Abstract
Compelling evidence continues to emerge suggesting that the glycocalyx surface layer on vascular endothelial cells plays a determining role in numerous physiological processes including inflammation, microvascular permeability, and endothelial mechanotransduction. Previous research has shown that enzymes degrade the glycocalyx, while inflammation causes shedding of the layer. To track the endogenous recovery of the glycocalyx in vivo, we used fluorescent micro-particle image velocimetry (µ-PIV) in mouse cremaster-muscle venules to estimate the hydrodynamically relevant glycocalyx thickness 1, 3, 5, and 7 days after enzymatic or cytokine-mediated degradation of the layer. Results indicate that after acute degradation of the glycocalyx, 5–7 days are required for the layer to endogenously restore itself to its native hydrodynamically relevant thickness in vivo. In light of these findings, and since demonstrable evidence has emerged that standard cell-culture conditions are not conducive to providing the environment and/or cellular conditions necessary to produce and maintain a physiologically relevant cell-surface glycocalyx in vitro, we sought to determine if merely the passage of time would be sufficient to promote the production of a hydrodynamically relevant glycocalyx on a confluent monolayer of human umbilical vein endothelial cells (HUVECs). Using µ-PIV, we found that the hydrodynamically relevant glycocalyx was substantially absent 7 days post-confluence on HUVEC-lined cylindrical collagen microchannels maintained under standard culture conditions. Thus it remains to be determined how a hydrodynamically relevant glycocalyx surface layer can be synthesized and maintained in culture before the endothelial-cell culture model can be used to elucidate glycocalyx-mediated mechanisms of endothelial-cell function.
Keywords: cell culture, glycocalyx, mechanotransduction, microcirculation, vascular inflammation, vascular permeability
The endothelial-cell glycocalyx is currently the focus of intensive investigation, and significant progress has recently been made to determine its composition, function, and clinical relevance. Located on the apical surface of blood vessel endothelial cells, the glycocalyx has been implicated in a wide range of mechanisms and pathologies, including vascular permeability, inflammation, atherosclerosis, and diabetes1–5. The endothelial glycocalyx has been studied using a variety of imaging methods in vivo, ex vivo, and in vitro. One of these methods, referred to as microviscometry, involves the hydrodynamic analysis of micro-particle image velocimetry (µ-PIV) data6–10. Another method uses electron microscopy of perfusion-fixed tissue11–13. Both methods have been applied to measure a physical dimension of the glycocalyx and both clearly demonstrate that the endothelial glycocalyx observed in vivo (ex vivo) is substantially absent in vitro.
The accompanying Research Commentary of Chappell et al.13 provides additional evidence that suggests that the surface glycocalyx on cultured HUVECs in vitro is deficient when compared with similar measurements made ex vivo, which is consistent with earlier findings by these investigators using similar methods12. Results of Chappell et al.13 also corroborate earlier findings of Potter and Damiano9, which were based on their microviscometric analysis of the hydrodymanically relevant thickness of the endothelial glycocalyx in vivo and in vitro. In addition to their measurements of glycocalyx thickness, Chappell et al.13 used immunohistological staining of heparan sulfate and syndecan-1 to provide insight into the molecular structure of the glycocalyx ex vivo and in vitro. While the methods employed by Potter and Damiano9 allow for interrogation of the glycocalyx in vivo and in live tissue culture in vitro, the methods of Chappell et al.13 provide information about molecular composition of the glycocalyx in the same perfusion-fixed cell type ex vivo and in vitro. Despite the very different experimental approaches taken in these two studies, the qualitative and quantitative consistency of their findings both in vivo (ex vivo) and in vitro is quite remarkable.
In particular, Chappell et al.13 reported a mean glycocalyx thickness of 0.8–0.9 µm in perfusion-fixed ex vivo specimens taken from umbilical veins immediately after caesarean birth, and ≈0.03 µm in perfusion-fixed HUVECs in vitro. By comparison, Potter and Damiano9 reported a hydrodynamically relevant glycocalyx of 0.5–0.6 µm in vivo and ≈0.03 µm in steadily perfused live HUVECs in vitro. The explanation for this deficiency of the glycocalyx in the endothelial culture model is currently a matter of speculation. One possibility suggested by Chappell et al.13 is that the isolation and preparation of endothelial cells for culture may be responsible for the observed deficiencies in the glycocalyx in vitro. They further go on to say that the time required for the glycocalyx to reconstruct itself in vivo is not known, nor is it known if it is able to do so in vitro. It is precisely these two points that this study seeks to address. In particular, we use microviscometric analysis of µ-PIV data obtained from mouse cremaster-muscle venules to track the recovery time course of the hydrodynamically relevant glycocalyx thickness in vivo at regular intervals for up to 7 days after either enzymatic or cytokine-mediated degradation of the glycocalyx is brought about acutely on Day 0. This recovery time course is compared with the progression, over the same time period, of systemic white blood cell (WBC) count, which is used as a marker of inflammation. The time required for the hydrodynamically relevant glycocalyx to make a full recovery in vivo is then applied to cultured endothelial cells to determine if a hydrodynamically relevant glycocalyx would be observed provided a similar incubation period were allowed to elapse post-confluence.
