Abstract
The gastric pathogen Helicobacter pylori has developed resistance to virtually all current antibiotics; thus, there is a pressing need to develop new anti-H. pylori therapies. The goal of this work was to evaluate the antibacterial effect of oligo-acyl-lysyl (OAK) antimicrobial peptidomimetics to determine if they might represent alternatives to conventional antibiotic treatment of H. pylori infection. A total of five OAK sequences were screened for growth-inhibitory and/or bactericidal effects against H. pylori strain G27; four of these sequences had growth-inhibitory and bactericidal effects. The peptide with the highest efficacy against strain G27, C12K-2β12, was selected for further characterization against five additional H. pylori strains (26695, J99, 7.13, SS1, and HPAG1). C12K-2β12 displayed MIC and minimum bactericidal concentration (MBC) ranges of 6.5 to 26 μM and 14.5 to 90 μM, respectively, across the six strains after 24 h of exposure. G27 was the most sensitive H. pylori strain (MIC = 6.5 to 7 μM; MBC = 15 to 20 μM), whereas 26695 was the least susceptible strain (MIC = 25 to 26 μM; MBC = 70 to 90 μM). H. pylori was completely killed after 6 to 8 h of incubation in liquid cultures containing two times the MBC of C12K-2β12. The OAK demonstrated strong in vitro stability, since efficacy was maintained after incubation at extreme temperatures (4°C, 37°C, 42°C, 50°C, 55°C, 60°C, and 95°C) and at low pH, although reduced killing kinetics were observed at pH 4.5. Additionally, upon transient exposure to the bacteria, C12K-2β12 showed irreversible and significant antibacterial effects and was also nonhemolytic. Our results show a significant in vitro effect of C12K-2β12 against H. pylori and suggest that OAKs may be a valuable resource for the treatment of H. pylori infection.
Helicobacter pylori is a microaerophilic gram-negative bacterium that colonizes the gastric mucosa. It is known to be a principal gastric pathogen of humans and is associated with the development of gastritis, gastric ulcers, duodenal ulcers, and gastric cancer (46, 55, 56, 60). Approximately half of the world's population is infected with H. pylori (79). Thus, the bacterium poses a significant public health problem, which is further compounded by the fact that H. pylori has developed antimicrobial resistance to virtually all current antibiotics, a phenomenon that is hampering efforts to treat the infection (40, 51).
Since the original isolation of H. pylori in the early 1980s, treatment of the bacterial infection has undergone a significant evolutionary development from initial monotherapy to dual, triple, and in more recent trials quadruple therapy (8, 18). Current treatment strategies employ combination therapy, since single-antibiotic therapy often results in failure to eradicate the infection (21). The highest H. pylori eradication rates have been reported with triple therapy, which involves the utilization of two antibiotics in combination with bismuth or a proton pump inhibitor, PPI (34, 44). Amoxicillin (amoxicilline) with either clarithromycin or metronidazole is often the antibiotic combination of choice as a first- or second-line treatment regimen, respectively. However, in recent years the efficacy of the standard first-line triple therapy has also been decreasing dramatically, mainly due to development of resistance to the drugs (35, 59). Failure to cure H. pylori infection has been noted for more than 20 to 30% of patients (37). In addition, several studies have found an eradication rate lower than 75% (6, 11, 59), and values as low as 25 to 45% have also been recently reported (22, 24). Thus, prolonged standard triple therapy for up to 2 weeks has been recommended (9, 23, 34), and in cases of eradication failure, a quadruple therapy with a proton pump inhibitor, bismuth salt, tetracycline, and metronidazole has been advised as a second-line therapy (8, 13, 44). More recently, sequential therapy (PPI and amoxicillin for 5 days, followed by PPI, clarithromycin, and tinidazole for 5 days) has become very attractive for clinical practice since impressive efficacy was seen (36, 73). However, broad adoption of this strategy as standard first-line therapy for H. pylori infection is still debatable because of impending validation in other geographic locations and studies to demonstrate efficacy superior to that of quadruple therapy, which is still considered a simpler regimen than sequential therapy (74). Of note, all the aforementioned therapies including sequential therapies employ multiple drugs and relatively complex regimens for the treatment of H. pylori infection, hence the search for new/better antibiotics.
The bactericidal activity of amoxicillin results from interference with the interpeptide linkage of peptidoglycan by binding to penicillin binding proteins and blocking their function as transporters during cell wall synthesis. Clarithromycin, like other macrolides, binds to the 50S subunit of bacterial ribosomes, thus inhibiting translocation of tRNA during translation. Binding of clarithromycin to H. pylori ribosomes has been shown to be very strong and is irreversible (27). Finally, metronidazole is a 5-nitroimidazole drug whose mode of action is mediated by nitro metabolites, such as the radical anion (NO2·−) and perhaps nitroso (RNO) and hydroxylamine (RNHOH) derivatives (39). Such metabolites have been demonstrated to cause DNA damage that results in cell death.
H. pylori resistance to amoxicillin is very rare, while resistance to clarithromycin varies significantly and may range from 10 to 25% (14). However, in a recent study, it was reported that the first-line anti-H. pylori triple therapies containing clarithromycin failed in 7 to 49% of patients (19, 26), indicating the underlying significant increase in antimicrobial resistance and occurrence of refractory H. pylori infections (32, 50, 78). Currently, PPI-amoxicillin-metronidazole triple therapy is highly effective as a second-line regimen for the treatment of H. pylori infection in patients showing failure of the first-line regimen (PPI-amoxicillin-clarithromycin) (47). However, high rates of resistance have been reported for people with a history of metronidazole treatment (49). Given the immense challenge in rising antimicrobial resistance (38), there is an enormous need for new antibiotics for the treatment of H. pylori infection.
One of the pharmacodynamic parameters most studied for antibiotics is the postantibiotic effect (PAE), which describes the suppression of bacterial growth after a short exposure of bacteria to an antimicrobial agent (29). From a clinical standpoint, PAE provides a rationale for the modification of the dosing interval of antimicrobials and could be significant for the optimization of a treatment regimen and the minimization of drug-induced adverse effects. Similarly, the success of intermittent dosing with drugs that exhibit short half-lives has been attributed to the presence of significant PAE. A long and/or positive PAE is considered an attractive characteristic for an effective new antibiotic.
In the last decade, antimicrobial peptides (AMPs) have attracted attention as potential therapeutic agents mainly due to their ability to be promptly synthesized by the host upon induction and their capacity to subsequently lyse cell membranes of pathogens through direct interaction with them. Hence, AMPs are recognized as a cell-free host defense mechanism and are important component of the innate immune systems of living organisms, including plants (76), insects (30), amphibians (75), and mammals (80). These natural membrane-lytic peptides display immense diversity in terms of sequence, secondary structural motifs, charge (cationic and anionic), and/or the abundance of certain specific amino acids (16, 66). Despite the immense diversity, a common feature for cationic AMPs is that they all form amphipathic structures that allow them to bind to the membrane interface of microbes (5, 69). Peptides which are not cationic are known to exhibit less selectivity toward microbes than toward mammalian cells, since electrostatic interactions are critical for initial binding of the peptide to membrane containing anionic lipids (45).
