Abstract
A marine metabolite based on a previously undescribed carbon skeleton, aberrarone (1), is reported as a natural product from the Caribbean sea whip, Pseudopterogorgia elisabethae. The molecular structure of the crystalline metabolite was established by spectral analysis and subsequently confirmed by X-ray crystallographic analysis. Aberrarone shows in vitro antimalarial activity against a chloroquine-resistant strain of the protozoan parasite Plasmodium falciparum.
The West Indian gorgonian octocoral Pseudopterogorgia elisabethae (Bayer, 1961) has proved to be an exceptionally rich source of diterpene glycoside natural products initially isolated and characterized by Fenical and colleagues in the 1980’s.1 Some of these compounds display significant anti-inflammatory and wound healing properties and thus have been the focus of many investigations dealing with their isolation and structure characterization, others with synthesis, and others with their biological activity.2 Specifically, the pseudopterosins are of considerable interest given their commercial market as additives in skin cream products and the successful completion of clinical trials as topical anti-inflammatory agents.3 Since the pioneering work of the Fenical group, a myriad of unusual natural products have been steadily isolated from P. elisabethae by several research groups, which are representatives of several classes of previously undescribed terpene metabolites, typically based on unusual carbon skeletons.4 Unlike the pseudopterosins, these metabolites lack a sugar moiety indicating an interesting departure from the structures of the previously characterized diterpenes. As of 2009, nearly 66% of all of the natural products isolated from this prolific gorgonian species (in all 109 compounds) are diterpenoids based on the amphilectane or serrulatane (“biflorane”) carbon skeletons. On the other hand, about 29% of the remaining metabolites (32 compounds) are diterpenoids based on the so called “non-amphilectane” or “rearranged amphilectane” carbon skeletons.4 Diterpenoids belonging to the latter group are based on over 25 distinct carbon skeletal classes, and many are endowed with intricate architectures and interesting biological properties. These unusual features render P. elisabethae as the most chemically diverse gorgonian octocoral from the West Indian region.5 As we near towards completion of a 12-year long chemical scrutiny of this productive marine animal, we now wish to report on the discovery of aberrarone (1),6 an interesting C20 tetracyclic natural product based on yet another unusual carbon skeleton. When evaluated for in vitro antimalarial activity, aberrarone displayed inhibitory activity against the protozoan parasite Plasmodium falciparum.
The MeOH–CHCl3 extract of the gorgonian (<1 kg of dry wt) was partitioned between hexane and water to yield a green thick paste, a portion of which was loaded onto a large silica gel column and separated by stepwise elution with hexane– acetone mixtures. Fractions were routinely pooled on the basis of their TLC, NMR, and biological activity profiles. Aberrarone (1, 4.6 mg) was obtained pure after one of the primary fractions obtained above was subjected to a series of purification steps consisting mainly of normal-phase silica gel column chromatography and HPLC analysis. The molecular structure of this metabolite was proposed initially on the basis of comprehensive analysis of the 1D and 2D NMR (13C, 1H, 1H–1H COSY, HSQC, HMBC, and NOESY), IR, UV, and HRFAB-MS spectra. A single-crystal X-ray structure analysis was subsequently carried out in order to confirm the proposed molecular structure of 1.
