Abstract
Proton pump inhibitors (PPIs) have been associated with Clostridium difficile infection (CDI) in several recent studies. However, other studies have not shown this association, and the mechanism by which PPIs might promote CDI has not been elucidated. We hypothesized two possible mechanisms of causation: first, by raising pH, PPIs may prevent gastric contents from killing C. difficile spores; second, gastric contents of PPI-treated patients may promote germination and outgrowth of C. difficile spores. Survival rates of spores from six different strains of C. difficile in acidic gastric contents were assessed using quantitative cultures on selective media. Germination and outgrowth of spores were assessed by heat shock at 80°C, phase-contrast microscopy, and ethanol shock after incubation for 24 h in the gastric contents of patients and in the gastric, small intestinal, and cecal contents of mice. C. difficile spores survived and remained dormant in nonbilious gastric contents with acidic pH. Germination did not occur in unmodified gastric contents of patients but did occur with the addition of taurocholic acid and amino acids. In mice, germination did not occur in gastric contents but did occur in small intestinal and cecal contents. In summary, C. difficile spores survived in acidic gastric contents and did not undergo germination and outgrowth in gastric contents, probably due to lack of essential germinants, such as taurocholic acid. Our results suggest that the effects of PPIs in the stomach do not contribute to the pathogenesis of CDI.
Clostridium difficile is a gram-positive, anaerobic spore-forming bacillus that is the most common infectious cause of health care-associated diarrhea in developed countries (19). The recent emergence of an epidemic strain, termed North American pulsed-field gel electrophoresis type 1, or NAP1, has been associated with large outbreaks of C. difficile infection (CDI) in North America and Europe (14, 15). In addition to traditional risk factors, such as exposure to antibiotics and increased underlying disease severity (19), several recent studies have reported an association between proton pump inhibitors (PPIs) and nosocomial (1, 3, 4, 27) or community-associated (5, 6) CDI. Because PPIs are often used in the absence of clear indications (20), it might be feasible to reduce the use of these agents as a control strategy for C. difficile. However, the role of PPIs in the pathogenesis of CDI is controversial because some studies have not associated PPIs with C. difficile (10, 18, 21, 25) and the mechanisms by which acid-suppressive medications might promote CDI are unclear.
Gastric acid provides a host defense by killing ingested pathogens (20). PPIs could promote CDI by raising pH, thereby preventing gastric contents from killing ingested C. difficile. However, C. difficile exists primarily in the acid-resistant spore form in the environment (10), and animal models suggest that spores pass through the stomach and germinate in the small intestine, presumably due to stimulation by bile salts (26). Because bile salts can be detected in low concentrations in the stomachs of healthy volunteers and patients with reflux disease (2, 8), it is plausible that C. difficile spores could germinate in the gastric contents of PPI-treated patients. Germination in the stomach could potentially facilitate colonization by increasing the numbers of actively dividing C. difficile spores reaching the intestinal tracts of susceptible individuals (5, 10). We have previously demonstrated that the vegetative form of C. difficile survives in the gastric contents of PPI-treated patients with pHs greater than 5 (10). In addition, we demonstrated that spores from three strains of C. difficile were not killed in acidic gastric contents (20). Here, we examined the survival rates of spores from six different strains of C. difficile in the gastric contents of hospitalized patients not receiving PPIs and tested the hypothesis that germination occurs in the gastric contents of PPI-treated patients.
MATERIALS AND METHODS
Patients.
Aspirates of gastric contents were obtained from adult patients with a nasogastric tube placed as a part of their hospital care. Patients with known small bowel obstruction, bilious drainage, or significant upper gastrointestinal bleeding were excluded. For patients receiving PPI therapy, aspirates were collected from 2 to 12 h after the PPI dose. Only patients receiving PPI therapy for at least 3 days were enrolled. Information regarding demographics, indications for nasogastric tube placement, and medications, including PPIs, was obtained by medical record review. The experimental protocol was approved by the Cleveland Veterans Affairs Medical Center's institutional review board.