Oligomers of acylated lysines (OAKs) constitute a novel class of synthetic AMP mimics that consists of alternating amino acyl chains and cationic amino acids arranged to create an optimal molecular charge and hydrophobicity for enhanced potency (61, 65). This design has been reported to be advantageous over conventional AMPs by allowing the capacity for fine-tuning of the OAK structure to enhance potency against a broad spectrum of organisms while being devoid of apparent toxicity against mammalian cells (62, 64). This selective activity has been attributed to a design that lacks the secondary structures present in natural peptides (63) and to a mode of action that appears to target multiple sites, such as membranes and DNA (64). Circular dichroism studies of OAKs have demonstrated that they lack secondary structure in the presence of liposomes or hydrophobic media such as trifluoroethanol and sodium dodecyl sulfate (63). Additional characterization of OAKs with microbial pathogens other than H. pylori has demonstrated significant stability in the presence of serum and serum components and has shown no hemolysis of host erythrocytes (64). Two recent in vivo studies have also shown that administration of OAKs protected mice from an Escherichia coli lethal challenge (63, 64). Therefore, OAKs display characteristic features that are attractive for the development of a potent therapeutic drug.
Given the increasing antibiotic resistance rates of H. pylori and the current complicated treatment regime, the need for new potent antibiotics has never been greater. Given the potent effect of OAKs on other pathogens, we investigated the in vitro antibacterial activities of five representative OAKs against H. pylori. The selected sequences belong to two distinct groups: one group consisted of C12K-7α8 and its shorter analog, C12K-5α8, two-well characterized compounds (15, 63, 64) both of which are known for potent activity against gram-negative bacteria (25); the second group consisted of the less-characterized OAKs C12K-2β12 and two shorter analogs, C12-2β12 and 2β12, for which a preliminary study (62) predicted broad-spectrum activities at least for the longer analogs. Together, these representative OAKs were anticipated to provide a preliminary structure-activity assessment on the potential activity of OAKs against H. pylori. Our results indicate that four of the tested peptides show efficacy against the pathogen. Of these, C12K-2β12 demonstrated the most potent activity, was active against a spectrum of strains, and was remarkably stable at low pH and after exposure to extreme temperatures.
MATERIALS AND METHODS
Peptide synthesis, reagents, and antibiotics.
Oligo-acyl lysyls used in this study are listed in Table 1 and were synthesized as described previously (61-63). Briefly, a solid-phase method was used to synthesize peptides applying 9-fluorenylmethoxy carbonyl active ester chemistry. Peptide purity was 98 to 99% on assessment of chromatographic homogeneity by reverse-phase high-performance liquid chromatography (HPLC) (Alliance-Waters). Using a linear gradient of acetonitrile in water (1%/min), with both solvents containing 0.1% trifluoroacetic acid, semipreparative columns (Vydac, C4, 250 mm by 4.6 mm) were used to perform HPLC runs. Amino acid analysis and electrospray mass spectrometry were used to confirm the composition of purified peptides. Peptides were lyophilized and stored as powder at −20°C. Prior to experimentation, fresh solutions were prepared by two rounds of 2-min vortexing and sonication in a water bath to make a stock solution (1 mg/ml) that was then used in subsequent experiments.
TABLE 1.
Antimicrobial OAK peptide sequences and biophysicochemical characteristics
| OAKa | Sequenceb | MWc | Qd | He |
|---|---|---|---|---|
| C12K-7α8 | C12K-oKoKoKoKoKoKoK | 2,212.9 | 8 | 47.5 |
| C12K-5α8 | C12K-oKoKoKoKoK | 1,674.1 | 6 | 49.7 |
| C12K-2β12 | C12K-KlK-KlK | 1,234.8 | 5 | 51.0 |
| C12-2β12 | C12-KlK-KlK | 1,106.6 | 4 | 53.3 |
| 2β12 | KlK-KlK | 924.3 | 5 | 38.1 |
OAK designation, where α8 and β12 represent aminooctanoyl-lysyl and lysyl-aminododecanoyl-lysyl subunits, respectively; C12, dodecanoic acid; K, lysine.
o, aminooctanoic acid; l, aminododecanoic acid. For other symbols, see footnote a.
Molecular weight.
Net charge.
Hydrophobicity measured using reverse-phase HPLC.
Amoxicillin, ampicillin, vancomycin, and chloramphenicol purchased from Sigma-Aldrich (St. Louis, MO), USB Corporation (Cleveland, OH), and EMD Chemicals (Darmstadt, Germany), respectively, were reconstituted according to the manufacturer's protocol and used at the concentrations indicated throughout this article.
Bacterial strains and growth conditions.
H. pylori strains used in this study are listed in Table 2. All bacterial strains were maintained as frozen stocks at −80°C in brain heart infusion medium supplemented with 20% glycerol and 10% fetal bovine serum (FBS). Horse blood agar (HBA) plates containing 4% Columbia agar base (Oxoid), 5% defibrinated horse blood (HemoStat Labs, Dixon, CA), 0.2% ß-cyclodextrin (Sigma), 10 μg/ml of vancomycin, 5 μg of cefsulodin/ml (Sigma), 2.5 U of polymyxin B/ml (Sigma), 5 μg of trimethoprim /ml (Sigma), and 8 μg of amphotericin B/ml (Sigma), were used to grow bacteria under microaerophilic conditions at 37°C. For liquid culture, H. pylori was grown in Brucella broth (Difco) containing 10 μg/ml vancomycin and 10% fetal bovine serum (Gibco-BRL) with shaking in a microaerobic environment. A microaerobic atmosphere with a 5% O2, 10% CO2, and 85% N2 gas mixture was achieved using an Anoxomat instrument (Spiral Biotech, Norwood, MA).
TABLE 2.
Antimicrobial activity of C12K-2β12
| H. pylori strain | Reference | OAK concn (μM)a
|
|
|---|---|---|---|
| MIC | MBC | ||
| G27 | 3 | 6.5-7.0 | 15-20 |
| 7.13 | 20 | <9.0 | 14.5-15 |
| J99 | 1 | 10.5-11.0 | 20-25 |
| HPAG1 | 57 | 10.5-11.0 | 40-45 |
| SS1 | 43 | 25.5-26 | 45-50 |
| 26695 | 72 | 25-26 | 70-90 |
MIC (100.1% survival) and MBC (99.9% killing) data represent results from at least three independent experiments.
Determination of MIC and MBC.
Bacteria harvested from HBA plates were used to inoculate Brucella broth liquid starter cultures containing 10 μg/ml vancomycin and 10% fetal bovine serum (complete Brucella broth medium [CBBM]). These cultures were grown for 16 to 18 h to an optical density at 600 nm (OD600) wavelength of between 0.4 and 0.6. The starter culture was then diluted in fresh CBBM to an OD600 of 0.05 and aliquoted in sterile 15-mm-diameter glass tubes to make 1-ml cultures containing various concentrations of OAK peptide (range of 0 to 100 μM). The inoculated tubes were incubated at 37°C with shaking at 110 rpm under microaerobic conditions for 24 h. The MICs and minimum bactericidal concentration (MBCs) were determined by enumeration of starting and ending CFU on HBA plates. The lowest concentration of OAK peptide at which there was no evidence of growth (100.1% CFU survival) was recorded as the MIC, whereas the MBC was defined as the lowest concentration of peptide that killed 99.9% of the starting CFU (0.1% CFU survival). MICs and MBCs were determined for six H. pylori strains in three to five independent biological assays.
Bactericidal kinetics.
The killing kinetics of C12K-2β12 were determined after exposing H. pylori strains to twofold the MBC of the peptide. Briefly, 1-ml liquid bacterial cultures were diluted to an OD600 of 0.05 in CBBM and exposed to the OAK peptide. After 0, 1, 2, 4, 6, 8, 10, 22, and 24 h of culture in the presence of peptide, 10-fold serial dilutions were plated on HBA plates. The plates were incubated at 37°C for 3 to 4 days, at which point CFU were enumerated.
RBC hemolysis assay.