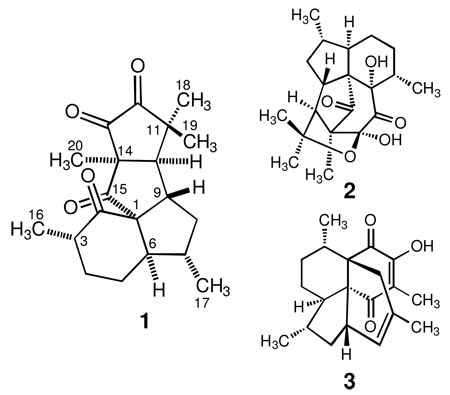
Aberrarone (1), isolated as a UV-active, light orange crystalline solid, was assigned a molecular formula of C20H26O4 based on the HRFAB-MS and NMR data, implying eight degrees of unsaturation. The presence of four ketone carbonyls (δC 215.3, 207.2, 202.4 and 199.0) was further corroborated by the IR absorption bands at 1768, 1753, 1724, and 1699 cm−1. The UV and 13C NMR spectra indicated that two of the carbonyls (δC 202.4 and 199.0) were those of a cyclic α-diketone functionality.7 The carbonyl groups accounted for four of the eight degrees of unsaturation as well as all of the oxygens required by the molecular formula of 1, and the absence of any further olefinic functionalities suggested a tetracyclic skeleton for this compound. Analysis of the 13C and DEPT NMR spectra of 1 confirmed the presence of 20 carbons, comprising four ketones, three quaternary, five methyl, five methine, and three methylene carbons. These data suggested a diterpenoid structure, but the tetracyclic nature of 1 implied rearrangement of the typical tricyclic amphilectane (or bicyclic serrulatane) skeleton, such as in elisapterosin A (2) or colombiasin A (3).8,9
The 1H NMR spectrum of 1 indicated the presence of five methyl groups with three-proton singlets at δ 1.54, 1.52, and 1.23, as well as two doublets with large couplings at δ 1.02 (J = 6.5 Hz) and 0.95 (J = 7.3 Hz). It also exhibited a doublet of a doublet of doublets at δ 2.87 (J = 8.4, 6.8, 1.6 Hz) and a broad doublet of doublets with fine splitting at δ 2.28 (J = 1.6 Hz), each integrating for one proton, which subsequently were attributable to the adjoining angular methines H-9 and H-10, respectively. The rest of the 1H NMR spectrum consisted of nine complex proton resonances between δ 0.78 and δ 2.21, suggestive of a polycyclic terpenoid structure.8,9
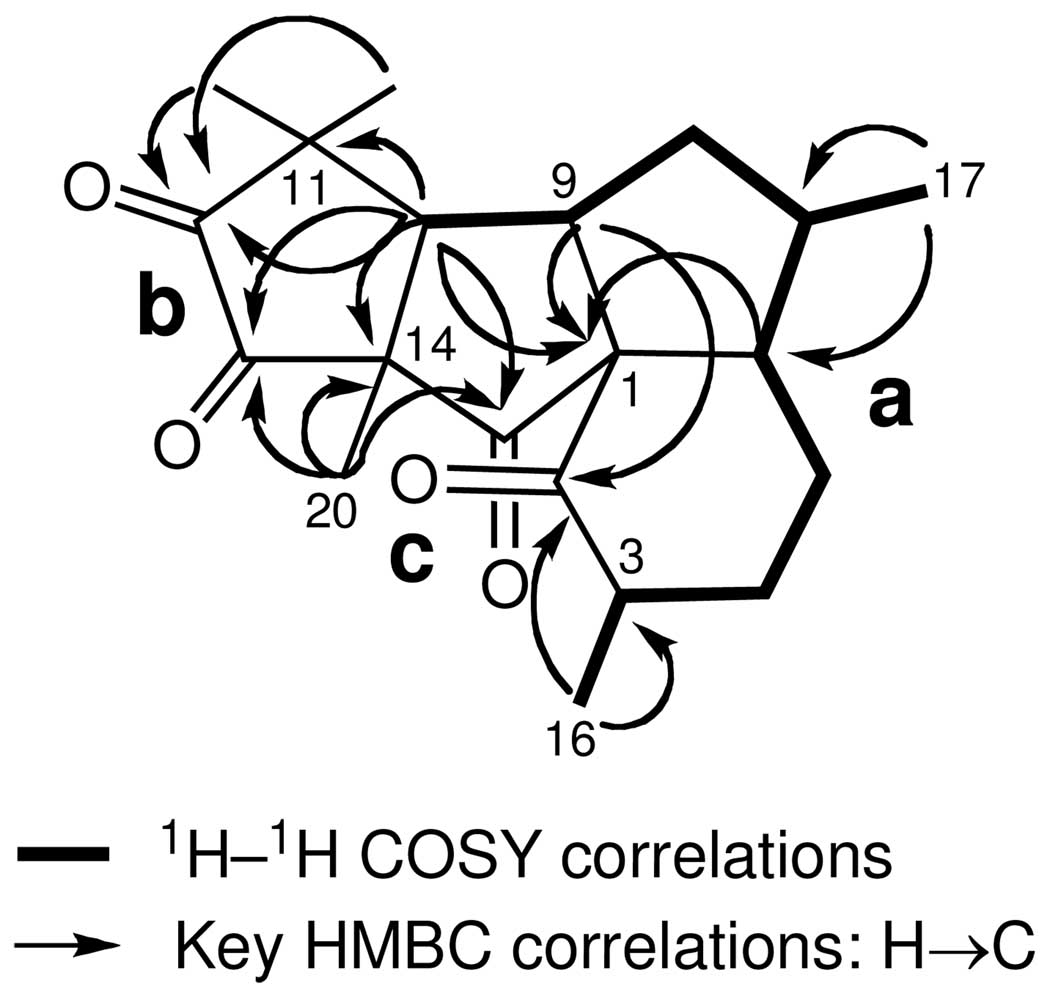
Correlations deduced from extensive analyses of the 2D NMR data of 1 (1H–1H COSY, TOCSY, HSQC, and HMBC NMR experiments) in CDCl3 enable initially the establishment of three partial structures (a–c) that were interconnected later on to yield the final structure of aberrarone (see Figure 1). The 1H and 13C NMR data are presented in Table 1.
