Abstract
There is growing interest in causal factors for pelvic floor disorders. These conditions include pelvic organ prolapse and urinary and fecal incontinence and are affected by a myriad of factors that increase occurrence of symptomatic disease. Unraveling the complex causal network of genetic factors, birth-induced injury, connective tissue aging, lifestyle and co-morbid factors is challenging. We describe a graphical tool to integrate the factors affecting pelvic floor disorders. It plots pelvic floor function in 3 major life phases: 1) development of functional reserve during an individual’s growth, 2) variations in the amount of injury and potential recovery that occur during and after vaginal birth, and 3) deterioration that occurs with advancing age. This graphical tool accounts for changes in different phases to be integrated to form a disease model to help assess the overlap of different causal factors.
Keywords: Vaginal birth, pelvic floor, urinary incontinence, pelvic organ prolapse
Introduction
A woman has an 11% chance of experiencing pelvic floor dysfunction (PFD) so severely during her life that she will require surgery, based on data from a managed care population (1). Pelvic floor disorders (PFD), the umbrella term for conditions such as incontinence and pelvic organ prolapse, leads to over 300,000 operations each year (2) (3). Understanding the cause of these common conditions is critical to improving treatment and prevention; but clarifying causation is complex due to their multifactorial nature. Factors such as instrumented vaginal delivery, heavy lifting, rapid decline in tissue strength, damaged levator ani muscle, and nerve injury are cited as increasing the likelihood that a woman may develop prolapse or experience recurrence of PFD after surgery (4) (5). Rather than a single factor, it is more probable that combinations of anatomical, physiological, genetic, lifestyle, and reproductive factors interact throughout a woman’s lifespan to contribute to PFD. Understanding these complex causal questions can be facilitated by having a framework that integrates how different factors interact to result in the development of PFD.
We will present a conceptual model, The Lifespan Model, to help understand how PFDs might result from different combinations of biologic and lifestyle factors, and we will demonstrate how these conditions can be better understood by considering the individual elements “independently, interactively, and cumulatively during… childhood, adolescence, and adult life” (6). We will provide some examples of factors that may be involved, recognizing that these are only exemplars and not a complete list of involved factors.
Lifespan Model
The pelvic floor grows and develops during childhood reaching a maximal capability in early life. At this point, there is considerable functional reserve and symptoms are rare. Beyond this point, there is a normal decline in functional reserve with increasing age. In certain women this reserve is depleted to the point that a threshold is crossed and symptoms begin to occur. When, and if, this occurs will depend on the amount of reserve originally achieved in development, the rate of decline, the degree of stress that her lifestyle places on the pelvic floor, and the effects of any inciting events.
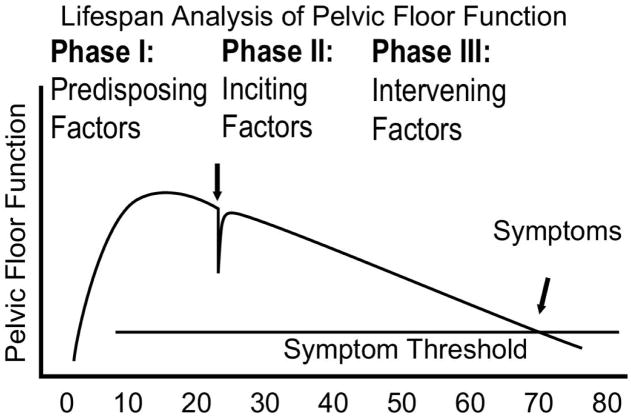
The Lifespan Model for Pelvic Floor Disorders is a simple graphical display that portrays a theoretical parameter, “pelvic floor function,” and tracks it over a woman’s life (Figure 1). There are three basic life phases in this analysis: Phase I, Predisposing Factors (e.g., growth and development); Phase II, Inciting Factors (e.g., birth induced changes); and Phase III, Intervening Factors (e.g., age-related changes).