Materials and Methods
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were cultured at 37°C in 5% CO2 in M199 (Invitrogen) and supplemented with 2 nM l-glutamine, 100 U/mL penicillin, 100 µg/ml streptomycin (1% GPS, Invitrogen), 20% heat-inactivated fetal bovine serum, 5 U/ml heparin (Sigma-Aldrich, Co.), and 25 µg/ml endothelial mitogen (ECGS, Biomedical Technologies). The HUVECs were passaged with trypsin (Fisher Scientific) and cultured in gelatin-coated tissue culture dishes. Cells were discarded after passage six.
Imaging and µ-PIV
Micro-particle image velocimetry (µ-PIV) was performed using a previously described technique6–9. Fluorescent microspheres were visualized using stroboscopic double-flash (1–9 ms apart, DPS-1 Video, Rapp Opto Electronic) epi-illumination on a Zeiss microscope (Axioskop II, Carl Zeiss, Inc., Thornwood, NY), and recordings were made with a CCD camera (Sensicam QE, Cooke, Inc.) connected to a G5 Power Mac (Apple, Inc.). A Dualview (Optical Insights, Inc.) beam splitter separates infrared transillumination from the fluorescent microsphere image, allowing for simultaneous acquisition of the vessel or channel wall and the microspheres. The midsagittal plane was defined as corresponding to the focal plane at which the contrast of the edge of the intraluminal wall reversed6, 14.
Images were captured and processed with IPlab (BD Biosciences) and analyzed with the public domain ImageJ program (http://rsb.info.nih.gov/ij/) as described6, 15. The flash-time interval for µ-PIV recordings was chosen such that the two images for a given microsphere were ≈3–10 µm apart. The collagen microchannel data were collected with both 40× and 63× saline immersion objectives (NA 0.8 and 1.0, respectively) to accurately capture both center-stream and near-wall microspheres. The distance between these two images, and the shortest distance between the microsphere center and the vessel wall, were measured for at least 40 microspheres in each venule or collagen microchannel. Since the velocity profile is known to be monotonically decreasing with increasing radial position, a microsphere in the midsagittal plane travels faster than any other microsphere at that measured radial location. In light of this, and considering that not all of the recorded microspheres travel in the midsagittal plane of the vessel, only the fastest microspheres at a given measured radial location were considered. As such, all µ-PIV data sets were filtered to satisfy this monotonicity condition as previously described6, 7, 9. Only monotonically filtered data were included in the analysis.
Intravital Experiments
All animal experiments were conducted under a protocol approved by the Boston University Institutional Animal Care and Use Committee (Protocol #2474). Wild-type (WT) male mice (C57Bl/6) were obtained from Charles River Labs (Wilmington, MA). All mice appeared healthy and were between 8 and 14 weeks of age.
For Day 1–Day 7 experiments, enzymes were administered via a single tail-vein bolus injection consisting of either 100 U hyaluronidase (Sigma-Aldrich Inc.) or 1 U heparinase III (Sigma-Aldrich Inc.) in 100 µL saline. For Day 0 experiments, a single bolus of either 100 U hyaluronidase, 1 U heparinase III, or 100 U denatured hyaluronidase in 100 µL saline was administered via a carotid cannula. Hyaluronidase was denatured by heating a 1 U/µL solution to 90°C for 10 minutes. The enzyme solution was then cooled to room temperature and used on the same day it was prepared. For cytokine treatments, 0.2 µg tumor necrosis factor-α (TNF-α) in 125 µL saline was administered via a single scrotal injection while the animal was briefly anesthetized. Blood was extracted via the lateral tail vein for manual WBC counts using a hemocytometer (Fisher Scientific Inc.).
To estimate the hydrodynamically relevant glycocalyx thickness in mouse cremastermuscle venules, the mouse (25–30g) was initially placed in a small-rodent induction chamber for ≈2 minutes where 3% isoflurane mixed with oxygen was delivered through an anesthesia machine (SergiVet 100 series vaporizer) at a flow rate of 500 cc/min. Once sedated, the mouse was placed on a custom-heated stage where anesthesia was maintained with 1.5% isoflurane in oxygen through endotracheal intubation. The respiration rate and tidal volume were controlled by a ventilator (Inspira ASV, Harvard Apparatus) and auto-adjusted based on the animal’s body mass. A carotid cannula was placed for administration of the microsphere solution, enzyme solutions, and a P-selectin antibody (RB40.34, BD Biosciences). The cremaster muscle was prepared for intravital microscopy as described16. Briefly, the cremaster muscle was exteriorized, pinned to the stage, and superfused with thermocontrolled bicarbonate-buffered saline equilibrated with 5% CO2 in N2 at 36–38°C.
Intravital microscopic observations were made on a Zeiss microscope (Axioskop II, Carl Zeiss, Inc., Thornwood, NY) with a 63× saline immersion objective (NA 1.0). A small volume (< 0.03 ml) of polychromatic red microspheres (1.75±0.055 µm, ρ =1.05 g/cm3, Polysciences, Inc., Warrington, Pennsylvania) was slowly injected through the carotid cannula until 10–20 microspheres per second passed through the vessel. Measurements were not included if rolling or firmly adherent leukocytes were present anywhere within the 15-µm measurement window.
EC-Lined Collagen Microchannel Experiments
The flow chamber and in vitro culture system consist of an endothelial-cell (EC)-lined cylindrical microchannel (with a circular cross section) through a collagen gel contained within a cured elastomer scaffold17. Both ends of the microchannel were immersed in media reservoirs, which were connected, via ployethylene tubing, to culture dishes filled with media.