FIGURE 1.
Partial structures for aberrarone (1) generated from 1H— 1H COSY and HMBC spectral correlations.
TABLE 1.
1H NMR (500 MHz), 13C NMR (125 MHz),1H—1H COSY, NOESY, and HMBC Spectral Data for Aberrarone (1)a
| position | δH, mult, intgt (J, Hz) | δCb | 1H–1H COSY | NOESY | HMBCc |
|---|---|---|---|---|---|
| 1 | 76.1 (C) | H-3, H-5α, H-6, H-8β, H-9, H-10 | |||
| 2 | 215.3 (C) | H-3, H-9, H3-16 | |||
| 3 | 2.07, m, 1H | 44.5 (CH) | H-4αβ, H3-16 | H3-16 | H3-16 |
| 4α | 1.77, m, 1H | 28.6 (CH2) | H-3, H-4β, H-5αβ | H-4β, H3-16 | H3-16 |
| 4β | 1.87, m, 1H | H-3, H-4α, H-5αβ | H-4α | ||
| 5α | 2.21, m, 1H | 27.3 (CH2) | H-4αβ, H-5β, H-6 | H-5β | |
| 5β | 1.07, m, 1H | H-4αβ, H-5α, H-6 | H-5α | ||
| 6 | 2.18, m, 1H | 50.5 (CH) | H-5αβ, H-7 | H3-17 | H-4αβ, H-8β, H3-17 |
| 7 | 1.63, m, 1H | 43.0 (CH) | H-6, H-8αβ, H3-17 | H-9, H3-17 | H-8α, H3-17 |
| 8α | 0.78, q, 1H (12.3) | 44.3 (CH2) | H-7, H-8β, H-9 | H-8β, H-10, H3-17 | H-10, H3-17 |
| 8β | 2.16, m, 1H | H-7, H-8α, H-9 | H-8α, H-9 | ||
| 9 | 2.87, ddd, 1H (8.4, 6.8, 1.6) | 45.9 (CH) | H-8αβ, H-10 | H-7, H-8β, H3-19 | H-8α, H-10 |
| 10 | 2.28, br d, 1H (1.6) | 55.7 (CH) | H-9 | H-8α, H3-18, H3-20 | H-8α, H3-18, H3-19, H3-20 |
| 11 | 45.0 (C) | H-10, H3-18, H3-19 | |||
| 12 | 202.4 (C) | H-10, H3-18, H3-19 | |||
| 13 | 199.0 (C) | H-10, H3-20 | |||
| 14 | 63.5 (C) | H-10, H3-20 | |||
| 15 | 207.2 (C) | H-6, H-9, H-10, H3-20 | |||
| 16 | 0.95, d, 3H (7.3) | 17.6 (CH3) | H-3 | H-3, H-4α | |
| 17 | 1.02, d, 3H (6.5) | 17.9 (CH3) | H-7 | H-6, H-7, H-8α | |
| 18 | 1.23, s, 3H | 29.8 (CH3) | H-10, H3-19, H3-20 | H-10, H3-19 | |
| 19 | 1.54, s, 3H | 20.9 (CH3) | H-9, H3-18 | H3-18 | |
| 20 | 1.52, s, 3H | 24.2 (CH3) | H-10, H3-18 | H-10 |
Spectra were recorded in CDCl3 at 25 °C. Chemical shift values are in parts per million relative to the residual CHCl3 (7.25 ppm) or CDCl3 (77.0 ppm) signals. Assignments were aided by 2D NMR experiments, spin-splitting patterns, number of attached protons, and chemical shift values.