The hemolysis assay was performed basically as described previously (54) with minor modifications. Briefly, red blood cells (RBC) from defibrinated horse blood were washed three times in sterile phosphate-buffered saline (PBS) by centrifugation for 5 min at 2,700 × g and resuspension to a 50% hematocrit value in fresh PBS. Fifty microliters of the RBC suspension was added to microtiter wells containing 200 μl of serial twofold dilutions of OAK peptide solutions in PBS (range, 0.625 to 80 μM), PBS alone (for baseline values), or distilled water (for 100% hemolysis). After incubation for 1, 6, or 10 h with agitation at 37°C, samples were centrifuged. The hemolytic activity was assessed as a function of hemoglobin leakage by measuring the absorbance of 200 μl of supernatant at a 405-nm wavelength. Data were obtained from at least three independent experiments performed in triplicate or quadruplicate. The hemolysis percentage was calculated using the following equation: % hemolysis = [(A405 in the peptide solution − A405 in PBS)/(A405 in water)] × 100, where A405 is the absorbance measured at the 405-nm wavelength.
Peptide stability: pH and temperature.
The stability of C12K-2β12 at low pH and after incubation at various temperatures was examined using the bactericidal kinetic assay described above. The OAK peptide was first preincubated at 4°C, 37°C, 42°C, 50°C, 55°C, 60°C, or 95°C for 1 h. The bactericidal kinetics were then determined over 24 h of culture at 37°C in a 1-ml liquid culture containing twofold the MBC of peptide (40 μM C12K-2β12 for strain G27). To test OAK peptide for stability at low pH, 4 M HCl was used to adjust the pH of the CBBM to pH 4.5. This CBBM was subsequently filter sterilized to remove precipitates. A bacterial starter culture was grown as described above and then diluted in a 40-ml volume to an OD600 of 0.05 and the C12K-2β12 peptide added to a 40 μM final concentration. The liquid bacterial culture was incubated while shaking at 37°C for 24 h. The killing kinetics for both experiments (pH and temperature) were evaluated by enumeration of CFU of samples after plating of serial 10-fold dilutions on HBA plates. Additionally, in order to monitor variations in pH for the pH stability assay during the 24-h culture period, samples were collected to determine the pH at each time point.
Reversibility of OAK peptide effect.
The reversibility of the C12K-2β12 effect on H. pylori was examined by evaluation of the killing rate following removal of the peptide. One milliliter of bacterial liquid culture with an OD600 of 0.05 was exposed to 40 μM C12K-2β12 for 2 h with shaking at 37°C. At this point the bacterial suspension was washed three times in culture medium by repeated centrifugation for 2 min at 2,000 × g and resuspension in Eppendorf tubes. Following resuspension in fresh culture medium, the bacterial culture was incubated with shaking at 37°C for 24 h and bactericidal kinetics determined by enumeration of surviving CFU as described above.
Effect of growth arrest on C12K-2β12 activity.
To determine if the activity of C12K-2β12 required active H. pylori growth, CFU were enumerated over a 24-h period from 1-ml bacterial liquid cultures containing 40 μM C12K-2β12 and 10 μg/ml chloramphenicol, which stops H. pylori growth but does not kill the bacterium. Incubation of H. pylori culture with chloramphenicol or C12K-2β12 alone was included as a control.
RESULTS
Screening of OAK peptides against H. pylori and determination of MIC and MBC.
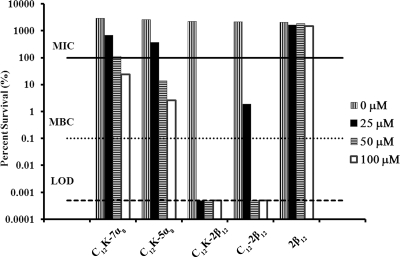
Given that OAKs have been shown to be effective against a number of bacterial pathogens and given the increasing problem of H. pylori antibiotic resistance, we screened five OAK sequences for activity against H. pylori strain G27. The names, sequences, molecular weights, net charges, and hydrophobicities of these AMP mimics are given in Table 1. Concentrations of each peptide ranging between 10 and 100 μM were initially screened. Four of the five OAKs examined exhibited growth-inhibitory and/or bactericidal effects against H. pylori strain G27 (Fig. 1). Whereas 2β12 did not show any efficacy at the concentrations tested, the remaining four peptides showed various effects that could be divided into two groups: group I, strong-efficacy peptides (C12K-2β12 and C12-2β12) that showed an MBC range of 0 to 50 μM and a MIC of 0 to 25 μM; and group II, weak-efficacy peptides (C12K-7α8 and C12K-5α8) that showed an MBC of greater than 100 μM and a MIC of 25 to 50 μM.
FIG. 1.
Screening of five OAK sequences for antibacterial activity. The percent survival of H. pylori strain G27 is shown at the indicated peptide concentration. The bacterial CFU/ml was determined by plating following growth for 24 h in liquid culture containing 10 μM, 25 μM, 50 μM, or 100 μM of each OAK. Percent survival was calculated using the following equation: % survival = CFUt24/CFUt0 × 100, where CFUt0 represents CFU at the beginning of the experiment and CFUt24 represents CFU at 24 h of exposure to the peptide. The three dotted horizontal lines represent the MIC, MBC, and limit of detection (LOD). Data are representative of three independent experiments.
The peptide with the strongest efficacy, C12K-2β12, was selected for further in-depth characterization. We chose 2β12 to be used as a control peptide in subsequent experiments since it lacked efficacy against the bacteria. MICs and MBCs were determined against six H. pylori strains: G27, 7.13, J99, HPAG1, SS1, and 26695. These strains were chosen based on the availability of a complete genome sequence (G27, J99, HPAG1, and 26695) and the ability to colonize H. pylori animal models (7.13 and SS1 infect gerbils and mice, respectively). Based on data shown in Fig. 1, tighter OAK doses were designed, with 0.5 μM and 5 μM intervals for the MIC and MBC, respectively. Table 2 summarizes the results for determination of the MICs and MBCs for C12K-2β12 against the strains tested. All six strains of H. pylori tested were sensitive to treatment with C12K-2β12. G27 was the most sensitive (MIC = 6.5 to 7 μM; MBC = 15 to 20 μM), whereas 26695 was the least susceptible (MIC = 25 to 26 μM; MBC = 70 to 90 μM), suggesting that there are strain differences that may affect either the mode of action or the kinetics of interaction of the OAK. Importantly, efficacy of the OAK was dose dependent (Fig. 1 and data not shown), indicating that the peptides interact with a fixed number of targets on the bacteria.
OAK killing kinetics.
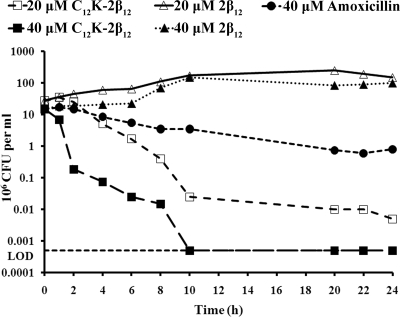
The rate at which an antibiotic is effective is important due to the ability to maintain suitable concentrations for an affective length of exposure. Therefore, we performed time-kill assays to determine the rate of OAK-dependent killing of strains G27, 7.13, and J99. As shown in Fig. 2, C12K-2β12 exhibited rapid killing of strain G27 at both concentrations tested (20 μM and 40 μM). At 40 μM (twofold the MBC of strain G27), no colonies were recovered after 10 h of treatment in liquid cultures containing the peptide. Similar results were obtained with strains 7.13 and J99 at the same peptide concentration (data not shown). It was also observed by determining the slope of the kill curves that C12K-2β12 exhibited concentration-dependent killing kinetics (Table 3 and Fig. 2). Following mathematical modeling of the curves, it was demonstrated that at the MBC, the OAK could kill 90% of bacteria in liquid culture two times slower than at concentrations twofold higher than the MBC. Thus, 90% of the bacteria for strains G27, 7.13, and J99 could be killed after incubation with the MBC of peptide in 6, 6, and 4 h, respectively, but the bactericidal rates could be increased to 3, 3.5, and 2 h upon incubation with twofold the MBC of the peptide, respectively (Table 3). A comparison of the killing rates between amoxicillin and C12K-2β12 demonstrated superior efficacy by the OAK (Fig. 2). Additionally, testing of the killing rate of C12K-2β12 in liquid medium without vancomycin to rule out any potential interaction of the drugs revealed similar efficacy (data not shown). Taken together, these data indicate dose-dependent killing of H. pylori by C12K-2β12.