The microchannels were constantly perfused with media via a static gravity-induced pressure head. Culture media flowed from the tissue culture dish feeding the microchannel to another culture dish, which collected the media and was located 5 cm below the feeding culture dish. At confluence the channels were ≈110–150 µm in diameter. The culture media was supplemented with 4% 70 kDa Dextran to increase the viscosity of the media and thereby reduce the amount of media consumed. Between experiments, the channels remained in an incubator at 37°C in 5% CO2.
The µ-PIV data were collected after Fluoresbrite YG microspheres (0.47±0.01 µm, ρ = 1.05 g/cm3, Polysciences, Inc., Warrington, Pennsylvania) were added to the culture media. The microchannels were thermocontrolled with the same superfusion appartatus used in the in vivo experiments. The superfusion solution was constantly pooled and aspirated away on the glass surface of the microchannel chamber and was maintained at 23–24°C. Data collection for a single collagen microchannel typically required 15 minutes.
Analytical Methods
Analysis of µ-PIV data in vivo and in vitro was identical to methods described in Potter and Damiano9. Briefly, the velocity profile over the vessel or microchannel cross-section was extracted directly from fluid-particle velocities estimated from the µ-PIV data, as described previously7–10. Using a fitting function for the velocity profile that consists of a constant term, a quadratic term, and a growing and a decaying exponential term, a nonlinear regression analysis is employed to determine the values of two constants that minimize the least-squares error in the fit to the monotonically filtered fluid-particle velocity data. Further details regarding this regression analysis are given by Damiano et al.17. The variation, relative to a trial glycocalyx thickness, in the least-squares error associated with the fit to the fluid-particle velocity data tracks the “quality” of the fit relative to each trial thickness. The minimum in this variation in the least-squares error corresponds to the best fit of all trial thicknesses and provides our calculated estimate for the hydrodynamically relevant glycocalyx thickness.
Statistical Analysis
Data are expressed as mean ± one standard deviation. ANOVA analysis was used to determine if a statistically significant variation exists within a group, where a group includes all of the untreated control data and all of the data from one post-treatment group (e. g. all of the data from Day 0, 1, 3, 5, and 7 in the case of enzyme treatments and Day 1, 3, 5, and 7 in the case of cytokine treatments). A difference arising from a comparison between data from the untreated control data and data from different days within a group was determined via a post hoc Tukey test and regarded as statistically significant if p < 0.05. When a group included untreated control data and data from only one day post-treatment (e.g. the comparison between the untreated control group and the group treated with denatured-hyaluronidase), a difference was determined via a two tailed student t-test and regarded as statistically significant if p < 0.05.
Results and Discussion
Recovery Time Course of the Glycocalyx in vivo
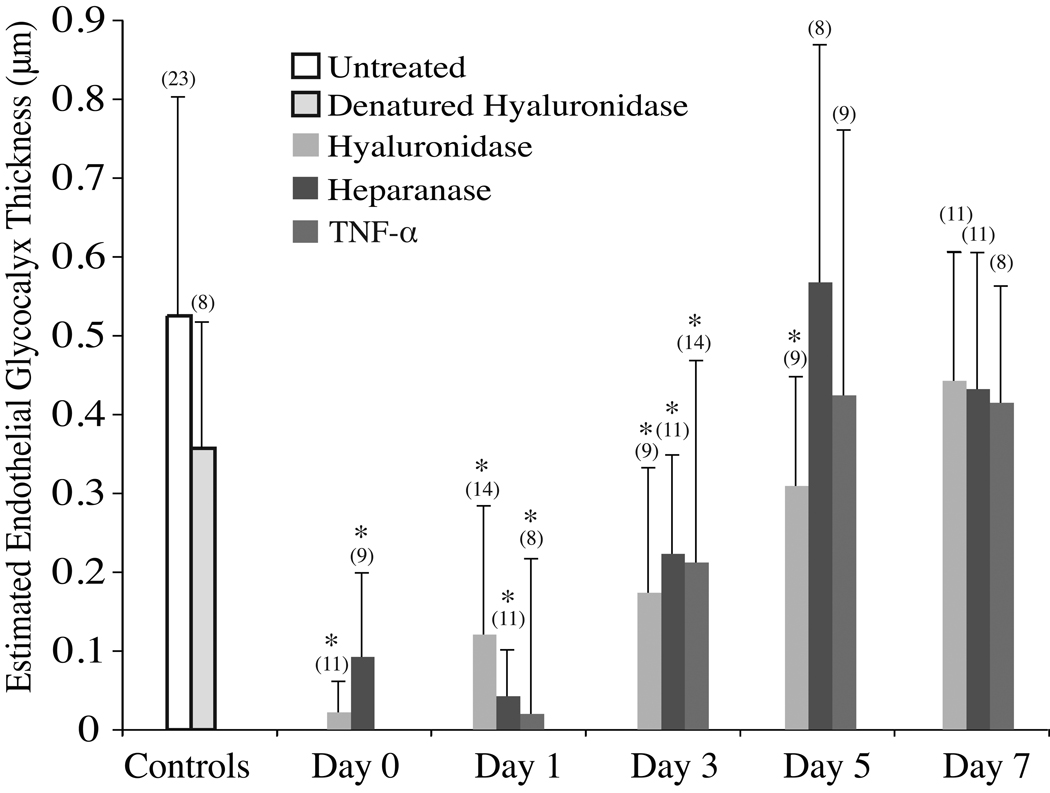
Representative monotonically filtered µ-PIV data sets obtained from cremaster-muscle venules of WT mice are shown in Figure 1 at a series of discrete time points (up to 7 days) after the administration of a single bolus injection on Day 0 of either hyaluronidase, heparinase III, or TNF-α. The corresponding velocity profile extracted from each µ-PIV data set is also shown in each panel of Figure 1 as is the maximum viscous shear stress, |τmax| (arising at the plasma–glycocalyx interface), the vessel diameter, and the estimated hydrodynamically relevant glycocalyx thickness. Results show that for all three treatments, a steady increase in the hydrodynamically relevant glycocalyx thickness occurs over the course of 5–7 days. This trend is supported with statistical significance when microviscometric analysis is applied to µ-PIV data obtained from groups of Day-0, 1, 3, 5, and 7 mice in each of the three treatment protocols (see Figure 2). The mean hydrodynamically relevant glycocalyx thickness was found to be significantly different from, and less than, the untreated control group for up to 3 days after TNF-α treatment and enzyme treatment to degrade the glycocalyx. By Day 7, the mean hydrodynamically relevant glycocalyx thickness recovered in all three treatment groups so as to be insignificantly different from the control group. Furthermore, treatment with denatured hyaluronidase resulted in a mean hydrodynamically relevant glycocalyx thickness that was insignificantly different from the control group.