13C NMR multiplicities were obtained from a DEPT-135 experiment.
Protons correlated to carbon resonances in the 13C column. Parameters were optimized for 2,3JCH = 6 and 8 Hz.
Connectivities from C-3 to C-10 (substructure a) were inferred from the 1H–1H COSY cross-peaks, including correlations from H-3 to H3-16 and H-7 to H3-17. This extended spin system, encompassing half the carbon atoms present in 1 across three rings, was quickly recognized as it is present in other compounds isolated from the same gorgonian specimen (i.e. compounds 2 and 3). Confirmation of the proton connectivity network already established from the 1H– 1H COSY and TOCSY experiments was obtained directly from long range 1H–13C couplings (Table 1). The structure elucidation of substructures b and c, comprising the other half of carbon atoms in 1, was more difficult to achieve as they deviated substantially from those found in other rearranged diterpenes, such as 2 or 3.
The HMBC experiment showed connectivity between the C-1 carbon [δC 76.1 (C)] and the protons of C-3, C-5, C-6, C8, C-9 and C-10. Thus the pivotal C-1 quaternary carbon must be attached to C-2, C-6, and C-9, thereby establishing the perhydroindane substructure within 1 (substructures a and c). Furthermore, these units were linked by a strong correlation between C-15 [δC 207.2 (C)] and protons H-6, H-9, H-10, H3-20, and the strong couplings between C-2 [δC 215.3 (C)] and protons H-3, H-9 and H3-16. Since C-10 [δC 55.7 (CH)] correlated strongly with H3-18, H3-19, and H3-20, and C-14 [δC 63.5 (C)] was strongly coupled to H-10 and H3-20, C-14 has to be flanked by C-10, C-15 and C-20. On the other hand, substructure b was connected to partial structure c by the observation of strong HMBC correlations of C-13 [δC 199.0 (C)] to H-10 and H3-20, and to unit a from the correlations of C-10 [δC 55.7 (CH)] to geminal methyls H3-18 and H3-19, and between C-11 [δC 45.0 (C)] with H-10. The observation of a strong HMBC correlation of C-12 [δC 202.4 (C)] and the protons of C-10, C-18, and C-19 showed that C-11 and C-12 must themselves be linked to one another. This strongly implied that the carbonyl carbons C-12 and C-13 (the last connecting points remaining) had to be linked to one another thus comprising the 1,2-cyclopentanedione ring system within aberrarone (1). Applying these combined 2D NMR methods resulted in the unambiguous assignment of all protons and carbons as listed in Table 1 and allowed the complete planar structure for 1 to be assigned.