FIG. 2.
Bactericidal kinetics of C12K-2β12 against H. pylori. Bacterial suspensions of H. pylori were added to liquid culture medium containing 20 μM or 40 μM C12K-2β12. The OAK 2β12 and amoxicillin were used as negative and positive controls, respectively. Bacterial samples were taken at the indicated time intervals and plated to determine surviving CFU. Plotted values are representative of 11 independent experiments. The horizontal dotted line marks the limit of detection (LOD) (500 bacteria).
TABLE 3.
Dose-dependent killing kinetics of C12K-2β12
| H. pylori strain | Time-kill curvea slope (h for 90% killing)
|
|
|---|---|---|
| 1 MBC | 2 MBC | |
| G27 | 0.163 (6.1) | 0.377 (2.7) |
| 7.13 | 0.164 (6.1) | 0.279 (3.5) |
| J99 | 0.260 (3.8) | 0.567 (1.8) |
The data represent results from at least three independent experiments.
Mode of action: reversibility studies and killing kinetics under bacteriostatic conditions.
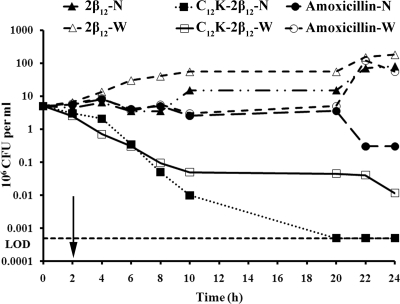
Whether suppression of bacterial growth persists after limited exposure to an antibiotic has been established as an important pharmacodynamic parameter that is usually considered in choosing antibiotic dosing regimens. This is because in a host, there is often a gradual decrease in the antibiotic concentration to subinhibitory levels. Thus, the question of whether the damage to the bacteria is irreversible or not comes into play once antibiotic levels fall below the effective concentration. Therefore, in an attempt to evaluate whether H. pylori could recover from the damage imposed by exposure to C12K-2β12, the killing kinetics were evaluated under conditions in which H. pylori bacteria were exposed to the peptide for 2 h and the peptide withdrawn by washes. The time-kill curves were then compared to those for conditions in which the peptide was not withdrawn. The data from these studies are presented in Fig. 3 and demonstrate that although complete killing was abrogated, the killing rate remained similar to that when the bacteria were grown under conditions where the peptide was not withdrawn. These data suggest that the killing effects of C12K-2β12 are irreversible. In contrast, amoxicillin showed no PAE, an observation that is consistent with previously reported findings (70).
FIG. 3.
PAE of the C12K-2β12 against H. pylori strain G27. Bacteria were exposed to C12K-2β12 at a 40 μM concentration for 2 h at 37°C for PAE determination. Equimolar concentrations of 2β12 and amoxicillin were used as negative and positive controls, respectively. Each line indicates the kinetics as determined by enumeration of CFU at the indicated times. The PAE curves in which the peptide was withdrawn (W) were compared with curves in which the peptide was not withdrawn (N). The arrow symbolizes the time point of peptide withdrawal, which was accomplished by three washes with Brucella broth medium. The data presented are representative of three independent experiments. The horizontal dotted line marks the limit of detection (LOD) (500 bacteria).
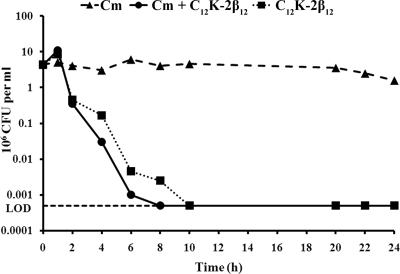
Since some antibiotics are ineffective against slowly growing bacteria and since some bacteria grow slowly at sites of infection (17, 48), we next sought to determine whether the OAK efficacy was maintained when H. pylori growth was inhibited. Therefore, the killing profile of the peptide was examined under chloramphenicol-induced bacteriostatic conditions. Notably, the OAK effects were increased and antibacterial activity was sustained under bacteriostatic conditions (Fig. 4). Sequential addition of C12K-2β12 after 4 h of growth under chloramphenicol-induced bacteriostatic conditions was unable to further increase the activity of C12K-2β12 (data not shown). Taken together, these data indicate that the C12K-2β12 peptide does not require active bacterial growth to be effective.
FIG. 4.
Determination of OAK efficacy under static and exponential growth conditions. Bactericidal kinetics of C12K-2β12 against H. pylori strain G27 were determined when the bacterial growth was inhibited by the addition of chloramphenicol (Cm) (C12K-2β12 + Cm) and compared to results under exponential growth conditions (C12K-2β12 alone). Bacteria cultured with chloramphenicol alone were used as a control. Each line indicates the kinetics as determined by enumeration of CFU at the indicated times. The data presented are representative of seven independent experiments. The horizontal dotted line marks the limit of detection (LOD) (500 bacteria).
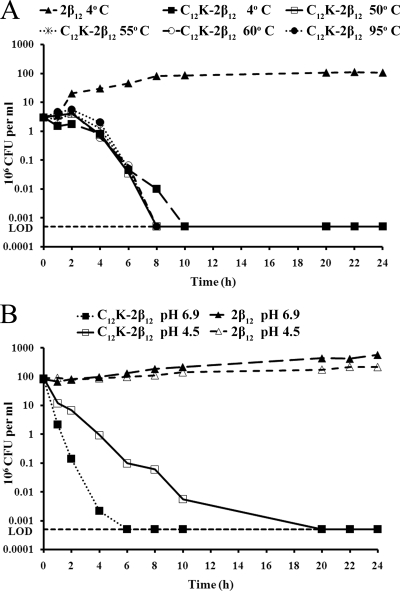
OAK stability after exposure to different ambient temperatures and at low pH.
Inactivation by low pH in the gastric environment as well as poor stability in ambient temperatures during transport, storage, and/or after delivery in vivo may be factors that contribute to the limited clinical efficacies of antimicrobial agents that are active in vitro against H. pylori. Therefore, we tested the effect of exposure to various temperatures and low pH on the stability of the OAK. As shown in Fig. 5, incubation of C12K-2β12 at 4°C, 50°C, 55°C, 60°C, or 95°C for 1 h prior to testing had no effect on the efficacy of the peptide against strain G27 (Fig. 5A). Similar data were obtained with peptide preincubated at 37°C or 42°C (data not shown). Similarly, the OAK showed antimicrobial efficacy against strain G27 at pH 4.5 and pH 6.9 despite a reduced rate of killing at pH 4.5 (Fig. 5B). Taken together, these data suggest that C12K-2β12 is stable at extreme temperatures and shows a reduced rate of killing at low pH.
FIG. 5.
Effects of temperature and pH on OAK activity and stability. (A) Temperature stability of C12K-2β12 was evaluated after preincubation of the peptide at 50°C, 55°C, 60°C, and 95°C for 1 h prior to the experiment. Cultures containing 2β12 and C12K-2β12 preincubated at 4°C were used as negative and positive controls, respectively. The plotted results are representative data from two independent experiments. (B) To evaluate the stability of C12K-2β12 at low pH, the activity was determined at pH 4.5 and compared to that at pH 6.9. The 2β12 peptide was used as a control. Samples at time t0 h were plated prior to peptide exposure. The plotted results are representative data from three independent experiments. The horizontal dotted line marks the limit of detection (LOD) (500 bacteria).