Figure 1. Integrated lifespan analysis of pelvic floor function.
This graphical display of the abstract concept of pelvic floor function tracks the functional reserve throughout different phases of a woman’s lifespan. Initially, pelvic floor structure growth in late teens leads to a fully developed pelvic floor. Vaginal birth affects pelvic floor function. Finally, age-related deterioration occurs until a symptom threshold is reached where the functional reserve present earlier in life is lost. (© DeLancey 2007)
This “pelvic floor function,” as an abstract concept, can have several different meanings depending on the condition being considered. Generally, it can be viewed as the sum of the activities of many elements of the pelvic floor that each contribute in different ways to overall pelvic organ support and urinary and fecal control. In this sense, it is a concept rather than one specific measurable parameter. The concept can include the combined actions of the levator ani muscles, urethra, bladder, anal sphincters with their controlling nerves and supporting connective tissue, or alternatively can also be used to consider any single parameter (for example, levator ani muscle maximal strength, anterior vaginal wall support, or urethral closure pressure).
In the Lifespan Model for Pelvic Floor Disorders, we consider the composite function of the pelvic floor as a conceptual entity. Although the pelvic floor is a single unit, it is comprised of several separate structures that relate to clinical symptoms and problems such as prolapse and incontinence. These involve muscles that have different origins and insertions, specific levels at which neural control occurs, and unique connective tissue features that connect specific parts of the pelvic floor to the bony pelvis and to one another that allows the actions of individual structural units to be integrated. Many of the structures that relate to stress incontinence are different than those involved with fecal incontinence. On the other hand there are elements such as the levator ani muscles and the pelvic nerves that affect all aspects of the pelvic floor.
Each of these structures has its own tissue composition. Dense regular connective tissue of the arcus tendineus fascia pelvis is similar to tendon and very different than the smooth muscle and irregular connective tissue makeup of the vaginal wall, or the striated muscle of the levator ani. The injury, recovery, and disease mechanisms that affect each type of tissue and structure must be individually considered. For example, during birth, the striated muscle could sustain irrecoverable injury due to rupture of the muscle from excess stretching, denervation from nerve compression or elongation, or ischemic injury due to compartment syndrome. Connective tissue change with advancing age could be due to gradual stretching due to mechanical deterioration, or it could rupture during a single episode of structural overload. Each type of tissue has specific types of injury; specifying the proposed mechanism of impairment can focus research on forward progress.
Women present with specific symptoms that relate to a subset of these structures. Each structure must be considered in the role that it plays in pelvic organ support and urinary and fecal control. Additionally, each of these structures functions differently in the context of aging and lifestyle factors over time.
Phase I: Predisposing Factors
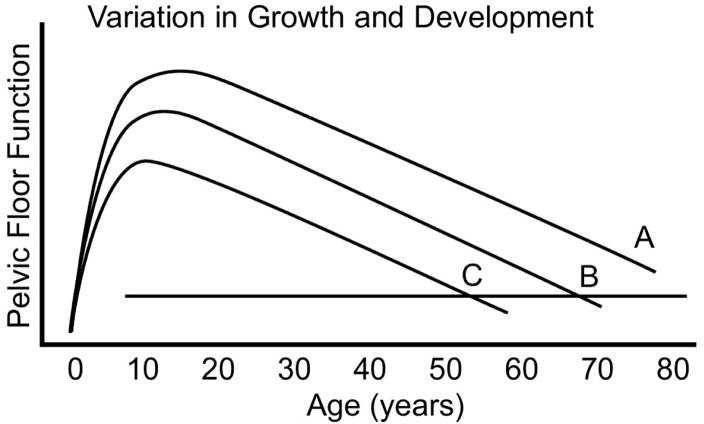
There are normal variations in initial growth and development of all parts of the body. Variation in height is perhaps the most familiar. Pelvic floor development, like height, is influenced by factors such as an individual’s genetic code, nutrition, and environment. An individual who develops excellent pelvic floor function may never have sufficient deterioration to develop PFD symptoms throughout her lifespan, despite inciting or lifestyle events. However, another woman whose growth and development are less robust may become symptomatic during her lifetime solely on the basis of normal age-related decline (Figure 2). Conceptually then, the role of genetics must be considered in the analysis of PFD causation.