Figure 1.
Representative velocity profiles from mouse cremaster-muscle venules in vivo corresponding to each experimental condition. Dots represent monotonically filtered µ-PIV data and each curve corresponds to the velocity profile found using a nonlinear regression analysis which minimizes the least-squares error in the fit to the µ-PIV data. The shaded region (if present) represents the hydrodynamically relevant glycocalyx. The vessel diameter, D, hydrodynamically relevant endothelial glycocalyx (EG) thickness, and shear stress, |τmax|, at the plasma–glycocalyx interface are indicated for each experiment (note, vessels shown vary from 22.8–57.4 µm in diameter).
Figure 2.
The mean hydrodynamically relevant glycocalyx thickness determined by microviscometric analysis of µ-PIV data obtained from mouse cremaster-muscle venules in vivo before and 0, 1, 3, 5, and 7 days after a single intravenous bolus injection of either hyaluronidase, heparinase III, TNF-α, or denatured hyaluronidase (Day 0 only). Experiments were performed with 49 mice in 23 untreated control vessels (20–100 µm ID) and 143 treated vessels (18–66 µm ID). Vessels were analyzed from 5 WT mice for each day and treatment combination, except for the Day 0 hyaluronidase group and the untreated control group, which included vessels from 5 and 8 animals, respectively. Asterisks denote significant difference from untreated control vessels via a two-tailed unpaired t-test (p < 0.05). Day-0 experiments involving enzyme-treated vessels were preformed one hour after enzyme administration via a carotid cannula. For later time points, the enzyme was administered via a single tail-vein injection and the cytokine was administered as a single scrotal injection on Day 0. Note, in untreated control venules the mean glycocalyx thickness was found to be 0.52±0.27 µm, and ~5–7 days was required for the mean hydrodynamically relevant glycocalyx thickness to fully recover after either enzymatic or cytokine degradation. The number of experiments conducted in each experimental group is indicated in parentheses.
While these results suggest that 5–7 days is required for the glycocalyx to make a full recovery after various methods of degradation, determinants of this recovery rate are unknown at the present time, and could be influenced by a variety of direct and/or indirect factors. Certainly, the rate of hyaluronan and proteoglycan synthesis must be a determinant; however, the kinetics of hyaluronan synthase renders any scenerio in which synthesis is the rate-limiting step unlikely, since hyaluronan synthase is capable of producing a hyaluronan molecule of 240 kDa within ≈5 minutes19. Unless the synthesis rate decreases as the molecular weight increases or the expression of hyaluronan synthase is low, it seems likely that an endothelial cell should be able to produce enough hyaluronan to consitute a hydrodynamically relevant layer within 24 hours. On the other hand, the acute trauma brought about by enzymatic degradation of the glycocalyx may initiate a state of systemic inflammation that could compete with glycocalyx synthesis and thus prolong the recovery process long enough to account for the extended 7-day progression observed here. Other rate-limiting factors might include the role of secretory organs, such as the liver. Since components of the endothelial glycocalyx aid in binding and neutralizing potentially toxic substances, such as LDL20, glycocalyx degradation could lead to a systemic build up of oxidative substances, which, in turn, could have deleterious consequences on the liver and other organs that could indirectly feedback on the glycocalyx and retard its recovery. Furthermore, the mere presence of systemically elevated blood-borne free radicals would be a direct agonist for glycocalyx shedding. If these factors are indeed determinants of the glycocalyx recovery rate, then recovery after local, rather than systemic, degradation could be faster; such speculations, however, remain to be tested.