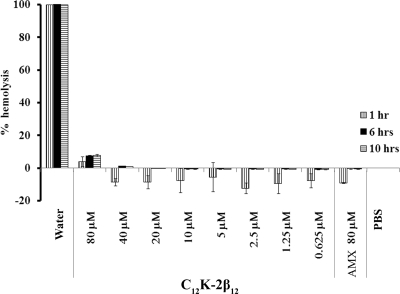
Hemolytic assay.
Most peptides are cationic, and hence their interaction with anionic membrane phospholipids provides a ready explanation for their specificity for bacterial membranes (7, 10). However, some AMPs exhibit hemolytic activities (42), and delivery of such peptides becomes problematic, especially via an intravascular route. Therefore, we tested the hemolytic activity of C12K-2β12. As shown in Fig. 6, the OAK was nonhemolytic at concentrations from 0.625 to 40 μM. We observed only minor hemolytic activity (4 to 8%) following incubation of 80 μM peptide with erythrocytes for 10 h. These results suggest that C12K-2β12 is nonhemolytic and may be able to be administered via various routes.
FIG. 6.
Hemolytic activity of C12K-2β12. Erythrocytes at a 10% hematocrit value were exposed for 1 h, 6 h, or 10 h to peptide solution containing serial twofold dilutions of C12K-2β12 in PBS (range = 0.625 to 80 μM) at 37°C. Erythrocytes incubated in distilled water (100% hemolysis), PBS (no hemolysis), or amoxicillin solution (AMX) (80 μM) were included as controls. Hemoglobin leakage was determined by measurement of absorbance at the 405-nm wavelength. Percent hemolysis was calculated using the following equation: % hemolysis = [(A405 in the peptide solution − A405 in PBS)/(A405 in water)] × 100, where A405 is the absorbance measured at the 405-nm wavelength. Plotted values represent the means ± standard deviations obtained from three independent experiments performed in triplicate or quadruplicate.
DISCUSSION
Considering the increasing resistance observed among H. pylori strains worldwide (38, 81), therapeutic options are becoming significantly limited. Therefore, the aim of the present study was to evaluate the antimicrobial activity of OAKs against H. pylori. OAKs are a class of AMPs with a novel design of linear peptidomimetic sequences consisting of alternating acyl chains and cationic amino acids (63). They have previously been tested against several strains of both gram-positive and gram-negative bacteria, including clinically challenging species (Acinetobacter, Klebsiella, and Pseudomonas spp.), but not against Helicobacter spp. (64). Here we provide in vitro experimental evidence suggesting that the OAK C12K-2β12 displays potent antimicrobial activity against H. pylori.
We evaluated the in vitro susceptibility of H. pylori to OAKs by using time-kill assays. This methodology is superior to measuring the 3-log10 decrease in the number of CFU per milliliter in a 24-h period (71), since it has been shown that the 3-log10 difference method may sometimes leave enough bacteria to establish culture. All H. pylori strains tested were sensitive to treatment with C12K-2β12, which was the most potent of the five OAKs displaying antimicrobial activity. H. pylori was unable to resume growth on untreated agar plates after a 6- to 10-h treatment with concentrations equivalent to the MBC. Moreover, in a molar-to-molar comparison, the antimicrobial activity of C12K-2β12 was superior to that of the commercial antibiotic, amoxicillin. Notably, the six H. pylori strains tested exhibited various levels of sensitivity to C12K-2β12. While the reason for this difference is not completely clear, it is possible that the strain-specific sensitivity observed may be related to differential expression of molecules in the anionic phospholipid cell membrane, such as lipopolysaccharides and/or some unknown specific glycoprotein(s) that interacts with C12K-2β12. Like other cationic peptides, C12K-2β12 is believed to form pores by interacting with anionic phospholipids. It is therefore expected that the stability and permeability of the cell membrane would play a fundamental role in the adaptation to different peptides/antibiotics and that these properties would be closely related to the lipid and fatty acid content. In keeping with this idea, lipopolysaccharide has been demonstrated to protect Bordetella bronchiseptica from the activity of AMPs (4). To our knowledge this has not been tested for H. pylori; however, it should be noted that strain-specific differences in sensitivity and resistance to other antibiotics have been demonstrated. Differential sensitivity to metronidazole has been shown to be due to changes in expression of pyruvate oxidoreductase (33) or gene mutations in the NADP (NADPH)-dependent nitroreductase (encoded by rdxA) (28) and the NADPH flavin oxidoreductase enzyme (encoded by frxA) (41). This suggests that H. pylori strain-specific effects can occur with various classes of antibiotics, and future work from our group will seek to understand the nature of these differences for C12K-2β12.
One of the five peptides evaluated, 2β12, failed to show any antimicrobial activity against H. pylori. This OAK also was inactive when tested against a panel of gram-positive and gram-negative bacteria (62). It is therefore possible that this short peptide may not display enough charge and/or hydrophobicity to interact well with the anionic H. pylori membrane, which is consistent with the fact that optimal charge and/or hydrophobicity is critical for potency (2, 53, 62, 64). It is also noteworthy to mention that although the alpha-OAKs C12K-7α8 and C12K-5α8 showed only weak efficacy against H. pylori, these peptides demonstrated very strong antibacterial activity against various gram-negative bacteria, including E. coli (assessed by both in vitro and in vivo studies), Salmonella spp., and Klebsiella pneumoniae (63, 64). Although differences in cell membrane composition could explain the disparity in these results, the differential antimicrobial activity among the gram-negative bacteria may signal the underlying differences in specificity of peptide-target interaction in different pathogens.
An advantage of OAK peptide design is the possibility to fine-tune the structure to potentiate activity by manipulation of the acyl length and/or lysine residues (63, 64). This is evidenced in our study by comparing the antibacterial effects of C12K-2β12 and C12-2β12; C12K-2β12 showed stronger efficacy. Thus, the differential cationic charge created by the addition of an extra lysine residue results in increased efficacy of C12K-2β12. These results are similar to those obtained by Rotem and colleagues when they compared the effects of C12K-5α8 and C12K-7α8 against Pseudomonas aeruginosa: the two extra α subunits in C12K-7α8 were able to enhance potency (64). Taken together, these data confirm the possibility of modifying OAK peptides to optimize potency and enhance activity.
As with most bacteria, doubling times of H. pylori at the site of colonization are likely longer than those in vitro. Cozens and colleagues have extensively studied the influence of growth rate on the susceptibilities of members of the family Enterobacteriaceae and of P. aeruginosa to antimicrobial agents and have shown that the susceptibilities of slowly growing bacteria to antimicrobial agents are greatly reduced (12). This phenomenon may explain the failure of many promising antimicrobial agents when tested in vivo. To address this concern, we asked the following question: is the efficacy of C12K-2β12 against H. pylori influenced by the growth rate? Our data demonstrate that C12K-2β12 exhibits antibacterial activity regardless of whether the bacteria are actively growing, indicating that slow growth in the stomach may not influence efficacy of the peptide.
We determined the OAK stability by looking at the effect of extreme temperatures (range, 0 to 95°C) and pHs (4.5 and 6.9) on activity. Our data demonstrated that C12K-2β12 is stable at the afore-mentioned temperatures and at low pH, albeit reduced activity was observed at low pH. pH influences the ionization of charged groups and consequently affects downstream interactions. This fact may explain the slightly reduced kinetics of the C12K-2β12 peptide at low pH. The robust stability suggests slow OAK peptide degradation, sustained antimicrobial activity in the acidic environment of the stomach, and the possibility of overcoming cold-chain storage problems in a clinical setup. Indeed, these results are in accordance with those in studies with the cationic peptide nisin, which have suggested low-pH-induced reduction of peptide net charge and reduced potency (76). In the host, however, H. pylori survives strong acidity of the stomach using the enzyme urease to convert gastric urea into ammonia, which then neutralizes the bacterial cytoplasm and microenvironment (52, 58, 67, 68, 77). Thus, the reduced kinetics observed in vitro might not apply in the host, since the local microenvironment inhabited by the bacteria would have a near-neutral pH.