Figure 2. Phase I Variation in Growth and Development.
Differences in the degree of functional reserve development during early life, and the effect it can have on when symptoms may develop are demonstrated. An individual with more functional reserve (A) may not develop symptoms during her lifespan while somewhat less reserve may lead to symptoms late in life and even less reserve, symptoms early in life. (© DeLancey 2007)
Phase II: Inciting factors
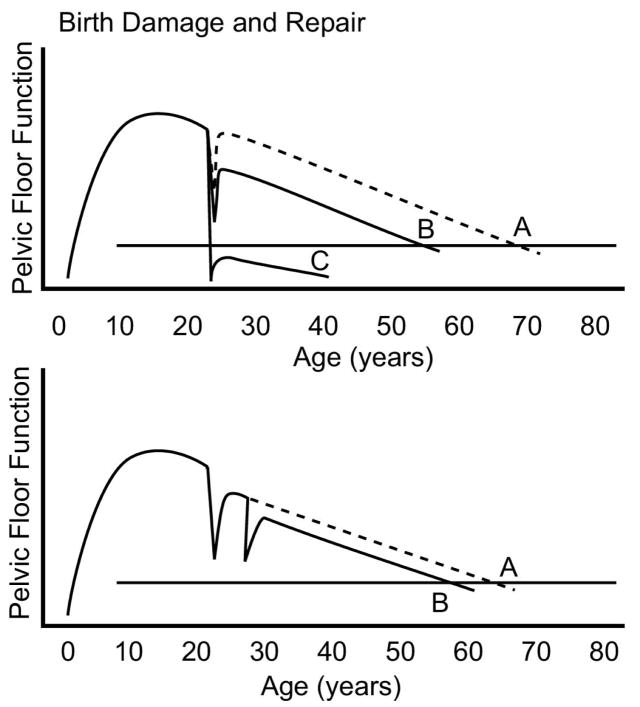
The pelvic floor may be altered by events such as pregnancy and childbirth. No other part of a woman’s body has such dramatic and dynamic changes than the pelvic floor during the second stage of labor. The degree of pelvic floor injury and recovery is shown in Figure 3. Birth of a small baby (e.g., 2,400 grams) may cause few changes in pelvic floor structures, while delivery by forceps of a 5,000 gram infant may cause considerable changes, and even damage. If little damage occurs a woman’s body may completely recover and she may not experience symptoms of pelvic floor disorders. Even with substantial, dynamic changes to the pelvic floor during childbirth, return to normal functional capacity occurs for most women. The fact that one woman can deliver several large babies without developing PFD while another can have significant problems after a single birth, or a relatively small infant, indicates how little we know about this process. Depending on Phase I events, a woman may have no increase in the risk of developing pelvic floor disorders, despite the experience of potentially inciting events.
Figure 3. Variations in Birth Damage and Repair.
Different degrees of functional impairment after vaginal birth are shown. A delivery that does not permanently injure any structure beyond the body’s ability for repair (A) may not affect an individual’s development of symptoms later in life. A more significant injury (B) that is partially healed may decrease functional reserve leading to earlier occurrence of symptoms. Severe injury (C) may cause immediate problems that cannot be repaired, leading to symptomatic disease following birth that does not resolve. A second birth may further affect the rate of decline, depending again on degree of cumulative injury. (© DeLancey 2007)
In another instance with the delivery of a macrosomic infant by forceps, there may be types of injury that cannot be fully recovered from. Permanent nerve injury, avulsion of a muscle, and rupture of connective tissue under the skin are examples of injuries that lead to lasting changes in the pelvic floor. Because the pelvic floor has excess capacity in young women, this damage may not become immediately evident in all cases, however the loss of capacity, when added to deterioration with advancing age, can lead to symptoms later in life. Certain instances, for example neuromuscular damage to the urethra or its connective tissue supports, may result in immediate symptoms.