C. difficile strains.
Six C. difficile strains were studied. Five of the strains were cultured from patients with CDI in Cleveland and were characterized by pulsed-field gel electrophoresis and by restriction endonuclease analysis (REA) typing; two were epidemic NAP1 (REA type BI) strains (VA 17 and VA 15), and three were REA J-type strains (VA 11, VA 10, and VA 19). The sixth isolate (ATCC 43593) was a nontoxigenic strain from the American Type Culture Collection (ATCC).
Preparation of spores.
Spores were prepared by growth on Duncan and Strong agar medium as previously described (7, 16). Spores were stored at 4°C in sterile distilled water until use. Spores were confirmed by phase-contrast microscopy and malachite green staining to be >98% free of vegetative cells or cell debris.
Survival of C. difficile spores in gastric contents.
Survival of spores in gastric acid in the gastric contents of subjects not receiving PPI therapy was assessed. The gastric contents were used within 4 h of collection or stored for up to 1 month at −20°C. Preliminary experiments demonstrated that identical results were obtained with fresh gastric aspirates versus aspirates stored at −20°C for up to 1 month. Samples were prereduced in an anaerobic chamber for at least 4 h prior to inoculation with 106 CFU of the six different strains of C. difficile spores. After 24 h of incubation, the number of viable spores was assessed by diluting samples 1:1 in absolute alcohol, followed by plating of serial dilutions on prereduced cycloserine-cefoxitin-fructose agar containing 0.1% taurocholic acid and 5 mg/liter lysozyme (CCFA-TAL).
Germination and outgrowth of C. difficile spores in gastric contents.
Initial experiments were performed to assess germination and outgrowth of spores from the six C. difficile strains in gastric contents. A spore germination medium containing 18 amino acids (i.e., cysteine, isoleucine, leucine, proline, tryptophan, valine, arginine, glycine, histidine, methionine, tyrosine, phenylalanine, alanine, lysine, serine, aspartic acid, glutamic acid, and threonine) and minerals (i.e., potassium phosphate, sodium phosphate, sodium chloride, calcium chloride, magnesium chloride, manganese chloride, ammonium sulfate, ferrous sulfate, cobalt chloride, and sodium bicarbonate), prepared as previously described by Karasawa et al. (11), plus 0.1% taurocholic acid was used as a positive control, and sterile water was used as a negative control. Samples were allowed to prereduce in the anaerobic chamber for at least 4 h, and 200-μl aliquots were inoculated with 106 CFU of spores. Aliquots at baseline and after 24 h of incubation were analyzed for germination and outgrowth by using three methods. First, aliquots of each sample were viewed under a phase-contrast microscope to assess whether the spores remained dormant (bright phase) or germinated (dark phase). Second, aliquots were heat shocked at 80°C for 5 min and spores were enumerated by plating serial dilutions on CCFA-TAL to assess whether germination was initiated. Dormant spores remain resistant to heat at 80°C, while spores that have initiated germination are killed at this temperature (7). Finally, aliquots were diluted 1:1 in absolute alcohol or phosphate-buffered saline (PBS) and then serially diluted in PBS and plated on CCFA-TAL to enumerate spores and vegetative cells, respectively. Spores that have initiated germination are killed by heat but not alcohol, whereas spores that have begun outgrowth are killed by heat or alcohol (9, 11).
Additional experiments were performed using one of the C. difficile strains (VA 17, an epidemic BI strain) to assess whether germination of spores could be induced in gastric contents by the addition of germinants. The gastric contents were modified to include either 0.1% taurocholic acid and minerals; a solution of 18 amino acids and minerals prepared as noted above; or a combination of taurocholic acid, amino acids, and minerals (i.e., the spore germination medium noted above).
Mouse model of C. difficile spore germination.
