Abstract
[125I]IodoDPA-713 [125I]1, which targets the translocator protein (TSPO, 18 KDa), was synthesized in seven steps from methyl-4-methoxybenzoate as a tool for quantification of inflammation in preclinical models. Preliminary in vitro autoradiography and in vivo small animal imaging were performed using [125I]1 in a neurotoxicant-treated rat and in a murine model of lung inflammation, respectively. The radiochemical yield of [125I]1 was 44 ± 6% with a specific radioactivity of 51.8 GBq/μmol (1,400 mCi/μmol) and > 99% radiochemical purity. Preliminary studies showed that [125I]1 demonstrated increased specific binding to TSPO in a neurotoxicant-treated rat and increased radiopharmaceutical uptake in the lungs of an experimental inflammation model of lung inflammation. Compound [125I]1 is a new, convenient probe for preclinical studies of TSPO activity.
Keywords: pyrazolopyrimidine, peripheral benzodiazepine receptor, translocator protein, autoradiography, small animal imaging
Introduction
Inflammation is becoming an increasingly important target for imaging, particularly in the context of central nervous system disease [1], [2]. Imaging agents are also being sought to study autoimmune disease and the inflammatory arthritides, the cardiovascular system and cancer. For nearly twenty years the standard radiopharmaceutical for imaging inflammation has been the isoquinoline [11C]PK11195, a ligand for the translocator protein (TSPO, 18 KDa), a.k.a. the peripheral benzodiazepine receptor (PBR), which is upregulated in activated glial and immune cells [3], [4]. But [11C]PK11195 tends to demonstrate significant nonspecific binding and poor brain uptake [5], [6], so superior radioligands for TSPO have been aggressively sought [2]. One such class of compounds that could be derivatized for imaging TSPO includes the pyrazolopyrimidines, in particular N,N-diethyl-2-[2-(4-methoxy-phenyl)-5,7-dimethyl-pyrazolo[1,5-a]pyrimidin-3-yl]-acetamide, DPA-713 [7], [8]. DPA-713 is 10-fold less lipophilic than PK11195 and has nearly twice the affinity for TSPO. James et al. synthesized [11C]DPA-713, which demonstrated higher signal-to-noise ratios than [11C]PK11195 in a rodent model of brain injury [8]. We have recently undertaken the first human study of [11C]DPA-713, which demonstrated a higher binding potential than [11C]PK11195 [5]. However, 11C-labeled radiopharmaceuticals are limited to centers in which there is a cyclotron on site, can be difficult to handle due to the 20 min physical half-life of the radionuclide, and the synthesis of such compounds can be prohibitively costly for preclinical (small animal) imaging studies. Here we report the synthesis of the corresponding radioiodinated compound, [125I]iodoDPA-713 [125I]1 for use in autoradiography and small animal imaging studies.
Materials and Methods
General
Chemicals and solvents obtained from commercial sources were analytical grade or better and used without further purification. Iodine-125 (125I) was obtained as a 0.1 N solution of NaOH (high concentration) from MP Biomedicals (Solon, Ohio). Analytical thin-layer chromatography (TLC) was performed using Aldrich aluminum-backed 0.2 mm silica gel plates and visualized by UV light (254 nm) and I2. Flash column chromatography was performed on silica gel (60Ǻ) from MP Biomedicals. Radio-HPLC purification were performed using a Waters (Milford, MA) system consisting of two Waters 510 pumps, a Waters 490E variable wavelength UV/V is detector set at 254 nm, a BioScan FlowCount radioactivity detector, a Waters radial-PAK C18 reverse phase analytical column (8 × 100 mm) with H2O/CH3CN/TFA solvent systems, and Win Flow (LabLogic) chromatography software. 1H NMR was recorded on a Bruker (Billerica, MA) Ultrashield™ 400 MHz spectrometer. ESI mass spectra were obtained with a Bruker Daltonics Esquire 300 plus spectrometer. Radioactivity was measured in a Capintec CRC-12 dose calibrator. The specific radioactivity was calculated as the radioactivity eluting at the retention time of [125I]1 during HPLC purification divided by the mass corresponding to the area under the curve of UV absorption.
