Abstract
Single point mutations of the amyloid precursor protein generate Aβ variants bearing amino acid substitutions at positions 21–23. These mutants are associated with distinct hereditary phenotypes of cerebral amyloid angiopathy, manifesting varying degrees of tropism for brain vessels, and impaired microvessel remodeling and angiogenesis. We examined the differential effects of E22Q (Dutch), and E22G (Arctic) variants in comparison to WT Aβ on brain endothelial cell proliferation, angiogenic phenotype expression triggered by fibroblast growth factor (FGF-2), pseudo-capillary sprouting, and induction of apoptosis. E22Q exhibited a potent anti-angiogenic profile in contrast to E22G, which had a much weaker effect. Investigations on the FGF-2 signaling pathway revealed the greatest differences among the peptides: E22Q andWT peptides suppressed FGF-2 expression while E22G had barely any effect. Phosphorylation of the FGF-2 receptor, FGFR-1, and the survival signal Akt were abolished by E22Q and WT peptides, but not by E22G. The biological dissimilar effect of the mutant and WT peptides on cerebral EC cannot be assigned to a particular Aβ structure, suggesting that the toxic effect of the Aβ assemblies goes beyond mere multimerization.
Keywords: Aβ peptides, Cerebral amyloid angiopathy, Endothelial cells, Angiogenesis, FGF-2
Introduction
Cerebral amyloid angiopathy (CAA), a common feature of Alzheimer's disease (AD), is characterized by the deposition of amyloid peptides (Aβ), mainly Aβ40, in cortical and leptomeningeal vessels causing disruption of brain capillaries and dysfunction of endothelium [1,2]. Besides the dominant sporadic form, numerous hereditary variants occur in humans [3–5]. These rare forms originate mainly from mutations in the DNA encoding the amyloid precursor protein (APP), giving rise to mutated peptides which cause a wide spectrum of diseases differing in clinical manifestations, age of onset and prognosis. At one extreme is the well known Dutch variant, an early onset autosomal dominant disorder in which a point mutation at codon 693 of APP yields a single amino acid substitution (Glu to Gln) at position 22 of Aβ [6,7]. The disease, known as Hereditary Cerebral Hemorrhage with Amyloidosis-Dutch type (HCHWA-D) is characterized by recurrent strokes, vascular dementia in the absence of neurofibrillar pathology, and fatal cerebral bleeding due to massive amyloid deposition in leptomeningeal vessels and cortical arteries and arterioles. At the other end of the spectrum is the Arctic mutation, in which a single nucleotide change at the same codon 693 translates into a Glu to Gly substitution also at position 22 resulting in early onset memory impairment rather than stroke [8]. The clinical phenotype is typical of AD as a result of abundant parenchymal plaques and mild amyloid angiopathy co-existing with neurofibrillary tangles. Interestingly, when compared to the wild type Aβ40 peptide, the Dutch mutation exhibits enhanced fibrillization properties [9] whereas the Arctic variant induces the formation of protofibrils [8] within comparable time frame.
Several studies have documented the action of Aβ peptides on the brain endothelium, showing alteration of its morphology and impairment of its functions [3]. Particularly affected is the endothelium ability to survive [10], to maintain its vasoactive tone [11], and to evolve in the angiogenic phenotype [12,13]. Here we report studies on the actions on primary human brain microvascular endothelial cells (HBMEC) of the three amyloid peptides mentioned above, two of them bearing a point mutation in the amino acid localized at position 22 of the Aβ40 sequence, namely the wild type peptide (WT), the Dutch (E22Q), and Artic (E22G) variants. We investigated the influence of the peptides on endothelial functions, focusing on the FGF-2 pathway, which through an autocrine–paracrine mechanismis central for endothelial survival. We demonstrate that Aβ peptides prevent the formation of the angiogenic phenotype by down-regulating the FGF-2 axis through an interference with its membrane receptor, FGFR-1. Differences among the peptides in causing endothelial damage, noted in this study, may likely explain the diverse clinical phenotypes of amyloid angiopathies.