A mouse model was used to provide an additional assessment of the site of germination of spores in the intestinal tract. Female CF1 mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 25 to 30 g were treated with subcutaneous protonix (a PPI) at 0.8 mg/day in 0.2 ml for 3 days. Four hours after the final dose, the mice were killed by CO2 asphyxiation. The stomach, small intestines, and cecum were removed, and the pH was measured. The samples were placed in an anaerobic chamber (Coy Laboratories, Grass Lake, MI) and prereduced for at least 4 h. Each sample was inoculated with 106 CFU of C. difficile spores (VA 11, J strain, and VA 17, BI strain). The number of heat-resistant spores was assessed at baseline and after 60 min by heat shocking aliquots of each sample at 80°C for 5 min. Samples before and after heating were serially diluted and enumerated as described above.
Statistical analysis.
Student's t test was used to compare means of results from different experimental groups (Microsoft Corporation, Redmond, WA).
RESULTS
Characteristics of subjects.
Samples of gastric contents were obtained from 20 patients, including 13 receiving PPI therapy and 7 not receiving PPI or other acid-suppressive therapy. The indications for the nasogastric tubes included gastric decompression in postsurgical or ventilator-dependent patients (n = 9), initiation of enteral feeding (n = 4), evaluation for upper gastrointestinal bleeding (n = 4), and administration of medications (n = 3). The pHs of gastric contents from patients on PPI therapy ranged from 4.65 to 7.28 (mean pH = 6.43). Patients not receiving PPI therapy had gastric contents that ranged from pH 0.95 to 4.89 (mean pH = 2.34).
Survival of C. difficile spores in gastric contents.
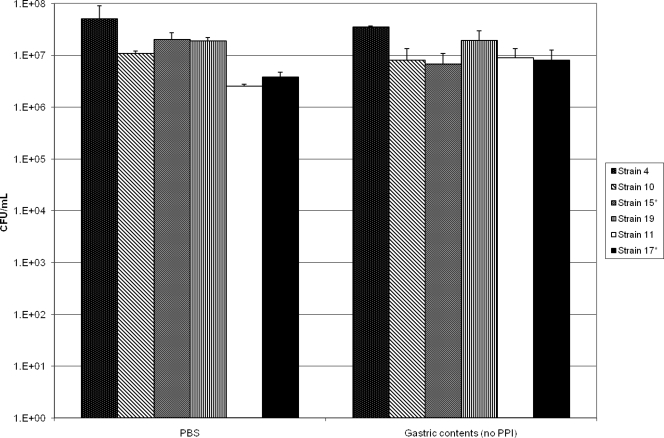
Figure 1 shows the results for assessment of C. difficile spore survival in gastric contents of patients not receiving PPI therapy. After 24 h of incubation, there was no significant difference in the concentrations of viable spores for any of the six test strains in gastric contents versus in PBS (P = 0.44).
FIG. 1.
Survival of Clostridium difficile spores in PBS versus gastric contents of patients not receiving PPI therapy. Survival was assessed by alcohol shock treatment after 24 h of incubation, followed by plating onto selective media.
Germination and outgrowth of C. difficile spores in gastric contents.
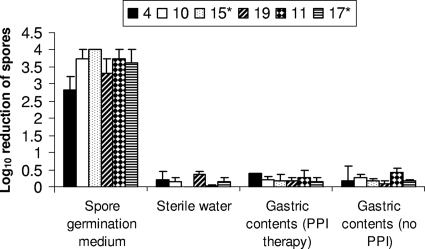
Figure 2 shows the results for the assessment of germination and outgrowth of spores in gastric contents, as indicated by killing by alcohol. In the spore germination medium (positive control), the concentration of C. difficile was reduced by 2.8 to 4 logs by alcohol shock, indicating initiation of germination and outgrowth. Phase-contrast microscopy confirmed outgrowth based on the presence of vegetative rods after 24 h of incubation. In contrast, there was no reduction of the spore counts induced by alcohol shock for spores incubated in sterile water or gastric contents. Phase-contrast microscopy confirmed that spores remained dormant (i.e., no evidence of initiation of germination), as indicated by spores remaining in bright phase after 24 h of incubation. In sterile water and gastric contents, spores remained resistant to heat shock at 80°C, providing further confirmation that germination had not been initiated (data not shown).