Chemical Synthesis
N,N-Diethyl-2-[2-(4-hydroxy-phenyl)-5,7-dimethyl-pyrazolo[1,5-a]pyrimidin-3-yl]-acetamide 3
BBr3 in CH2Cl2 (3 mL, 3 mmol) was added dropwise to a solution of 2 (0.22 g, 0.58 mmol) in CH2Cl2 at −78 °C. After stirring overnight the pH of the reaction mixture was adjusted to pH 8–9 using NaHCO3 and extracted with CH2Cl2. The organic layer was collected and dried over anhydrous Na2SO4. The solvent was removed under vacuum and the residue was purified by silica gel column chromatography (CHCl3/MeOH, 40:1 (v/v), as eluent) to yield 3 (0.18g, 85%). 1H NMR (CDCl3, 400 MHz) δ 1.06–1.18 (m, 6H), 2.54 (s, 3H), 2.73 (s, 3H), 3.34–3.51 (m, 4H), 3.96 (s, 2H), 6.49 (s, 1H), 6.79–6.82 (d, J = 8.7 Hz, 2H), 7.61–7.64 (d, J = 8.4 Hz, 2H). ESI MS m/z: [M+H]+. C20H25N4O2, calculated 353.2, found 353.2.
N,N-Diethyl-2-[2-(4-hydroxy-3-iodophenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide 4
To a solution of 3 (100 mg, 0.28 mmol) in MeOH (15 mL) was added NaI (52 mg, 0.35mmol) and chloroamine-T hydrate (80 mg, 0.35mmol). The reaction mixture was stirred for 1 h and then concentrated under vacuum. The residue was purified by column chromatography (CH2Cl2/MeOH, 20:1 (v/v), as eluent) to yield 4 (60 mg, 45% yield). 1H NMR (CD3OD, 400 MHz) δ 1.16 (t, J = 7.2 Hz, 3H), 1.29 (t, J = 7.2 Hz, 3H), 2.57 (s, 3H), 2.76 (s, 3H), 3.44 (q, J = 7.2 Hz, 2H), 3.59 (q, J = 7.2 Hz, 2H), 3.97 (s, 2H), 6.80 (s, 1H), 6.92 (d, J = 8Hz, 1H), 7.57 (d, J = 8Hz, 1H), 8.01 (s, 1H). ESI MS m/z: [M+H]+. C20H24IN4O2 calculated 479.1, found 479.1.
N,N-diethyl-2-[2-(3-iodo-4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide (iodoDPA-713) 1
To a solution of 4 (30 mg, 0.063 mmol) in DMF (5 mL) was added K2CO3 (300 mg, 2.2 mmol) and MeI (100 μL × 2M, 0.2 mmol). The reaction was stirred overnight and the solvent was removed under vaccum and the residue was purified by silica gel column chromatography (EtOAc as eluent) to give 1 (29 mg, 0.60 mmol, 95%). 1H NMR (CD3OD, 400 MHz) δ 1.15(t, J = 7.2 hz, 3H), 1.29 (t, J = 7.2 Hz, 3H), 2.57 (s, 3H), 2.77 (s, 3H), 3.43 (q, J = 7.2 Hz, 2H), 3.58 (q, J = 7.2 Hz, 2H), 3.92 (s, 3H), 3.97 (s, 2H), 6.80 (s, 1H), 7.06 (d, J = 8 Hz, 1H), 7.74 (d, J = 8 Hz, 1H), 8.09 (s, 1H). ESI MS m/z: [M+H]+. C21H26IN4O2 calculated 493.1, found 493.1.