Relationship Between Glycocalyx Recovery and Systemic WBC Count
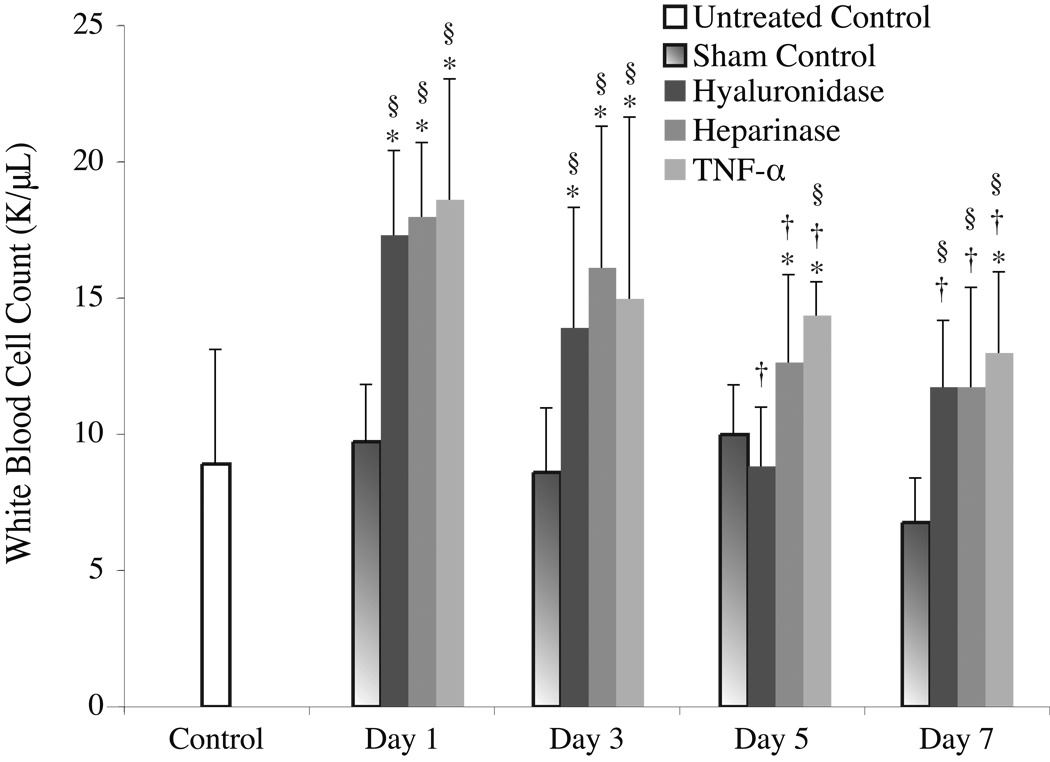
Evidence in support of the hypothesis that glycocalyx degradation is linked with inflammation is provided in Figure 3, which shows a two-fold elevation in systemic WBC count relative to control one day after any of the enzyme or cytokine treatments. Neutrophilia is a classical indicator of acute inflammation arising from, among other causes, infection or acute trauma21, 22. Acute and rapid increases in WBC count are generally indicative of a drug, disease, toxin, or trauma that has produced an inflammatory reaction21. Although nonspecific, the observed rapid elevation in WBC count, along with its slow return toward baseline over the 7 days following enzymatic degradation, is consistent with the rapid onset of glycocalyx shedding and subsequent recovery time course of the glycocalyx over the same time period. While shedding of the glycocalyx may cause leukocyte attachment and rolling, which tends to decrease the number of circulating WBCs in the short term, inflammatory stimuli can initiate rapid mobilization of WBCs from the bone marrow. The exact time scales associated with these competing phenomena are not known for this particular process, but the present data suggest that a net accumulation of WBCs into the vascular space occurs within one day after the enzyme or cytokine challenge.
Figure 3.
Systemic WBC count measured in 20 WT mice before (Day 0) and after administration with either either 100 µL saline, 100 U hyaluronidase, 1U heparinase III, or 0.2 µg of TNF-α. Each substance was administered to 5 WT mice, and each mouse was checked 1, 3, 5, and 7 days after a single intravenous or scrotal (TNF-α only) bolus injection. After administration, the WBC count of all treatment groups were significantly higher than the baseline for the length of the experiment except for hyaluronidase treated mice, which were not statistically different from control on Days 5 and 7. Similarly all experimental groups had a statistically significantly higher WBC count than the sham control over all seven days, except for the hyaluronidase- and heparanase-treated groups on Day 5 only. Relative to Day 1, the WBC count of all treatment groups fell significantly over time. Note, the * indicates a statistically significant difference (p < 0.05) from the untreated control, the † indicates a statistically significant difference from that group’s corresponding Day-1 WBC count, and the § indicates a statistically significant difference from the sham control on that day.
One explanation for these observations might be that the enzyme treatment acutely initiates an inflammatory response, which simultaneously activates leukocytes and causes shedding of the glycocalyx. However, in light of the short half life of these enzymes in blood23, 24, it is hard to reconcile how the elevated WBC count could be sustained for a period of days unless some other underlying pathology were dragging out the process. An alternative and far more profound explanation for these observations might be that enzymatic degradation of the glycocalyx results in trauma to the blood vessel wall, which sets up a wound-healing response. In this case, it is not the mere presence of the enzyme in the blood that is causing the inflammatory response, but rather it is the fact that the enzyme rapidly degrades the glycocalyx leaving a denuded endothelium in its wake. In this scenario, the tissue trauma would initially result in leukocyte rolling and adhesion, which is dependent on P-selectin via a mast-cell dependent process25–27. However, soon thereafter, possibly within a matter of minutes23, 24, the enzyme is cleared from the blood, but the injury to the endothelium remains, and the mere absence of glycocalyx alone is sufficient for the endothelial cells themselves to continue to mount an inflammatory response. In essence, we may be observing a wound-healing response that takes place over the course of a week, over which time the glycocalyx recovers. Associated with this trauma is an elevation in the systemic WBC count, which is typical of an immune response that mounts in the presence of a wound. In this case, the wound occupies the entire luminal endothelial-cell surface exposed to blood.