FIG. 2.
Germination and outgrowth of Clostridium difficile spores in gastric contents as indicated by killing by alcohol. Six different strains were tested (ATCC 43593 [strain 4], VA 10, VA 15, VA 19, VA 11, and VA 17). *, epidemic North American pulsed-field gel electrophoresis type 1 strain. For spores in gastric contents, the absence of initiation of germination was confirmed by phase-contrast microscopy showing persistence of bright-phase spores and lack of killing by heat at 80°C (see text).
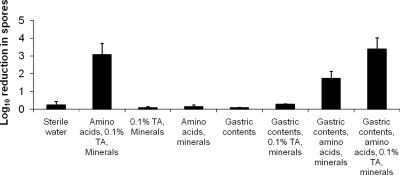
As shown in Fig. 3, the combination of amino acids, taurocholic acid, and minerals induced germination and outgrowth of spores of strain VA 17, as indicated by killing by alcohol, whereas amino acids or taurocholic acid (in combination with minerals) separately was insufficient to induce germination and outgrowth. The addition of amino acids, taurocholic acid, and minerals to gastric contents resulted in germination and outgrowth of seven of nine samples tested. Interestingly, the addition of taurocholic acid and minerals to gastric contents did not induce germination and outgrowth (P = 0.32 for comparison to water control), whereas amino acids and minerals added to gastric contents stimulated germination and outgrowth in 6 of 11 gastric content samples tested. The addition of amino acids and minerals resulted in an average reduction of 1.7 logs in the spore count versus a 3.4-log reduction with the addition of amino acids, taurocholic acid, and minerals (P = 0.04), as indicated by killing by alcohol. The pH ranges of the gastric content samples that did and did not show germination were similar (4.0 to 6.8 and 4.6 to 6.4, respectively). The addition of amino acids, minerals, and taurocholic acid did not affect the pH of the gastric contents.
FIG. 3.
Germination and outgrowth of Clostridium difficile spores in unmodified and modified gastric contents as indicated by killing by alcohol. One epidemic North American pulsed-field gel electrophoresis type 1 strain (VA 17) was tested. TA, taurocholic acid. See text for listing of amino acids and minerals.
Mouse model of C. difficile spore germination.
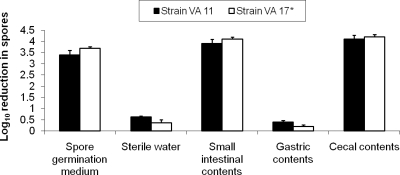
The results of C. difficile spore germination in a mouse model are presented in Fig. 4. The pH values of the gastric, small intestine, and cecal contents were 5.4, 6.4, and 6.3, respectively. Spores of both test strains did not initiate germination or begin outgrowth in gastric contents of mice, as indicated by resistance to killing by heat. In small-intestinal contents and in cecal contents, the spores of both strains initiated germination to the same degree as in the spore germination medium.
FIG. 4.
Initiation of germination of Clostridium difficile spores in gastric, small-intestinal, and cecal contents of mice as indicated by killing by heat at 80°C. Two different strains were tested (VA 11 and VA 17). *, epidemic North American pulsed-field gel electrophoresis type 1 strain.
DISCUSSION
We found that C. difficile spores were not killed in acidic gastric contents and did not germinate or begin outgrowth in the gastric contents of hospitalized patients receiving or not receiving PPIs. The addition of taurocholic acid, amino acids, and minerals stimulated germination and outgrowth of spores in seven of nine gastric content samples tested, suggesting that the absence of germination was due to lack of sufficient concentrations of germinants rather than due to inhibition of germination. In mice, initiation of germination occurred in small-intestinal and cecal contents but not in gastric contents. These findings do not support the hypotheses that PPI therapy might promote CDI by reducing killing of spores or by promoting germination of spores in the stomach.