Persistent suppression of bacterial growth after a short exposure to an antibacterial agent, PAE, was first noted and described more than 65 years ago. Since then, several studies have been undertaken to determine the PAE of numerous antimicrobial agents that have further established the significance of PAE in drug development. Today, it is one of the minimum recommendations in a preclinical evaluation of all new antimicrobial agents that determination of the PAE be performed. This is essentially because PAE is a factor that influences optimal antimicrobial dosing intervals. It is generally an accepted clinical observation that antibiotics without a PAE would usually require more-frequent administration than agents exhibiting PAE. PAE is thus an important pharmacodynamic predictor of clinical application of antibiotic dosage in the drug development process. We performed a PAE assay for C12K-2β12 against H. pylori and made a direct comparison of time-kill curves of treated and untreated bacterial cultures using the classical viable-count procedure. Our data demonstrate a significant and irreversible PAE of the OAK against H. pylori, a characteristic feature that makes the peptide promising for future drug development. The cellular and molecular events involved in the significant PAE observed here are largely unknown. However, it is reasonable to suggest that the irreversible significant PAE observed might be related to the level of damage done by the peptide to the bacterial cell. Thus, this might indicate that the damage to the bacterial cell is very profound or at least irreparable, a speculation that is consistent with the fact that if the damage is not fixable, then the bacterial cell dies. It is also noteworthy that the amoxicillin data are consistent with the results of a previous study (31) in which other β-lactams were shown to lack PAE against slowly growing H. pylori.
In conclusion, our data demonstrate strong in vitro antimicrobial activity of C12K-2β12 against H. pylori. Our results suggest that the activity of the OAK is irreversible and sustainable regardless of bacterial growth. Additionally, the peptide is stable at a wide range of temperature and pH conditions. This is the first characterization of synthetic OAK peptides against H. pylori, and our results indicate that C12K-2β12 may, in principle by itself, have strong therapeutic potential against H. pylori. Future work will investigate the in vivo efficacy of C12K-2β12 using H. pylori animal models.
Acknowledgments
Research in the laboratory of Amram Mor was supported by the Israel Science Foundation (grant 283/08). Research in the laboratory of D. Scott Merrell is made possible by grants R073LA from USUHS and AI065529 from the NIAID.
Contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or the DOD.
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Andra, J., D. Monreal, G. Martinez de Tejada, C. Olak, G. Brezesinski, S. S. Gomez, T. Goldmann, R. Bartels, K. Brandenburg, and I. Moriyon. 2007. Rationale for the design of shortened derivatives of the NK-lysin-derived antimicrobial peptide NK-2 with improved activity against Gram-negative pathogens. J. Biol. Chem. 282:14719-14728. [DOI] [PubMed] [Google Scholar]
- 3.Baltrus, D. A., M. R. Amieva, A. Covacci, T. M. Lowe, D. S. Merrell, K. M. Ottemann, M. Stein, N. R. Salama, and K. Guillemin. 2009. The complete genome sequence of Helicobacter pylori strain G27. J. Bacteriol. 191:447-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banemann, A., H. Deppisch, and R. Gross. 1998. The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides. Infect. Immun. 66:5607-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 6.Calvet, X., M. Lopez-Lorente, M. Cubells, M. Bare, E. Galvez, and E. Molina. 1999. Two-week dual vs. one-week triple therapy for cure of Helicobacter pylori infection in primary care: a multicentre, randomized trial. Aliment. Pharmacol. Ther. 13:781-786. [DOI] [PubMed] [Google Scholar]
- 7.Chan, D. I., E. J. Prenner, and H. J. Vogel. 2006. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim. Biophys. Acta 1758:1184-1202. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, H., and F. L. Hu. 2009. Furazolidone, amoxicillin, bismuth and rabeprazole quadruple rescue therapy for the eradication of Helicobacter pylori. World J. Gastroenterol. 15:860-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chey, W. D., and B. C. Wong. 2007. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 102:1808-1825. [DOI] [PubMed] [Google Scholar]
- 10.Clausell, A., M. Garcia-Subirats, M. Pujol, M. A. Busquets, F. Rabanal, and Y. Cajal. 2007. Gram-negative outer and inner membrane models: insertion of cyclic cationic lipopeptides. J. Phys. Chem. B 111:551-563. [DOI] [PubMed] [Google Scholar]
- 11.Comet, R., X. Calvet, M. Navarro, N. Garcia, and I. Sanfeliu. 1998. Seven-day omeprazole, clarithromycin, and amoxicillin for the therapy of Helicobacter pylori infection. Gastroenterol. Hepatol. 21:81-83. (In Spanish.) [PubMed] [Google Scholar]
- 12.Cozens, R. M., E. Tuomanen, W. Tosch, O. Zak, J. Suter, and A. Tomasz. 1986. Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob. Agents Chemother. 29:797-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer, W. A., R. J. van Etten, R. W. Schade, M. E. Ouwehand, P. M. Schneeberger, and G. N. Tytgat. 1996. 4-day lansoprazole quadruple therapy: a highly effective cure for Helicobacter pylori infection. Am. J. Gastroenterol. 91:1778-1782. [PubMed] [Google Scholar]
- 14.Della Monica, P., A. Lavagna, G. Masoero, L. Lombardo, L. Crocella, and A. Pera. 2002. Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Aliment. Pharmacol. Ther. 16:1269-1275. [DOI] [PubMed] [Google Scholar]
- 15.Epand, R. M., S. Rotem, A. Mor, B. Berno, and R. F. Epand. 2008. Bacterial membranes as predictors of antimicrobial potency. J. Am. Chem. Soc. 130:14346-14352. [DOI] [PubMed] [Google Scholar]
- 16.Epand, R. M., and H. J. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11-28. [DOI] [PubMed] [Google Scholar]
- 17.Eudy, W. W., and S. E. Burrous. 1973. Generation times of Proteus mirabilis and Escherichia coli in experimental infections. Chemotherapy 19:161-170. [DOI] [PubMed] [Google Scholar]
- 18.Felga, G. E., F. M. Silva, R. C. Barbuti, T. Navarro-Rodriguez, S. Zaterka, and J. N. Eisig. 2008. Quadruple therapy with furazolidone for retreatment in patients with peptic ulcer disease. World J. Gastroenterol. 14:6224-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fennerty, M. B., D. A. Lieberman, N. Vakil, N. Magaret, D. O. Faigel, and M. Helfand. 1999. Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Arch. Intern. Med. 159:1562-1566. [DOI] [PubMed] [Google Scholar]