Segments of the relative stereochemistry of aberrarone were readily assigned by NOE NMR spectral methods (Table 1). However, while the relative configuration about the spirocyclic carbon (C-1) in 1 could be assigned indirectly on the basis of the overall correlations observed in the NOESY spectrum, we could not confidently assigned the relative configuration at C-3 with the NMR data already at hand. Thus, confirmation of the entire structure of aberrarone by single-crystal X-ray diffraction analysis was highly desirable. Recrystallization of 1 by slow evaporation from a mixture of MeOH/H2O gave crystals of excellent quality that were amenable to X-ray crystallographic analysis. The X-ray structure, which defines only the relative configuration, is shown in the Supporting Information. Hence, the overall relative stereochemistry for the seven stereocenters about the complex tetracarbocyclic ring system of aberrarone was assigned as 1S*, 3S*, 6R*, 7S*, 9S*, 10R*, and 14S*. The assigned configurations, which were in full agreement with the correlations observed in the NOESY spectrum of 1, were confirmed through interpretation of NMR coupling data (Table 1).10
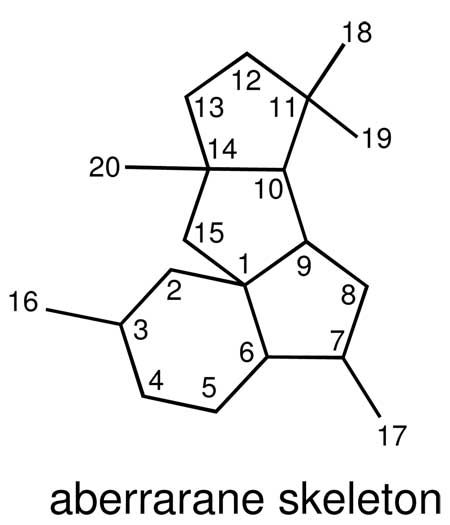
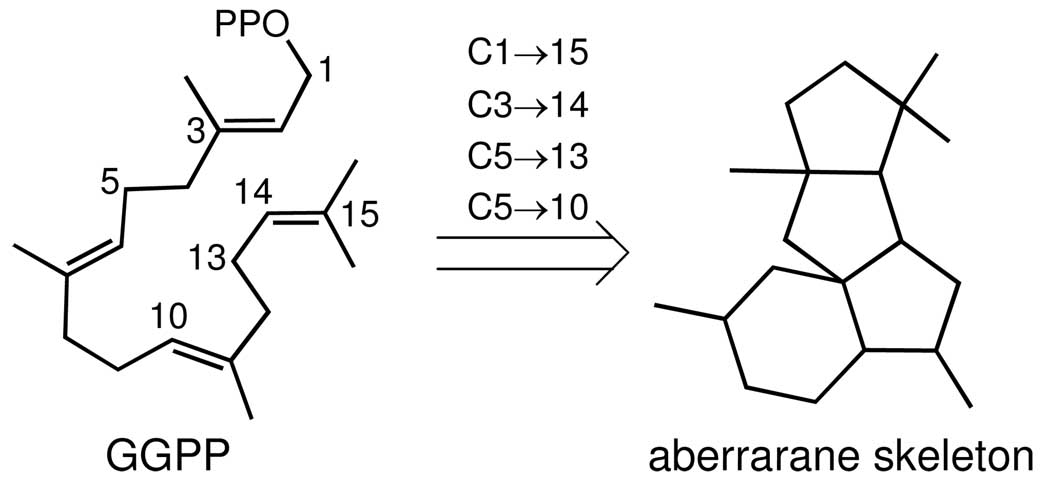
Diterpenes with the same tetracyclic ring system found in 1 have not been previously encountered in Nature.11 We therefore suggest the general name aberrarane for this class of diterpene. While the biosynthesis of this regular diterpene remains to be demonstrated, aberrarone can be considered as deriving from geranylgeranyl pyrophosphate (GGPP) via C1/C15, C3/C14, C5/C13, and C5/C10 cyclizations (Scheme 1). Nonetheless, the presence of various serrulatane-, amphilectane-, and elisabethane-based diterpenes within the same gorgonian specimen provides circumstantial support for a biosynthetic pathway in which the carbon skeleton of the latter class of metabolites, for instance, is the precursor to the aberrarane skeleton (via cleavage of the C2/C17 bond followed by concomitant C10/C15 and C11/C17 cyclizations (see Scheme 2 in the Supporting Information).12,13
SCHEME 1.
Proposed Biogenesis of the Aberrarane Carbon Skeleton
Compounds 1–3 were tested against the most common etiologic agent of malaria, Plasmodium falciparum (W-2 chloroquine-resistant strain). The antimalarial activity was determined using a novel fluorometric method, based on the intercalation of the fluorochrome PicoGreen® in the parasite DNA, as described by Corbett et al.14 After a 24 and 48 h incubation, all P. falciparum parasites were killed by these compounds, with α-diketone 1 and colombiasin A (3) showing the highest antimalarial activity (IC50 = 10 µg/mL).
Experimental Section
Biological Material
The biological specimens used for this investigation corresponded to a deep-water morphotype of Pseudopterogorgia elisabethae Bayer (order Gorgonacea, family Gorgoniidae, phylum Cnidaria) collected in May 1996 by scuba from deep reef waters (−28 m) off San Andrés island, Colombia (located at 12°33’N 81°43’W). A voucher specimen (No. PESAI-01) has been deposited at the Chemistry Department of the University of Puerto Rico.