The signals triggering C. difficile spore germination after ingestion are not completely understood. On the basis of findings in a hamster model, Wilson et al. (26) suggested that germination occurred in the small intestine and was stimulated by exposure to bile salts. Sorg and Sonenshein (22) have subsequently demonstrated that bile salts alone do not induce germination but that bile salts in combination with the amino acid glycine served as cogerminants for C. difficile spores. Our findings are consistent with those of Sorg and Sonenshein (22) in that the addition of taurocholic acid alone to gastric contents was insufficient to induce germination of spores but that addition of taurocholic acid in combination with 18 amino acids (including glycine) did induce germination. Interestingly, addition of amino acids alone induced germination in 6 of 11 gastric samples, suggesting that germination may not occur in the stomach, primarily due to insufficient levels of cogerminants, such as amino acids.
In the absence of a clear mechanism by which PPIs might promote CDI, it is possible that these agents do not promote CDI and that the association demonstrated in some studies is due to unidentified confounding factors. Alternatively, it has been proposed that PPIs may be associated with CDI due to effects in areas other than the stomach. For example, H+/K+ ATPases, the target of PPIs, have been identified in the colon (12). However, it is not known if these medications have any impact on the pathogenesis of CDI in the colon. It has also been demonstrated that PPIs may have antibacterial activity (13). However, we found that PPI therapy, in the absence of concurrent antibiotic therapy, did not promote colonization by vancomycin-resistant enterococci and Klebsiella pneumoniae, suggesting that these agents do not have significant adverse effects on the indigenous intestinal microflora (23).
Our study has limitations. First, we examined germination and outgrowth in vitro in gastric and intestinal contents rather than in vivo. Second, only male patients, most of whom were elderly, were included in the study population. Third, we collected gastric samples only from subjects with nasogastric tubes placed for clinical indications. We cannot exclude the possibility that there are differences between the subjects studied and patients who do not have nasogastric tubes. For example, the subjects were all fasting, which might reduce the likelihood of germination being stimulated by dietary amino acids.
In summary, C. difficile spores were not killed in acidic gastric contents and did not germinate or begin outgrowth in gastric contents of hospitalized patients. In mice, initiation of germination did not occur in gastric contents but did occur in small-intestinal and cecal contents. These findings do not suggest a mechanism by which PPI therapy might promote CDI.
Acknowledgments
This research was conducted with support from the Investigator-Sponsored Study Program of AstraZeneca and by the Geriatric Research, Education and Clinical Center, Cleveland VA Medical Center, Cleveland, OH.
We thank Peter Setlow for assistance in the development of methods for preparing spores and monitoring germination.
Footnotes
Published ahead of print on 10 August 2009.
REFERENCES
- 1.Al-Tureihi, F. I. J., et al. 2005. Albumin, length of stay, and proton pump inhibitors: key factors in Clostridium difficile-associated disease in nursing home patients. J. Am. Med. Dir. Assoc. 6:105. [DOI] [PubMed] [Google Scholar]
- 2.Collins, B. J., G. Crothers, R. J. McFarland, and A. H. G. Love. 1985. Bile acid concentrations in the gastric juice of patients with erosive esophagitis. Gut 26:495-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham, R., B. Dale, B. Undy, and N. Gaunt. 2003. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J. Hosp. Infect. 54:243. [DOI] [PubMed] [Google Scholar]
- 4.Dalton, B. R., T. Lye-Maccannell, E. A. Henderson, D. R. Macccannell, and T. J. Louie. 2009. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment. Pharmacol. Ther. 29:626-634. [DOI] [PubMed] [Google Scholar]