- 20.Franco, A. T., D. A. Israel, M. K. Washington, U. Krishna, J. G. Fox, A. B. Rogers, A. S. Neish, L. Collier-Hyams, G. I. Perez-Perez, M. Hatakeyama, R. Whitehead, K. Gaus, D. P. O'Brien, J. Romero-Gallo, and R. M. Peek, Jr. 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 102:10646-10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gisbert, J. P., J. L. Gisbert, S. Marcos, I. Jimenez-Alonso, R. Moreno-Otero, and J. M. Pajares. 2008. Empirical rescue therapy after Helicobacter pylori treatment failure: a 10-year single-centre study of 500 patients. Aliment. Pharmacol. Ther. 27:346-354. [DOI] [PubMed] [Google Scholar]
- 22.Gisbert, J. P., L. Gonzalez, X. Calvet, N. Garcia, T. Lopez, M. Roque, R. Gabriel, and J. M. Pajares. 2000. Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta-analysis of eradication of Helicobacter pylori. Aliment. Pharmacol. Ther. 14:1319-1328. [DOI] [PubMed]
- 23.Gisbert, J. P., S. Marcos, J. L. Gisbert, and J. M. Pajares. 2001. Helicobacter pylori eradication therapy is more effective in peptic ulcer than in non-ulcer dyspepsia. Eur. J. Gastroenterol. Hepatol. 13:1303-1307. [DOI] [PubMed] [Google Scholar]
- 24.Gisbert, J. P., R. Pajares, and J. M. Pajares. 2007. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter 12(Suppl. 2):50-58. [DOI] [PubMed] [Google Scholar]
- 25.Glukhov, E., M. Stark, L. L. Burrows, and C. M. Deber. 2005. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J. Biol. Chem. 280:33960-33967. [DOI] [PubMed] [Google Scholar]
- 26.Glupczynski, Y., F. Megraud, M. Lopez-Brea, and L. P. Andersen. 2001. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 20:820-823. [DOI] [PubMed] [Google Scholar]
- 27.Goldman, R. C., D. Zakula, R. Flamm, J. Beyer, and J. Capobianco. 1994. Tight binding of clarithromycin, its 14-(R)-hydroxy metabolite, and erythromycin to Helicobacter pylori ribosomes. Antimicrob. Agents Chemother. 38:1496-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin, A., D. Kersulyte, G. Sisson, S. J. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 29.Gudmundsson, S., S. Einarsson, H. Erlendsdottir, J. Moffat, W. Bayer, and W. A. Craig. 1993. The post-antibiotic effect of antimicrobial combinations in a neutropenic murine thigh infection model. J. Antimicrob. Chemother. 31(Suppl D):177-191. [DOI] [PubMed] [Google Scholar]
- 30.Haine, E. R., Y. Moret, M. T. Siva-Jothy, and J. Rolff. 2008. Antimicrobial defense and persistent infection in insects. Science 322:1257-1259. [DOI] [PubMed] [Google Scholar]
- 31.Hassan, I. J., R. M. Stark, J. Greenman, and M. R. Millar. 1998. Absence of a post-antibiotic effect (PAE) of beta-lactams against Helicobacter pylori NCTC 11637. J. Antimicrob. Chemother. 42:661-663. [DOI] [PubMed] [Google Scholar]
- 32.Heep, M., M. Kist, S. Strobel, D. Beck, and N. Lehn. 2000. Secondary resistance among 554 isolates of Helicobacter pylori after failure of therapy. Eur. J. Clin. Microbiol. Infect. Dis. 19:538-541. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman, P. S., A. Goodwin, J. Johnsen, K. Magee, and S. J. Veldhuyzen van Zanten. 1996. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J. Bacteriol. 178:4822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howden, C. W., and R. H. Hunt. 1998. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am. J. Gastroenterol. 93:2330-2338. [DOI] [PubMed] [Google Scholar]
- 35.Iacopini, F., P. Crispino, O. A. Paoluzi, A. Consolazio, R. Pica, M. Rivera, D. Palladini, F. Nardi, and P. Paoluzi. 2005. One-week once-daily triple therapy with esomeprazole, levofloxacin and azithromycin compared to a standard therapy for Helicobacter pylori eradication. Dig. Liver Dis. 37:571-576. [DOI] [PubMed] [Google Scholar]
- 36.Jafri, N. S., C. A. Hornung, and C. W. Howden. 2008. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann. Intern. Med. 148:923-931. [DOI] [PubMed] [Google Scholar]
- 37.Janssen, M. J., A. H. Van Oijen, A. L. Verbeek, J. B. Jansen, and W. A. De Boer. 2001. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment. Pharmacol. Ther. 15:613-624. [DOI] [PubMed] [Google Scholar]
- 38.Jones, K. R., J.-H. Cha, and D. S. Merrell. 2008. Who's winning the war? Molecular mechanisms of antibiotic resistance in Helicobacter pylori. Curr. Drug Ther. 3:190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorgensen, M. A., J. Manos, G. L. Mendz, and S. L. Hazell. 1998. The mode of action of metronidazole in Helicobacter pylori: futile cycling or reduction? J. Antimicrob. Chemother. 41:67-75. [DOI] [PubMed] [Google Scholar]
- 40.Kuo, C. H., H. M. Hu, F. C. Kuo, P. I. Hsu, A. Chen, F. J. Yu, P. Y. Tsai, I. C. Wu, S. W. Wang, C. J. Li, B. C. Weng, L. L. Chang, C. M. Jan, W. M. Wang, and D. C. Wu. 2009. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. J. Antimicrob. Chemother. 63:1017-1024. [DOI] [PubMed] [Google Scholar]
- 41.Kwon, D. H., M. Kato, F. A. El-Zaatari, M. S. Osato, and D. Y. Graham. 2000. Frame-shift mutations in NAD(P)H flavin oxidoreductase encoding gene (frxA) from metronidazole resistant Helicobacter pylori ATCC43504 and its involvement in metronidazole resistance. FEMS Microbiol. Lett. 188:197-202. [DOI] [PubMed] [Google Scholar]
- 42.Lai, R., Y. T. Zheng, J. H. Shen, G. J. Liu, H. Liu, W. H. Lee, S. Z. Tang, and Y. Zhang. 2002. Antimicrobial peptides from skin secretions of Chinese red belly toad Bombina maxima. Peptides 23:427-435. [DOI] [PubMed] [Google Scholar]
- 43.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 44.Malfertheiner, P., F. Megraud, C. O'Morain, F. Bazzoli, E. El-Omar, D. Graham, R. Hunt, T. Rokkas, N. Vakil, and E. J. Kuipers. 2007. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 56:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marki, F., E. Hanni, A. Fredenhagen, and J. van Oostrum. 1991. Mode of action of the lanthionine-containing peptide antibiotics duramycin, duramycin B and C, and cinnamycin as indirect inhibitors of phospholipase A2. Biochem. Pharmacol. 42:2027-2035. [DOI] [PubMed] [Google Scholar]
- 46.Marshall, B. J., J. R. Warren, D. B. McGechie, G. J. Francis, P. J. Utley, P. A. Rogers, and R. J. Glancy. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 47.Matsuhisa, T., T. Kawai, T. Masaoka, H. Suzuki, M. Ito, Y. Kawamura, K. Tokunaga, M. Suzuki, T. Mine, S. Takahashi, and N. Sakaki. 2006. Efficacy of metronidazole as second-line drug for the treatment of Helicobacter pylori infection in the Japanese population: a multicenter study in the Tokyo Metropolitan Area. Helicobacter 11:152-158. [DOI] [PubMed] [Google Scholar]
- 48.Maw, J., and G. G. Meynell. 1968. The true division and death rates of Salmonella typhimurium in the mouse spleen determined with superinfecting phage P22. Br. J. Exp. Pathol. 49:597-613. [PMC free article] [PubMed] [Google Scholar]
- 49.McMahon, B. J., T. W. Hennessy, J. M. Bensler, D. L. Bruden, A. J. Parkinson, J. M. Morris, A. L. Reasonover, D. A. Hurlburt, M. G. Bruce, F. Sacco, and J. C. Butler. 2003. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann. Intern. Med. 139:463-469. [DOI] [PubMed] [Google Scholar]