While speculative, if this interpretation of these observations is correct, it has important implications for a variety of vascular disease processes including atherosclerosis and hyperglycemia in diabetes. Recurring disruption of the glycocalyx as a result of, for example, exposure to reactive oxygen species, such as hyperphysiological levels of the superoxide anion, which is known to be elevated in hyperglycemia28, 29, or dietary factors that could lead to elevated levels of oxidized low density lipoproteins in the blood, could result in cumulative trauma to the endothelium locally or systemically. While isolated transient occurrences of such injuries may be benign, the cumulative effect of repeated insults to the glycocalyx, along with the resulting injury to the underlying endothelium, may be at the root of many chronic vascular disease processes. For example, atherosclerosis is now understood to be a disease precipitated and propagated by inflammation. Risk factors for atherosclerosis, such as consuming a high-saturated-fat diet, smoking, hypertension, hyperglycemia, obesity, or insulin resistance, can initiate the expression of adhesion molecules by endothelial cells, thus allowing the attachment of leukocytes to the arterial wall30. It would seem that a necessary precipitating event to this pathological cascade, however, is that these factors must first degrade or compromise the glycocalyx sufficiently to allow leukocytes initial access to adhesion molecules on the endothelium6, 31, 32. Such a scenario reinforces the importance of the inflammatory barrier role of the endothelial glycocalyx in vascular health and disease.
Implications of Glycocalyx Recovery for Cell Culture
While these results clearly have important implications in vivo, they impact endothelial-cell culture studies as well. The recent microviscometric data from Potter and Damiano9, and the supporting electron microscopy data in perfusion-fixed tissue from Chappell et al.13, provide compelling evidence that standard cell-culture conditions are not conducive to providing the environment and/or cellular conditions necessary for endothelial cells to produce and maintain a physiologically relevant cell-surface glycocalyx in vitro. In order for future cell-culture studies to elucidate in vivo mechanisms of endothelial-cell function that require an intact glycocalyx, it is utterly essential that cell-culture protocols be developed to address the present shortcoming.
Based on the in vivo data presented here, showing an inverse relationship between the extent of glycocalyx recovery and the severity of systemic inflammation (Figure 2 and Figure 3), it is clear that if a physiologically relevant endothelial-cell glycocalyx is to be produced and maintained on cultured endothelial cells, it will evidently be necessary to first rescue the cells from their inflamed state, which inevitably arises after the trypsinisation process to re-suspend the cells prior to harvesting and seeding. When cells are passed, they are typically treated with proteolytic enzymes that separate the cells from the tissue-culture plate. These enzymes very likely cleave proteins that anchor the glycocalyx to the endothelial-cell surface. It is also known that this process inflames the cells as well, since cultured endothelial cells are subjected to an inflammatory stimulus at passage via their protease activated receptors33. Therefore, when cells are first plated they are likely to be severely inflamed with little or no endothelial glycocalyx on their surface.
The in vivo time course of glycocalyx recovery reported here is suggestive of a lower bound on the time required of post-confluent endothelial cells to synthesize a hydrodynamically relevant cell surface glycocalyx in vitro. We therefore sought to determine if the effect of time alone was sufficient to account for the observed deficiency in the endothelial-cell culture model9, 13. To do this, we acquired µ-PIV data from HUVEC-lined cylindrical collagen microchannels (148±18 µm diameter) that were 7 days post-confluence, but otherwise identical to those used in Potter and Damiano9. The mean hydrodynamically relevant thickness of the endothelial-cell surface layer was found not to be significantly different from 0 µm in microchannels after either 1 or 7 days post-confluence (see Figure 4). Allowing the cells 6 additional days to synthesize an endothelial surface layer was not sufficient to produce a hydrodynamically relevant layer. This suggests that there are one or more aspects of standard cell-culture conditions that prevent the formation of a hydrodynamically relevant glycocalyx surface layer. However, endothelial cells in culture produce all of the major components that are commonly thought to comprise the glycocalyx, including hyaluronan34, and heparan sulfate proteoglycans13, 35, as well as components that are thought to be the primary anchoring proteins, including glypican and syndecan−1, −2, and −413, 36–39. Nevertheless, these components fail to assemble themselves into a hydro-dynamically relevant glycocalyx on endothelial cells under standard culture conditions. This could reflect a fundamental defect in the endothelial-cell culture model as it has been shown that, when cultured in vitro, rat lung endothelial cells lack 41% of the proteins expressed on their apical surface in vivo40. Insofar as cultured HUVECs are similar in this regard to rat lung endothelial cells in vitro, it follows to reason that they might be fundamentally different from their in vivo counterparts, or perhaps from all blood endothelial cells in vivo. If such a defect in cultured HUVECs were to interfere with the cell’s ability to produce and maintain a glycocalyx in vitro, it would first be necessary to identify and activate pathways that would enable the cells to achieve this. Beyond this, it might further be necessary to adapt the culture environment to conditions more conducive to production and maintanence of glycocalyx than standard cell culture conditions provide. For example, it may first be necessary to coax them away from their growth-oriented phenotype and towards the non-proliferative and mature phe-notype of the endothelium in vivo. This may involve a shift in culture conditions that would, for example, remove most of the growth factors from the culture media and/or reduce PO2 to a normoxic incubation environment to prevent the production of free oxygen radicals41 that could activate cleavage of the glycocalyx42. It may also be necessary to add to culture media physiological concentrations of some or all of the circulating plasma proteins and glycoproteins (e.g. fibrinogen, fibronectin, albondin, etc.) in order to reconstitute the glycocalyx in vitro43.