To date, the cause of injury and pattern of recovery revealing the underlying origin of lasting structural damage has not been well elucidated. It is possible to presume that if a torn muscle, myogenic injury, were to occur the defect would be visible immediately and persist. In contrast, a neurogenic injury in a muscle may appear normal early on, but then degenerate over time due to denervation-related atrophy (7). However, there is limited understanding of the varied recovery processes. What is certain is that, for most women, the experience of childbirth and, in particular, vaginal birth is a lifespan event from which they initially recover. The Lifespan Model for PFD, however, offers a method of conceptualizing the process of initial recovery and subsequent PFD as well as the opposite: no injury, yet subsequent PFD. The model challenges our research efforts to broadly consider the specific events or factors within the larger context and reality of a woman’s lifespan in order to enhance our ability to accurately identify prevention and treatment strategies.
There are several aspects of childbirth that have been cited as factors leading to PFD, but all relate to the amount of injury that occurs and the ability of the body to recover from these injuries. Whether a woman sustains injury is influenced by her pelvic floor structure (e.g., pelvic size and shape), strength of her soft tissues (e.g., fragile or strong), size of the baby (e.g., small or large), mechanism of delivery (e.g., occipito-anterior or -posterior), obstetrical interventions (e.g., forceps delivery), and potentially the management of second stage labor. It is necessary to be clear about which factors might be causative (e.g., large infant), and which factors are markers of difficult birth (e.g., anal sphincter laceration). For example, obstetrical management of end stage bradycardia that requires performance of an episiotomy and subsequent manual manipulation to accomplish delivery may also result in a fourth degree extension. In addition to obstetrical factors, events such as spinal cord injury or hysterectomy might also play an inciting role.
To gain a more detailed understanding of birth-induced injury, the type of injury (e.g., muscle impairment) and the mechanism of injury (stretch induced avulsion vs. nerve compression vs. nerve stretch) must be determined. Each of these factors will lead to different approaches for intervention and the degree to which the body can recover from these injuries needs to be established. For example, if the length of time a nerve is compressed is believed to be the mechanism of injury, it might lead to forceps to shorten the second stage. If, however, it is the rate and degree of nerve stretch that is most often actually responsible, then adding forceps may in fact decrease the length of the second stage, but increase injury. For this reason, the exact nature of the injury must be carefully considered.
Phase III: Intervening factors
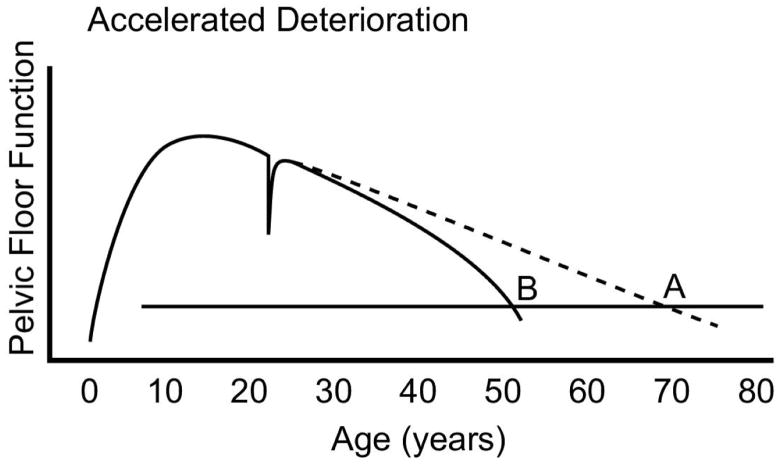
We have reviewed the development of initial pelvic floor functional reserve and the pelvic floor injury and recovery that occur during vaginal birth. Most women live more than half their life after reproduction has been completed and it is during these 40 to 50 years when the rate of pelvic floor decline influences the likelihood of experiencing pelvic floor disorders. Pelvic organ prolapse, for example, increases with age (1). Several factors influence the rate at which decline in pelvic floor function occurs and it should be noted that the effect of these factors may not occur for many years after they begin to influence pelvic floor function. The more rapidly age-related impairment occurs, the sooner symptom threshold occurs (Figure 4). For example, a woman may have a normally developed pelvic floor and may not have any birth related damage, yet may develop pelvic floor disorder as she ages. For any individual, the rate of aging may vary. These genetically programmed variations of aging influence when a woman reaches the symptom threshold.