Collection, Extraction, and Isolation
The gorgonian specimens were partially sun-dried and kept frozen prior to its extraction. The freeze-dried animal (1.0 kg) was cut into small pieces and blended with 1:1 MeOH/CHCl3 (11 × 1 L). The combined organic extracts were filtered and then concentrated, and the green residue obtained (284 g) was suspended in water and extracted with hexane, CHCl3 and EtOAc. A large portion (128 g) of the entire crude hexane extract (178 g) was loaded onto a column of silica gel (780 g) and separated with stepwise elution of acetone in hexane (0–100%), and then with 100% CH3OH. Fractions were routinely pooled on the basis of their TLC, NMR, and biological activity profiles to yield 7 primary fractions, denoted I–VII. Fractions IV (83 g) was re-chromatographed over silica gel (600 g) using a step gradient eluent system composed of EtOAc–hexane mixtures leading to 16 secondary fractions (A–P). Further purification of sub-fraction F (7.0 g) by silica gel (150 g) flash column chromatography using as eluant a 1:1 mixture of hexane–CHCl3 gave a series of smaller fractions, the most polar of which (113 mg) was dissolved in a small volume of 95:5 hexane–2-propanol, filtered, and purified by normal-phase HPLC on a 10 mm × 25 cm Magnum Partisil-10 column, 5 µm, eluted isocratically with 5% 2-propanol in hexane at 2.0 mL/min. One of the homogeneous components isolated during HPLC analysis was later identified as aberrarone (1) (4.6 mg, 6.4 × 10−4% yield) (dry gorgonian weight basis).
Biological Assay
The primary in vitro anti-microbial assay against Plasmodium falciparum was used as described before.14
Aberrarone (1)
light orange crystals; [α]D25 +2.2 (c 1.4, CHCl3); IR (film) νmax 2932, 2870, 1768, 1753, 1724, 1699, 1675, 1456, 1376, 1319, 1261, 1142, 1118, 1100 cm−1; UV (MeOH) λmax 203 (ε 4 700), 220 (ε 2 800), 380 (ε 30) nm; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz) (see Table 1); EIMS m/z 302 [M–CO]+ (6), 233 (17), 231 (17), 203 (11), 215 (22), 193 (52), 175 (23), 137 (23), 109 (19), 70 (100); HRFAB-MS m/z [M + H]+ 331.1922 (calcd for C20H27O4, 331.1909).
Single-Crystal X-Ray Structure Determination of Aberrarone (1) at 298(2) K
The X-ray data were collected at 298° K with a Bruker SMART 1 K CCD diffractometer equipped with a graphite monochromator and Mo-Kα radiation (λ = 0.71073 Å) using the SMART software. Final values of the cell parameters were obtained from least-squares refinement. The frames were processed using the SAINT software to give the hkl file corrected for Lorentz and polarization effects. No absorption correction was applied. The structures were solved by direct methods with the SHELX-90 program and refined by least-squares methods on F2, SHELXTL-93, incorporated in SHELXTL, Version 5.1. The initial E-maps yielded all non-hydrogen atom positions. All non-hydrogen atoms were refined anisotropically, and the H atoms were positioned geometrically and treated as riding, with C–H distances in the range 0.93–0.98 Å and with Uiso(H) = 1.2 or 1.5 Ueq(C). The crystallographic data for 1 reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under the reference number CCDC 741374. Copies of the data can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223 336033 or deposit@ccdc.cam.ac.uk). Aberrarone (1) was recrystallized by slow evaporation from a CH3OH/H2O mixture to yield light orange crystals of excellent quality. Crystal Data: C20H26O4, Mr = 330.41, orthorhombic, space group P212121 (No. 19), a = 10.375(2), b = 11.449(3), c = 15.153(4) Å, V = 1799.9(8) Å3, Z = 4, ρcalc = 1.219 Mgm−3, F000 = 712, λ(Mo Kα) = 0.71073 Å, µ = 0.084 mm−1. Data collection and reduction: crystal size, 0.19 × 0.14 × 0.11 mm, θ range = 2.23–23.29°, 8732 reflections collected, 2594 independent reflections (Rint = 0.0268), final R indices (I > 2σ(I)): R1 = 0.0341, wR2 = 0.0785 for 222 variable parameters, GOF = 1.025.
Supplementary Material
Acknowledgement
We gratefully acknowledge the assistance of the Instituto de Investigaciones Avanzadas y Servicios de Alta Tecnología in Panama for the antimalarial bioassays, and the Mass Spectrometry Laboratory of the University of Illinois at Urbana-Champaign for mass spectrometric analyses. We are indebted to Dr. Juan A. Sánchez and Dr. Raphael G. Raptis for assistance during collection of the animal material and the X-ray crystallographic data, respectively. This work was partly supported by grants from the NIH-MBRS and DOE-GAANN.