- 5.Dial, S., J. A. C. Delaney, A. N. Barkun, and S. Suissa. 2005. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 294:2989-2995. [DOI] [PubMed] [Google Scholar]
- 6.Dial, S., J. A. Delaney, V. Schneider, and S. Suissa. 2006. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ 175:745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan, C. L., and D. H. Strong. 1968. Improved medium for sporulation of Clostridium perfringens. Appl. Microbiol. 16:82-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorucci, S., E. Distrutti, F. DiMatteo, P. Brunori, L. Santocci, F. Mallozzi, U. Bigazzi, and A. Morelli. 1995. Circadian variations in gastric acid and pepsin secretion and intragastric bile acid in patients with reflux esophagitis and in healthy controls. Am. J. Gastroenterol. 90:270-276. [PubMed] [Google Scholar]
- 9.Hitzman, D. O., H. O. Halvorson, and T. Ukita. 1957. Requirements for production and germination of spores of anaerobic bacteria. J. Bacteriol. 74:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jump, R. L. P., M. J. Pultz, and C. J. Donskey. 2007. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with elevated pH. Antimicrob. Agents Chemother. 51:2883-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karasawa, T., S. Ikoma, K. Yamakawa, and S. Nakamura. 1995. A defined growth medium for Clostridium difficile. Microbiology 141:371-375. [DOI] [PubMed] [Google Scholar]
- 12.Kaunitz, J. D., and G. Sachs. 1986. Identification of a vanadate-sensitive potassium-dependent proton pump from rabbit colon. J. Biol. Chem. 261:14005-14010. [PubMed] [Google Scholar]
- 13.Loo, V. G., C. A. Fallone, E. DeSouza, J. Lavallee, and A. N. Barkun. 1997. In vitro susceptibility of Helicobacter pylori to ampicillin, clarithromycin, metronidazole and omeprazole. J. Antimicrob. Chemother. 40:881-883. [DOI] [PubMed] [Google Scholar]
- 14.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 15.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 16.Nerandzic, M. M., and C. J. Donskey. 2009. Effective and reduced-cost modified selective medium for isolation of Clostridium difficile. J. Clin. Microbiol. 47:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Pepin, J., N. Saheb, M. A. Coulombe, M. E. Alary, M. P. Corriveau, S. Authier, M. Leblanc, G. Rivard, M. Bettez, V. Primeau, M. Nguyen, C. E. Jacob, and L. Lanthier. 2005. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin. Infect. Dis. 41:1254-1260. [DOI] [PubMed] [Google Scholar]
- 19.Poutanen, S. M., and A. E. Simor. 2004. Clostridium difficile-associated diarrhea in adults. CMAJ 171:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao, A., R. L. Jump, N. J. Pultz, M. J. Pultz, and C. J. Donskey. 2006. In vitro killing of nosocomial pathogens by acid and acidified nitrite. Antimicrob. Agents Chemother. 50:3901-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah, S., A. Lewis, D. Leopold, F. Dunstan, and K. Woodhouse. 2000. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. Q. J. Med. 93:175-181. [DOI] [PubMed] [Google Scholar]
- 22.Sorg, J. A., and A. L. Sonenshein. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiefel, U., R. L. Jump, and C. J. Donskey. 2006. Suppression of gastric acid production by proton pump inhibitor treatment facilitates colonization of the large intestine by vancomycin-resistant Enterococcus and Klebsiella pneumoniae in mice. Antimicrob. Agents Chemother. 50:3905-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Wilcox, M. H., L. Mooney, R. Bendall, C. D. Settle, and W. N. Fawley. 2008. A case-control study of community-associated Clostridium difficile infection. J. Antimicrob. Chemother. 62:388-396. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, K. H., J. N. Sheagren, and R. Freter. 1985. Population dynamics of ingested Clostridium difficile in the gastrointestinal tract of the Syrian hamster. J. Infect. Dis. 151:355-361. [DOI] [PubMed] [Google Scholar]
- 27.Yearsley, K. A., L. J. Gilby, A. V. Ramadas, E. M. Kubiak, D. L. Fone, and M. C. Allison. 2006. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment. Pharmacol. Ther. 24:613-619. [DOI] [PubMed] [Google Scholar]