Radiochemical Synthesis
N,N-diethyl-2-[2-(4-hydroxy-3-[125I]-iodophenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide [125I]4
To a solution of 1 mg of 3 in 0.1 mL of CH3CN, 0.1 mL of Methanol and 0.1mL of phosphate-buffered saline was added 0.2 mg of Iodogen (Pierce, Rockford, IL), followed by 2 mCi of [125I]NaI. The reaction mixture was incubated at room temperature for 1.5 h and purified by reverse phase HPLC using 70% H2O/30% CH3CN/0.1% TFA with a flow rate of 2 mL/min on a Waters Radial-Pak analytical column (8 × 10 mm). The retention time of [125I]4 was 22.8 min (Figure 1A). The radioactive fraction corresponding to [125I]4 was collected, diluted with water and passed through a pre-conditioned C18 light Sep-Pak cartridge (Waters Corp, Milford, MA) eluted with 0.5 mL of ether. The ether was evaporated under a stream of nitrogen and the residue was used in the next step. Yield: 44% (n = 3), 0.041GBq (1.1 mCi).
Figure 1.
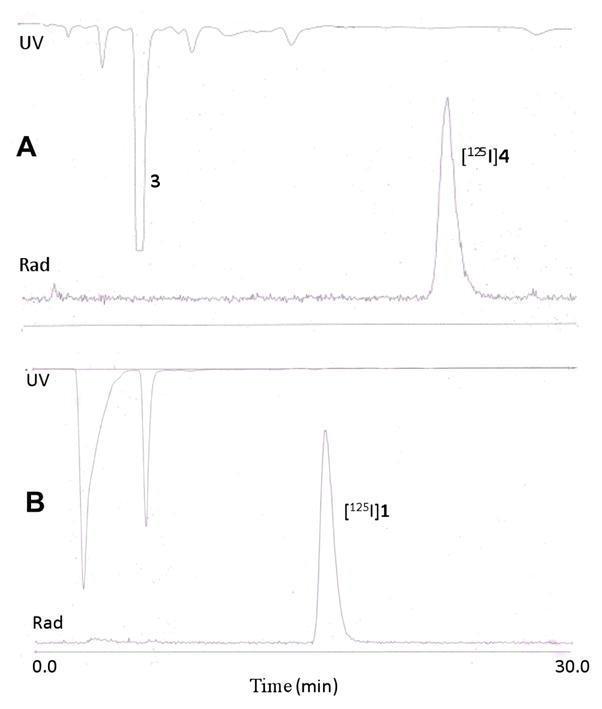
High-performance liquid chromatography (HPLC) traces in the synthesis of [125I]4 from 3 (A) and [125I]iodoDPA-713 ([125I]1) (B). Retention times for [125I]4 and [125I]1 were 22.8 and 15.5 min, respectively. The radiochemical yield of [125I]1 was 44 ± 6% with a specific radioactivity of 51.8 GBq/μmol (1,400 mCi/μmol) and > 99% radiochemical purity. Other yields and conditions are provided in the Materials and Methods.
N, N-diethyl-2-[2-(3-[125I]-iodo-4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5- a]pyrimidin-3-yl]acetamide ([125I]iodoDPA-713) [125I]1
Compound [125I]4 0.041GBq (1.1 mCi) was dissolved in 0.2 mL of anhydrous DMF in a 2 mL V-vial. To this was added 10 mg of K2CO3 followed by 10 μL of 2 M MeI. The reaction mixture was stirred overnight and diluted with 0.2 mL of water and purified by reverse phase HPLC using 50% H2O/50% CH3CN/0.1% TFA with a flow rate of 2 mL/min on a Waters Radial-Pak analytical column (8 × 100 mm). The retention time of [125I]1 was 15.5 min (Figure 1B). The radioactive fraction corresponding to [125I]1 was collected, diluted with water and passed through a pre-conditioned C18 light Sep-Pak and eluted with 0.5 mL of ethanol. Yield: 0.035 GBq (0.95 mCi), 86% from [125I]4 and 47% from 3. Specific radioactivity was 51.8 GBq/μmol (1,400 Ci/mmol).