Figure 4.
Estimates of the mean hydrodynamically relevant thickness of the endothelial glycocalyx surface layer in cremaster-muscle venules in vivo and in HUVEC-lined cylindrical collagen microchannels in vitro that were cultured for 1 day and 7 days after confluence. Whereas the hydrodynamically relevant thickness of the endothelial glycocalyx in vivo was found to be 0.52±0.27 µm, neither in vitro condition produced a hydrodynamically relevant surface layer with a thickness that was significantly different from zero.
These difficulties notwithstanding, we are not suggesting that an endothelial-cell culture model cannot be developed that possesses a physiologically relevant glycocalyx, but rather it should not be assumed that endothelial cells cultured under standard conditions possess a functional glycocalyx until decisively demonstrated otherwise. Although molecular components of the glycocalyx appear to be present in vitro13, they are evidently not organized in such a way as to provide a hydrodynamically relevant cell surface layer on cultured HUVECs9. Yet in vivo, the hydrodynamically quiescent environment near the endothelial-cell surface revealed by microviscometry has broad and far-reaching implications as it impacts such a diverse spectrum of phenomena relevant to cardiovascular physiology and pathophysiology. As such, it is incumbent upon those seeking to use the cell culture model as a research tool to either validate it as a platform upon which a hydrodynamically and physiologically relevant glycocalyx surface layer can be synthesized and maintained, or to somehow show that the presence of an intact and hydrodynamically relevant glycocalyx is not required to elucidate the particular in vivo mechanism of endothelial-cell function that they wish to examine.
Throughout this study, we have used the hydrodynamically relevant glycocalyx thickness as a means of characterizing the structure in vivo and in vitro. In a recent editorial of the Potter and Damiano9 study, Barakat44 stated that the findings of Potter and Damiano9 “…provide no information on the thickness, composition, or overall state of the glycocalyx layer…” On the contrary, the microviscometric method used by Potter and Damiano does indeed provide quantitative information about glycocalyx thickness. In particular, microviscometric analysis of µ-PIV data determines the hydrodynamically relevant glycocalyx thickness, which, in some applications (e.g. endothelial mechanotransduction, vascular permeability, and transport and exchange of small molecules), may, in fact, be more physiologically relevant and meaningful than the molecular or structural dimension of the glycocalyx measured using direct visualization methods. The hydrodynamically relevant thickness reported by Potter and Damiano9 captures a functional dimension, corresponding to the distance from the endothelium where non-Newtonian hydrodynamic effects dominate. It is much like a viscous boundary layer region in fluid mechanics, and as such, represents a physically real and quantitative characterization of glycocalyx thickness in the literal sense. As it also determines the physical extent of the non-circulating plasma layer bound up in the glycocalyx, it identifies the region over which the transport of small molecules is diffusion rather than convection limited. Furthermore, the hydrodynamically relevant thickness can certainly be used as one metric for the overall state of the glycocalyx, particularly in those instances where the absence of a hydrodynamically relevant layer (such as in culture or after enzyme or cytokine degradation) is indicative of either damage, degradation, or some other structural deficiency. The method does not, however, provide any information into the biomolecular composition of the glycocalyx in vivo. For this it will be necessary to develop staining methods that identify unique molecular constituents of the glycocalyx in conjunction with optical techniques, such as intravital two-photon excited fluorescence microscopy. When combined with microviscometry, such tools would provide new insight into the glycocalyx, by allowing for simultaneous acquisition of the biomolecular composition and hydrodynamical drag properties of the glycocalyx on live endothelial cells in vivo and in vitro.
Acknowledgments
We thank M.L. Smith, K. Ley, and J. Tien for valuable discussions.
Sources of Funding
Supported by NIH grant R01-HL076499.
Footnotes
Disclosures:
None.
References
- 1.Henry CBS, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 2.Platts SH, Duling BR. Adenosine A3 receptor activation modulates the capillary endothelial glycocalyx. Circ Res. 2004;94:77–82. doi: 10.1161/01.RES.0000108262.35847.60. [DOI] [PubMed] [Google Scholar]
- 3.Zuurbier CJ, Demirci C, Koeman A, Vink H. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. Am J Appl Physiol. 2005;99:1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg BM, Spaan JAE, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol. 2006;290:H915–H920. doi: 10.1152/ajpheart.00051.2005. [DOI] [PubMed] [Google Scholar]
- 5.Chappell D, Jacob M, Hofmann-Kiefer K, Bruegger D, Rehm M, Conzen P, Welsch U, Becker BF. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–784. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- 6.Smith ML, Long DS, Damiano ER, Ley K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys J. 2003;85:637–645. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damiano ER, Long DS, Smith ML. Estimation of viscosity profiles using velocimetry data from parallel flows of linearly viscous fluids: application to microvascular haemodynamics. J Fluid Mech. 2004;512:1–19. [Google Scholar]