Footnotes
Supporting Information Available: Copies of the 1D and 2D NMR spectra for aberrarone (1), Schemes 2 and 3, an ORTEP drawing for 1, and X-ray crystallographic data (in CIF format). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Look SA, Fenical W, Jacobs RS, Clardy J. Proc. Natl. Acad. Sci. USA. 1986;83:6238–6240. doi: 10.1073/pnas.83.17.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Look SA, Fenical W, Matsumoto GK, Clardy J. J. Org. Chem. 1986;51:5140–5145. [Google Scholar]; (c) Roussis V, Wu Z, Fenical W, Strobel SA, Van Duyne GD, Clardy J. J. Org. Chem. 1990;55:4916–4922. [Google Scholar]
- 2.(a) Mayer AMS, Jacobson PB, Fenical W, Jacobs RS, Glaser KB. Life Sci. 1998;62:PL401–PL407. doi: 10.1016/s0024-3205(98)00229-x. [DOI] [PubMed] [Google Scholar]; (b) Zhong W, Moya C, Jacobs RS, Little RD. J. Org. Chem. 2008;73:7011–7016. doi: 10.1021/jo801432t. [DOI] [PubMed] [Google Scholar]
- 3.(a) Rouhi M. Chem. Eng. News. 1995;20:42–44. [Google Scholar]; (b) Kijjoa A, Sawangwong P. Mar. Drugs. 2004;2:73–82. doi: 10.3390/md502040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Heckrodt TJ, Mulzer J. Top Curr. Chem. 2005;244:1–44. [Google Scholar]; (b) Berrue F, Kerr RG. Nat. Prod. Rep. 2009;26:681–710. doi: 10.1039/b821918b. [DOI] [PubMed] [Google Scholar]
- 5.Fenical W. J. Nat. Prod. 1987;50:1001–1008. doi: 10.1021/np50054a001. [DOI] [PubMed] [Google Scholar]
- 6.From the Latin word aberrare, not typical or usual.
- 7.For the UV spectal data of several non-enolizable cyclic 1,2-diketones, see: Lambert JB, Shurvell HF, Lightner DA, Cooks RG. Organic Structural Spectroscopy. New Jersey: Prentice-Hall, Inc.; 1998. p. 302.
- 8.Rodríguez AD, Ramírez C, Rodríguez II, Barnes CL. J. Org. Chem. 2000;65:1390–1398. doi: 10.1021/jo9914869. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez AD, Ramírez C. Org. Lett. 2000;2:507–510. doi: 10.1021/ol991362i. [DOI] [PubMed] [Google Scholar]
- 10.For instance, that C-9 and C-10 have the S* and R* configurations, respectively, is supported by the small 1.6 Hz coupling constant observed between H-9 and H-10, consistent with the trans orientation shown; see: Shi Y-P, Rodríguez II, Rodríguez AD. Tetrahedron Lett. 2003;44:3249–3253. Rodríguez II, Rodríguez AD. Tetrahedron Lett. 2009;50:5493–5496. doi: 10.1016/j.tetlet.2009.07.073.
- 11.While several fungal-derived diterpenes with the same 5-5-5-6 tetracyclic array have been described, the methylation pattern about the aberrarane skeleton is unprecedented in Nature; see: Roncal T, Cordobés S, Ugalde U, He Y, Sterner O. Tetrahedron Lett. 2002;43:6799–6802. Du L, Li D, Zhu T, Cai S, Wang F, Xiao X, Gu Q. Tetrahedron. 2009;65:1033–1039.
- 12.(a) Rodríguez AD, González E, Huang SD. J. Org. Chem. 1998;63:7083–7091. doi: 10.1021/jo981385v. [DOI] [PubMed] [Google Scholar]; (b) Rodríguez AD, Ramírez C, Rodríguez II. J. Nat. Prod. 1999;62:997–999. doi: 10.1021/np990090p. [DOI] [PubMed] [Google Scholar]; (c) Rodríguez AD, Ramírez C. J. Nat. Prod. 2001;64:100–102. doi: 10.1021/np000196g. [DOI] [PubMed] [Google Scholar]
- 13.Additional circumstantial evidence for this biosynthetic pathway stems from the notion that aberrarone (1), upon tandem oxidation (to the non-enolizable cyclic anhydride) and decarbonylation, might lead to the known γ-lactone elisabanolide (see Scheme 3 in the Supporting Information).
- 14.Corbett Y, Herrera L, González J, Cubilla L, Capson T, Colley PD, Kursar TA, Romero LI, Ortega-Barria E. J. Trop. Med. Hyg. 2004;70:119–124. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.