In Vitro Autoradiography
Fresh-frozen brains were sectioned (20 μm) on a freezing cryostat in the horizontal plane. Brain sections were thaw-mounted onto poly-L-lysine-coated slides (Sigma) and stored at −20 °C until used. Autoradiography using [125I]1 was performed on adjacent brain sections using the following procedures. Slides were thawed and dried at 37 °C for 30 min and prewashed in 50 mM Tris–HCl buffer (pH 7.4) for 5 min at room temperature. Sections were then incubated in 1.4 nM [125I]1 in 50 mM Tris-HCl buffer for 30 min at room temperature. For nonspecific binding, adjacent sections were incubated in the presence of 10 μM racemic PK11195. The reaction was terminated by two 3 min washes in cold buffer (4 °C) and two dips in cold deionized water (4 °C). Sections were air-dried and apposed to Kodak Bio-Max MR film for 1 h. Images were acquired using the MCID image analysis software (InterFocus Imagin Ltd., Cambridge, England).
SPECT-CT Imaging
8–10 wk old female immunocompetent CD-1 mice (Charles River Labs) were imaged. Mice were anesthetized by brief isoflurane sedation. While anesthetized, intranasal instillation was conducted by placing 10 μg/60 μL (167 μg/mL) of lipopolysaccharide (LPS) onto the nares [9]. The 60 μL sample was applied onto the nares as three 20 μL drops. Phosphate buffered saline (PBS) was administered in a similar fashion and used as the control.
For SPECT-CT imaging 18.5 MBq (500 μCi) of [125I]1 was injected via the tail vein into the LPS and PBS treated mice and imaged 1 h later, which corresponded to 24 h after the administration of LPS or PBS. That time point was chosen since it represents the time at which there is maximum inflammation in the lungs, as determined by a time course study with histological correlation (data not shown). Each mouse was anesthetized with isoflurane and maintained under 1–2% isoflurane in oxygen. The mouse was positioned on the X-SPECT (Gamma Medica, Northridge, CA) gantry and was scanned using two opposing low energy pinhole collimators (Gamma Medica) rotating through 360° in 3° increments for 20 seconds per increment. Images were reconstructed using Lunagem software that accompanies the X-SPECT. Immediately following SPECT acquisition, the mice were then scanned by CT over a 3 cm field-of-view using a 600 μA, 50 kV beam. The SPECT and CT data were then co-registered using the X-SPECT software and displayed using AMIDE (http://amide.sourceforge.net/). Data was reconstructed using the ordered subsets-expectation maximization (OS-EM) algorithm. The signal to background ratio was calculated from the images by drawing regions of interest over the lungs and muscle respectively.
Results and Discussion
N,N-Diethyl-2-[2-(3-iodo-4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide (iodoDPA-713) 1 was synthesized according to Scheme 1 in three steps from N-diethyl-2-[2-(4-methoxy-phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]-acetamide 2 (DPA-713). Compound 2 was prepared by the procedure of James et al. [7]. Attempts were made to iodinate 2 directly to 1 using NaI and N-chlorosuccinimide or chloramine-T as oxidants in acidic solvents (concentrated trifluoroacetic acid or trifluoromethanesulfonic acid). However, those conditions produced no or extremely low yields of 1. Consequently 2 was demethylated by reaction with BBr3 in methylene chloride to form the more reactive phenol derivative N,N-diethyl-2-[2-(4-hydroxyphenyl)- 5,7-dimethyl-pyrazolo[1,5-a]pyrimidin-3-yl]-acetamide 3. Compound 3 was smoothly iodinated using NaI with chloroamine-T to form N,N-diethyl-2-[2-(4-hydroxy- 3-iodophenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide 4. Finally, Omethylation of 4 gave 1, which could then serve as the cold standard for highperformance liquid chromatography (HPLC) for radiosynthesis.
Scheme 1.