Figure 4. Accelerated Deterioration.
Several factors may affect the rate at which pelvic floor function deteriorates. Genetic factors that affect rates of connective tissue and muscle aging, and changes that are influenced by an individual’s activity level or nutritional factors (illustrated by the B curve in the graphic) are a few examples. (© DeLancey 2007)
The normal decline of the pelvic floor across the lifespan may be influenced by other factors. Chronic constipation is an example of a condition that affects pelvic floor load by increasing stress and strain over time. Obesity is associated with increased rates of pelvic floor disorders independent of other associated factors. Finally scurvy, though rare today, impacts connective tissue integrity and such treatments as chronic steroid use can accelerate loss of connective tissue reserve. Generalized problems such as diabetes may affect peripheral motor and sensory nerves. Increased loading, such as occupations that involve heavy lifting or conditions that elicit heavy coughing, such as chronic bronchitis, may result in a type of “repetitive motion” trauma that challenges the pelvic floor. Any of these factors, or combination of factors, can accelerate loss of pelvic floor function over the course of a woman’s lifespan.
Lifestyle challenges to pelvic floor function
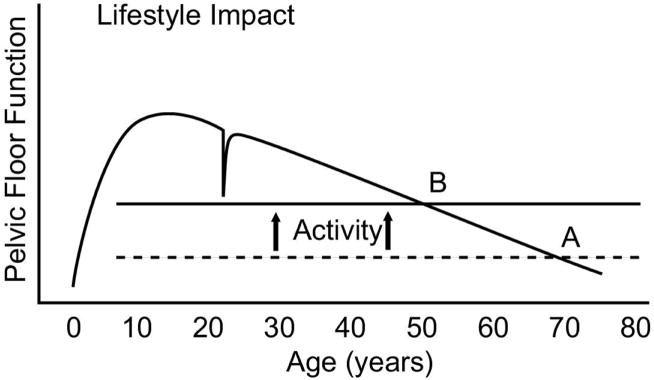
Lifestyle also affects development of pelvic floor disorder. For example, there may be two identical twin sisters who have exactly the same genetic make up, aging process, and physical make up including equal pelvic floor muscle, connective tissue and nerves. If one is an aerobics instructor she may experience urinary incontinence because her lifestyle places more demands on pelvic floor function than her sister who leads a sedentary lifestyle. In this way, the symptom threshold may be quite different despite the similarity in their background; however, increased activity levels may alter that threshold (Figure 5). These activities may not actually damage the pelvic floor, but will require more robust function to avoid symptoms. Paradoxically, these activities may actually strengthen the pelvic floor in healthy women. In women with existing damage, however, it may also lead to forces beyond the capabilities and capacity of the pelvic floor muscular and, in these predisposed women, may lead to increased damage.
Figure 5. Lifestyle Impact.