- 8.Long DS, Smith ML, Pries AR, Ley K, Damiano ER. Microviscometry reveals reduced blood viscosity and altered shear rate and shear stress profiles in microvessels after hemodilution. Proc Natl Acad Sci U S A. 2004;101:10060–10065. doi: 10.1073/pnas.0402937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potter DR, Damiano ER. The hydrodynamically relevant endothelial-cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 10.Savery MD, Damiano ER. The endothelial glycocalyx is hydrodynamically relevant in arterioles throughout the cardiac cycle. Biophys J. 2008;95:1439–1447. doi: 10.1529/biophysj.108.128975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Berg BM, Vink H, Spaan JAE. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 12.Jacob M, Rehm M, Loetsch M, Paul JO, Bruegger D, Welsch U, Conzen P, Becker BF. The endothelial glycocalyx prefers albumin for evoking shear stress-induced nitric oxidemediated coronary dilatation. J Vasc Res. 2007;44:435–443. doi: 10.1159/000104871. [DOI] [PubMed] [Google Scholar]
- 13.Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell — an impressive structure ex vivo, but not in culture. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.187831. (to appear) [DOI] [PubMed] [Google Scholar]
- 14.Gretz JE, Duling BR. Measurement uncertainties associated with the use of bright-field and fluorescence microscopy in the microcirculation. Microvasc Res. 1995;49:134–140. doi: 10.1006/mvre.1995.1011. [DOI] [PubMed] [Google Scholar]
- 15.Norman KE. An effective and economical solution for digitizing and analyzing video recordings of the microcirculation. Microcirculation. 2001;8:243–249. doi: 10.1038/sj/mn/7800082. [DOI] [PubMed] [Google Scholar]
- 16.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;185:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Damiano ER, Long DS, El-Khatib FH, Stace TM. On the motion of a sphere in a Stokes flow parallel to a Brinkman half-space. J Fluid Mech. 2004;500:75–101. [Google Scholar]
- 19.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 20.MacArthur JM, Bishop JR, Stanford KI, Wang L, Bensadoun A, Witztum JL, Esko JD. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J Clin Invest. 2007;117:153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stock W, Hoffman R. White blood cells I: Non-malignant disorders. Lancet. 2000;355:1351–1357. doi: 10.1016/S0140-6736(00)02125-5. [DOI] [PubMed] [Google Scholar]
- 22.von Vietinghoff Sibylle, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunology. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf RA, Chaung LY, O’Hara D, Smith TW, Muller JE. The serum kinetics of bovine testicular hyaluronidase in dogs, rats and humans. J Pharmacol Exp Ther. 1982;222:331–337. [PubMed] [Google Scholar]
- 24.Silver PJ. IBT 9302 (Heparinase III): a novel enzyme for the management of reperfusion injury-related vascular damage, restenosis and wound healing. Exp Opin Invest Drugs. 1998;7:1003–1014. doi: 10.1517/13543784.7.6.1003. [DOI] [PubMed] [Google Scholar]
- 25.Ley K. Histamine can induce leukocyte rolling in rat mesenteric venules. Am J Physiol. 1994;267:H1017–H1023. doi: 10.1152/ajpheart.1994.267.3.H1017. [DOI] [PubMed] [Google Scholar]
- 26.Thorlacius H, Raud J, Rosengren-Beezley S, Forrest MJ, Hedqvist P, Lindbom L. Mast cell activation induces P-selectin-dependent leukocyte rolling and adhesion in postcapillary venules. Biochem Biophys Res Commun. 1994;203:1043–1049. doi: 10.1006/bbrc.1994.2287. [DOI] [PubMed] [Google Scholar]
- 27.Gaboury JP, Johnston B, Niu X-F, Kubes P. Mechanisms underlying acute mast cell-induced leukocyte rolling and adhesion in vivo. J Immunol. 1995;154:804–813. [PubMed] [Google Scholar]
- 28.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Chien S, Weinbaum S. Dynamic contact forces on leukocyte microvilli and their penetration of the endothelial glycocalyx. Biophys J. 2001;80:1124–1140. doi: 10.1016/S0006-3495(01)76090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 33.Niu Q, Chen H, Chen Z, Fu Y, Lin J, He S. Induction of inflammatory cytokine release from human umbilical vein endothelial cells by agonists of proteinase-activiated receptor-2. Clin Exp Pharmacol Physiol. 2008;35:89–96. doi: 10.1111/j.1440-1681.2007.04755.x. [DOI] [PubMed] [Google Scholar]
- 34.Gouverneur M, Spaan JAE, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol. 2006;290:H258–H462. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 35.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a ”bumper-car” model. Proc Natl Acad Sci U S A. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mertens G, Cassiman JJ, van den Berghe H, Vermylen J, David G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J Biol Chem. 1992;267:20435–20443. [PubMed] [Google Scholar]
- 37.Halden Y, Rek A, Atzenhofer W, Szilak L, Wabnig A, Kungl AJ. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem J. 2004;377:533–538. doi: 10.1042/BJ20030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 39.Gharagozlian S, Borrebaek J, Henriksen T, Omsland TK, Shegarfi H, Kolset SO. Effect of hyperglycemic condition on proteoglycan secretion in cultured human endothelial cells. Eur J Nutr. 2006;45:369–375. doi: 10.1007/s00394-006-0608-9. [DOI] [PubMed] [Google Scholar]
- 40.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol. 2004;22:985–992. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 41.Jones CI, Han Z, Presley I, Varadharaj S, Zweier JL, Ilangovan G, Alevriadou BR. Endothelial cell respiration is affected by the oxygen tension during shear exposure: role of mitochondrial peroxynitrite. Am J Physiol. 2008;295:C180–C191. doi: 10.1152/ajpcell.00549.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol. 2006;290:H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 43.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 44.Barakat AI. Dragging along: The glycocalyx and vascular endothelial cell mechanotransduction. Circ Res. 2008;102:747–748. doi: 10.1161/CIRCRESAHA.108.174839. [DOI] [PubMed] [Google Scholar]