Synthesis of N,N-diethyl-2-[2-(3-iodo-4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide (iodoDPA-713) 1 and N,N-diethyl-2-[2-(3-[125I]-iodo-4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide ([125I]iodoDPA-713 [125I]1); (i) boron tribromide, dichloromethane, −78°C, overnight, 85%, (ii) chloroamine-T, phosphorus buffered saline (pH 7.4), methanol, 1 h, 45%, (iii) methyl iodide, potassium carbonate, dimethyl formamide, overnight, 95%, (iv) iodogen, [125I]NaI, CH3CN/MeOH/PBS 1.5 h, 47% (n = 3), (v) K2CO3/MeI/DMF, 92% (n =3) 51.8 GBq/μmol (1,400 Ci/mmol).
The synthesis of [125I]1 proceeded with an average radiochemical yield of 44 ± 6% by reaction of 3 with iodogen (Scheme 1) followed by methylation of the radioiodinated product. Compound [125I]1 was produced in specific radioactivities of 51.8 GBq/μmol (1,400 mCi/μmol) in > 99% radiochemical purity.
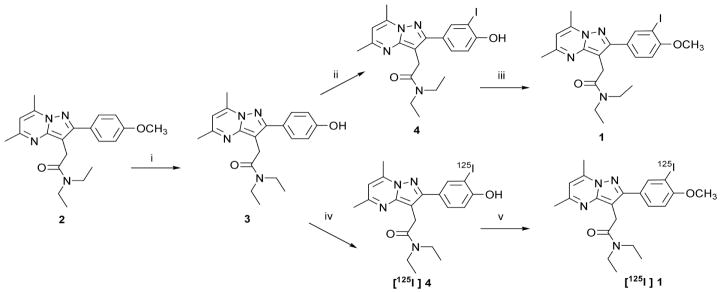
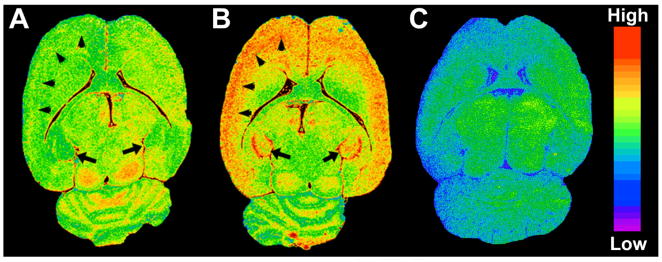
Compound [125I]1 underwent two preliminary tests to determine its utility for imaging inflammation. The first study involved a model of brain inflammation in which rats were exposed to a seizure-inducing neurotoxicant (Figure 2). Note that higher levels of [125I]1 were present in the neurotoxicant-treated rat brain (Figure 2B) than in control brain (Figure 2A). Exposure of the tissue specimens to [125I]1 to generate these images required only 60 min, as opposed to 4–5 weeks often needed for tritium-labeled compounds when exposing the samples to film. Rapid images can also be obtained with tritium-labeled compounds, including [3H]DPA-713, using an automated system such as a Beta-Imager [10], however the resolution of such images is generally inferior to that which can be obtained by exposing the tissue slices to film. Also note that uptake of [125I]1 could be almost completely blocked in the neurotoxicant-treated rat upon treatment with PK11195 (Figure 2C), demonstrating that most binding seen in this in vitro study was specific for TSPO. An in vivo small animal single photon emission computed tomography (SPECT-CT) study demonstrated 1.5-fold higher uptake of [125I]1 in inflamed mouse lungs than in normal lungs (Figure 3). Although only one result is presented here, that was a consistent finding. Together these two preliminary studies suggest that [125I]1 may be useful to study preclinical models of inflammation. Compound [125I]1 is particularly relevant as it is patterned after compounds that are currently undergoing early clinical testing [5], [6].
Figure 2.
