ideal” body weight equations. Ann. Pharmacother. 34:1066-1069. [DOI] [PubMed] [Google Scholar]
- 13a.Rowe, P.A., and T. J. Brown. 2001. Protein binding of 14C-oritavancin, abstr. A-2193. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 14.SAS Institute Inc. 2002-2003. SAS 9.1.3 service pack 3 for Windows. SAS Institute Inc., Cary, NC.
- 15.Scheinfeld, N. 2007. A comparison of available and investigational antibiotics for complicated skin infections and treatment-resistant Staphylococcus aureus and enterococcus. J. Drugs Dermatol. 6:97-103. [PubMed] [Google Scholar]
- 16.Schwalbe, R. S., A. C. McIntosh, S. Qaiyumi, J. A. Johnson, R. J. Johnson, K. M. Furness, W. J. Holloway, and L. Steele-Moore. 1996. In vitro activity of LY333328, an investigational glycopeptide antibiotic, against enterococci and staphylococci. Antimicrob. Agents Chemother. 40:2416-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upton, R. N. 2004. Calculating the hybrid (macro) rate constants of a three-compartment mamillary pharmacokinetic model from known micro-rate constants. J. Pharmacol. Toxicol. Methods 49:65-68. [DOI] [PubMed] [Google Scholar]