- 50.Megraud, F., and H. Lamouliatte. 2003. Review article: the treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 17:1333-1343. [DOI] [PubMed] [Google Scholar]
- 51.Miehlke, S., W. Schneider-Brachert, C. Kirsch, A. Morgner, A. Madisch, E. Kuhlisch, C. Haferland, E. Bastlein, C. Jebens, C. Zekorn, H. Knoth, M. Stolte, and N. Lehn. 2008. One-week once-daily triple therapy with esomeprazole, moxifloxacin, and rifabutin for eradication of persistent Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 13:69-74. [DOI] [PubMed] [Google Scholar]
- 52.Mobley, H. L., M. J. Cortesia, L. E. Rosenthal, and B. D. Jones. 1988. Characterization of urease from Campylobacter pylori. J. Clin. Microbiol. 26:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nan, Y. H., K. H. Park, Y. Park, Y. J. Jeon, Y. Kim, I. S. Park, K. S. Hahm, and S. Y. Shin. 2009. Investigating the effects of positive charge and hydrophobicity on the cell selectivity, mechanism of action and anti-inflammatory activity of a Trp-rich antimicrobial peptide indolicidin. FEMS Microbiol. Lett. 292:134-140. [DOI] [PubMed] [Google Scholar]
- 54.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nomura, A., G. N. Stemmermann, P. H. Chyou, I. Kato, G. I. Perez-Perez, and M. J. Blaser. 1991. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 325:1132-1136. [DOI] [PubMed] [Google Scholar]
- 56.Nomura, A., G. N. Stemmermann, P. H. Chyou, G. I. Perez-Perez, and M. J. Blaser. 1994. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann. Intern. Med. 120:977-981. [DOI] [PubMed] [Google Scholar]
- 57.Oh, J. D., H. Kling-Backhed, M. Giannakis, J. Xu, R. S. Fulton, L. A. Fulton, H. S. Cordum, C. Wang, G. Elliott, J. Edwards, E. R. Mardis, L. G. Engstrand, and J. I. Gordon. 2006. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc. Natl. Acad. Sci. USA 103:9999-10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owen, R. J., S. R. Martin, and P. Borman. 1985. Rapid urea hydrolysis by gastric campylobacters. Lancet i:111. [DOI] [PubMed] [Google Scholar]
- 59.Paoluzi, P., F. Iacopini, P. Crispino, F. Nardi, A. Bella, M. Rivera, P. Rossi, M. Gurnari, F. Caracciolo, M. Zippi, and R. Pica. 2006. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 11:562-568. [DOI] [PubMed] [Google Scholar]
- 60.Parsonnet, J., S. Hansen, L. Rodriguez, A. B. Gelb, R. A. Warnke, E. Jellum, N. Orentreich, J. H. Vogelman, and G. D. Friedman. 1994. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 330:1267-1271. [DOI] [PubMed] [Google Scholar]
- 61.Radzishevsky, I., M. Krugliak, H. Ginsburg, and A. Mor. 2007. Antiplasmodial activity of lauryl-lysine oligomers. Antimicrob. Agents Chemother. 51:1753-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radzishevsky, I. S., T. Kovachi, Y. Porat, L. Ziserman, F. Zaknoon, D. Danino, and A. Mor. 2008. Structure-activity relationships of antibacterial acyl-lysine oligomers. Chem. Biol. 15:354-362. [DOI] [PubMed] [Google Scholar]
- 63.Radzishevsky, I. S., S. Rotem, D. Bourdetsky, S. Navon-Venezia, Y. Carmeli, and A. Mor. 2007. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat. Biotechnol. 25:657-659. [DOI] [PubMed] [Google Scholar]
- 64.Rotem, S., I. S. Radzishevsky, D. Bourdetsky, S. Navon-Venezia, Y. Carmeli, and A. Mor. 2008. Analogous oligo-acyl-lysines with distinct antibacterial mechanisms. FASEB J. 22:2652-2661. [DOI] [PubMed] [Google Scholar]
- 65.Sarig, H., S. Rotem, L. Ziserman, D. Danino, and A. Mor. 2008. Impact of self-assembly properties on antibacterial activity of short acyl-lysine oligomers. Antimicrob. Agents Chemother. 52:4308-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schibli, D. J., R. F. Epand, H. J. Vogel, and R. M. Epand. 2002. Tryptophan-rich antimicrobial peptides: comparative properties and membrane interactions. Biochem. Cell Biol. 80:667-677. [DOI] [PubMed] [Google Scholar]
- 67.Scott, D. R., E. A. Marcus, D. L. Weeks, and G. Sachs. 2002. Mechanisms of acid resistance due to the urease system of Helicobacter pylori. Gastroenterology 123:187-195. [DOI] [PubMed] [Google Scholar]
- 68.Scott, D. R., D. Weeks, C. Hong, S. Postius, K. Melchers, and G. Sachs. 1998. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology 114:58-70. [DOI] [PubMed] [Google Scholar]
- 69.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1462:55-70. [DOI] [PubMed] [Google Scholar]
- 70.Sorberg, M., H. Hanberger, M. Nilsson, and L. E. Nilsson. 1997. Pharmacodynamic effects of antibiotics and acid pump inhibitors on Helicobacter pylori. Antimicrob. Agents Chemother. 41:2218-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stratton, C. W., K. E. Aldridge, and M. S. Gelfand. 1995. In vitro killing of penicillin-susceptible, -intermediate, and -resistant strains of Streptococcus pneumoniae by cefotaxime, ceftriaxone, and ceftizoxime: a comparison of bactericidal and inhibitory activity with achievable CSF levels. Diagn. Microbiol. Infect. Dis. 22:35-42. [DOI] [PubMed] [Google Scholar]
- 72.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 73.Vaira, D., A. Zullo, N. Vakil, L. Gatta, C. Ricci, F. Perna, C. Hassan, V. Bernabucci, A. Tampieri, and S. Morini. 2007. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann. Intern. Med. 146:556-563. [DOI] [PubMed] [Google Scholar]
- 74.Vakil, N., and D. Vaira. 2008. Sequential therapy for Helicobacter pylori: time to consider making the switch? JAMA 300:1346-1347. [DOI] [PubMed]
- 75.Vanhoye, D., F. Bruston, P. Nicolas, and M. Amiche. 2003. Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 270:2068-2081. [DOI] [PubMed] [Google Scholar]
- 76.van Kraaij, C., E. Breukink, M. A. Noordermeer, R. A. Demel, R. J. Siezen, O. P. Kuipers, and B. de Kruijff. 1998. Pore formation by nisin involves translocation of its C-terminal part across the membrane. Biochemistry 37:16033-16040. [DOI] [PubMed] [Google Scholar]
- 77.van Vliet, A. H., J. Stoof, S. W. Poppelaars, S. Bereswill, G. Homuth, M. Kist, E. J. Kuipers, and J. G. Kusters. 2003. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori fur repressor. J. Biol. Chem. 278:9052-9057. [DOI] [PubMed] [Google Scholar]
- 78.Wang, W. H., B. C. Wong, A. K. Mukhopadhyay, D. E. Berg, C. H. Cho, K. C. Lai, W. H. Hu, F. M. Fung, W. M. Hui, and S. K. Lam. 2000. High prevalence of Helicobacter pylori infection with dual resistance to metronidazole and clarithromycin in Hong Kong. Aliment. Pharmacol. Ther. 14:901-910. [DOI] [PubMed] [Google Scholar]
- 79.Yin, Y., L. H. He, and J. Z. Zhang. 2009. Successful isolation of Helicobacter pylori after prolonged incubation from a patient with failed eradication therapy. World J. Gastroenterol. 15:1528-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zanetti, M. 2005. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 7:179-196. [PubMed] [Google Scholar]
- 81.Zullo, A., F. Perna, C. Hassan, C. Ricci, I. Saracino, S. Morini, and D. Vaira. 2007. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment. Pharmacol. Ther. 25:1429-1434. [DOI] [PubMed] [Google Scholar]