Autoradiographic images of [125I]iodoDPA-713 [125I]1 to TSPO in the rat brain. Representative horizontal images of [125I]1 binding to TSPO in a normal rat brain (A) and in a neurotoxicant-injected rat brain (B). There is a significant increase in TSPO levels in the cerebral cortex (arrowheads) and hippocampus (arrows) in the neurotoxicant-injected rat brain (B) relative to the control brain (A). The image in (C) is representative of [125I]1 non-specific binding using 10 μM R-PK11195 as the blocking agent in a neurotoxicant-treated rat.
Figure 3.
SPECT-CT imaging at 1 h postinjection. The lung signal to background ratio in the control mouse (top row) was 14.6 to 1 and that in the LPS treated mouse (bottom row) was 21.2 to 1.
Conclusions
[125I]IodoDPA-713 ([125I]1) can be readily synthesized from DPA-713 in high radiochemical yield and specific radioactivity. It demonstrates specific binding to TSPO in vitro and in vivo, showing a higher level of uptake in inflamed than in the corresponding normal tissue. This agent provides a convenient and inexpensive alternative to other radiolabeled analogs of the pyrazolopyrimidine series for autoradiographic and preclinical imaging studies.
Acknowledgments
We thank Jennifer McGlothan for expertise in autoradiography and James Fox and Gilbert Green for providing the in vivo images. We also thank the Dana Foundation and NIH grants CA92871 and ES07062 for financial support. The funding sources had no impact on the scientific content of this contribution.
Abbreviations
- TSPO
translocator protein
- [125I]iodoDPA-713
N,N-diethyl-2-[2-(3-iodo-4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide
- PK11195
N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide
- SPECT
single photon emission computed tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wunder A, Klohs J, Dirnagl U. Non-invasive visualization of CNS inflammation with nuclear and optical imaging. Neuroscience. 2009;158:1161–73. doi: 10.1016/j.neuroscience.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Cagnin A, Kassiou M, Meikle SR, Banati RB. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics. 2007;4:443–52. doi: 10.1016/j.nurt.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarf AM, Ittner LM, Kassiou M. The translocator protein (18 kDa): central nervous system disease and drug design. J Med Chem. 2009;52:581–92. doi: 10.1021/jm8011678. [DOI] [PubMed] [Google Scholar]
- 5.Endres CJ, Pomper MG, James M, Uzuner O, Hammoud DA, Watkins CC, Reynolds A, Hilton J, Dannals RF, Kassiou M. Initial Evaluation of 11C-DPA-713, a Novel TSPO PET Ligand, in Humans. J Nucl Med. 2009;50:1276–1282. doi: 10.2967/jnumed.109.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauveau F, Van Camp N, Dolle F, Kuhnast B, Hinnen F, Damont A, Boutin H, James M, Kassiou M, Tavitian B. Comparative evaluation of the translocator protein radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a rat model of acute neuroinflammation. J Nucl Med. 2009;50:468–76. doi: 10.2967/jnumed.108.058669. [DOI] [PubMed] [Google Scholar]
- 7.James ML, Fulton RR, Henderson DJ, Eberl S, Meikle SR, Thomson S, Allan RD, Dolle F, Fulham MJ, Kassiou M. Synthesis and in vivo evaluation of a novel peripheral benzodiazepine receptor PET radioligand. Bioorg Med Chem. 2005;13:6188–94. doi: 10.1016/j.bmc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Boutin H, Chauveau F, Thominiaux C, Gregoire MC, James ML, Trebossen R, Hantraye P, Dolle F, Tavitian B, Kassiou M. 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48:573–81. doi: 10.2967/jnumed.106.036764. [DOI] [PubMed] [Google Scholar]
- 9.Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997;202:49–57. doi: 10.1016/s0022-1759(96)00236-0. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JC, Friel SL, Roman S, Perren M, Harper A, Davis JB, Richardson JC, Virley D, Medhurst AD. Autoradiographical imaging of PPARgamma agonist effects on PBR/TSPO binding in TASTPM mice. Exp Neurol. 2009;216:459–70. doi: 10.1016/j.expneurol.2009.01.002. [DOI] [PubMed] [Google Scholar]