The stresses placed on the pelvic floor vary from one individual to another. For example, a sedentary individual may never have stress incontinence where one that participates in high-impact aerobics may develop this condition. Thus, two women having the same functional capacity may reach symptoms earlier or later depending on variance in threshold of activities (B activity line versus A activity line in the graphic). (© DeLancey 2007)
Conclusion
The Lifespan Model presented here would suggest that the focus on preventive strategies should be on more refined identification of PFD risk in an individual woman as opposed to universal recommendations for all women. For example, focusing on specific events during the second stage management in individual women to reduce risk of muscle or nerve injury may result in successful prevention strategies that avoid exposing women who will not benefit to interventions such as cesarean section. In addition, improved understanding of predisposing factors such as the in-born structure and function of the pelvic floor connective tissue, or intervening factors such as chronic lifting or obesity, may aid in the development of measures to identify women at increased risk for injury during birth or to reduce the risk of pelvic floor disorders after a birth event.. In the end obstetrical events must be put in context of predisposing factors such as the inborn reserve of the muscles and ligaments before childbirth and the intervening factors that occur during the 40 to 50 years of life after delivery.
For PFD, a dynamic, multifaceted, potentially chronic health condition, the Lifespan Model of PFD, as we have described, may help highlight opportunities for research that can lead to primary and secondary prevention of this significant women’s health problem. The model described here is an approach and not a solution. It seeks to amplify the activities of the growing number of investigators focusing on PFD with a goal towards the development of prevention and improved treatment strategies. Because the pelvic floor is a multifaceted unit with many different tissues and PFD can be so disruptive to quality of life, researchers from varied disciplines and with a variety of areas of expertise are needed to create improved interdisciplinary approaches to PFD. It is hoped that this article can form a unifying model to help integrate the many important contributions needed to improve care for the 300,000 women each year who require surgery for pelvic floor disorder. Eventually, as data become available, it may be possible to quantify the degree to which each of these factors contributes to development of pelvic floor disorders.
Table 1.
Lifespan Phases and a few examples of potential causal factors
| Phase I: Predisposing Factors |
| Genetic constitution |
| Nutritional factors |
| Socialization (toilet training) |
| Phase II: Inciting Factors |
| Predisposing Maternal/Fetal Factors |
| Pelvic floor shape and size |
| Macrosomic Infant |
| Fetal Head Position |
| Effects of Obstetrical Interventions |
| Forceps |
| Prolonged 2nd stage |
| Occipito-posterior |
| Mechanism of injury |
| Muscle avulsion |
| Connective tissue rupture |
| Nerve avulsion |
| Nerve compression |
| Phase III: Intervening Factors |
| Variation in normal aging involving muscles, connective tissue and nerves from one individual to another (e.g., skin wrinkles) |
| Increased stresses on the pelvic floor (e.g., occupational lifting, obesity or chronic cough) |
| Factors that lead to weakening of the support tissues (e.g. chronic steroid use or disuse atrophy of muscles) |
| Lifestyle factors effect on symptoms (e.g., high impact aerobics, situations with restricted bathroom use) |
Acknowledgments
We gratefully acknowledge investigator support from the Office for Research on Women’s Health’s SCOR on Sex and Gender Factors Affecting Women’s Health and the National Institute of Child Health and Human Development through grants P50 HD044406 that has made this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188:108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 3.Boyles SH, Weber AM, Meyn L. Procedures for urinary incontinence in the United States, 1979–1997. Am J Obstet Gynecol. 2003;189:·70–5. doi: 10.1067/mob.2003.376. [DOI] [PubMed] [Google Scholar]
- 4.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007 Mar 24;369(9566):1027–38. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 5.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin N Amer. 1998;25:723–46. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 6.Kuh D, Hardy R, editors. A Life Course Approach to Women’s Health. Oxford University Press; 2002. [Google Scholar]
- 7.Fleckenstein JL, Watumull D, Conner KE, Ezaki M, Greenlee RG, Jr, Bryan WW, Chason DP, Parkey RW, Peshock RM, Purdy PD. Denervated human skeletal muscle: MR imaging evaluation. [Journal Article. Research Support, Non-U.S. Gov’t. Research Support, U.S. Gov’t, P.H.S.] Radiology. 1993 Apr;187(1):213–8. doi: 10.1148/radiology.187.1.8451416. [DOI] [PubMed] [Google Scholar]