Materials and methods
Aβ peptides
Aβ (1–40) peptides – WT or bearing the E22Q and E22G mutations – were synthesized either at Yale University (James I. Elliott) (see [10]) or at Espikem (University of Florence, Italy); reverse sequence Aβ40–1 was purchased from Bachem, Germany. In all cases peptides were purified by reverse phase high pressure liquid chromatography, eluting as a single peak, and their respective molecular masses corroborated by mass spectrometry. Peptides were first dissolved to 1 mM in cold (4 °C) hexafluoro-isopropanol (HFIP) in a chemical fume hood to break down β-sheet structures and disrupt hydrophobic forces in aggregated Aβ. HFIP was allowed to evaporate, and the resulting clear peptide films were vacuum dried. Before use, HFIP treated peptides were dissolved to 5 mM in anhydrous dimethyl sulfoxide (DMSO) [14] and further diluted (1–25 µM) in Endothelial Cell Medium (ECM, ScienCell, San Diego, CA) containing 0.1% Fetal Bovine Serum (FBS) and directly added to the cells. For the aggregation studies, as described below, HFIP-treated peptides were reconstituted in Hanks Balance Salt Solution (Invitrogen, Carlsbad, CA). Different peptide batches [WT Aβ40 #1821 and #1574; E22Q #1055 and #1576; E22G #343 and #2017] yielded comparable results after disruption of pre-existing β-sheet structures and adoption of α-helix conformations induced by the HFIP pre-treatment.
Aβ aggregation
Synthetic Aβ homologues, HFIP-treated as above, were reconstituted to 1 mg/ml in sterile Hanks' Balanced Salt Solution (231–235 µM, depending on the molecular masses of the respective peptides), and incubated at 37 °C for various time periods (up to 6 days). At any given time point the peptides' secondary structure was assessed by circular dichroism spectroscopy, the formation of fibrils and protofibrils evaluated by Thioflavin T binding, and electron microscopy whereas the amount of peptide remaining in solution was evaluated by absorbance at 280 nm after centrifugation at 14,000 rpm in an Eppendorf 5417R refrigerated micro-centrifuge (1 h; 10 °C).
Circular dichroism spectroscopy
The secondary structure of the different Aβ peptides was estimated by CD spectroscopy as we previously described [10,15]. Spectra in the far-UV light (195–260 nm; band width: 1 nm; 0.1 nm intervals; scan rate: 60 nm/min) of the different peptides at each time point were recorded at 24 °C with a Jasco J-720 spectropolarimeter (Jasco Corp.; Tokyo, Japan), using a 0.2 mm-path quartz cell and a peptide concentration of 1 mg/ml in Hanks balanced salt solution. For each sample 10 consecutive spectra were obtained, averaged and baseline-subtracted. Results were expressed in terms of mean residue ellipticity (degree cm2 dmol−1) [16].
Thioflavin T binding assay
Thioflavin T binding was assessed essentially as previously described [17,18]. Six microliter aliquots of each of the 1 mg/ml peptide aggregation time point samples were added to 10 µl of 0.1 mM Thioflavin T (Sigma) and 50 mM Tris–HCl buffer, pH 8.5 to a final volume of 200 µl. Fluorescence was recorded after 300 s in a Perkin Elmer LS-5B luminescence spectrometer with excitation and emission wavelengths of 435 nm (slit width = 10 nm) and 490 nm (slit width = 10 nm), respectively.
Electron microscopy
Three microliters of each of the peptide aggregation time point samples were placed onto carbon coated 400 mesh Cu/Rh grids (Ted Pella, Inc., Redding, CA) and stained with 1% uranyl acetate in distilled water (Polysciences, Inc., Warrington, PA). Stained grids were examined in a Philips CM-12 transmission electron microscope and photographed with a Gatan (4 k × 4 k) digital camera at the Image Core Facility of the Skirball Institute of Biomedical Medicine, NYU School of Medicine.
Cell culture
Human brain microvascular endothelial cells (HBMEC) were purchased from ScienCell (ScienCell Research) and maintained as recommended by the vendor. These cells were characterized for the expression of endothelial markers, CD31 (Dako, Denmark), vWF (Santa Cruz Biotechnology, Santa Cruz, CA), KDR (Santa Cruz), Flt1 (Santa Cruz), eNOS (BD Transduction Laboratories, San Jose, CA) as well as Occludin (Zymed Laboratories/Invitrogen, Carlsbad, CA) by immunofluorescence techniques using the standard procedures previously described [19]. Occludin was found to be overexpressed in HBMEC relative to non-cerebral endothelial cells e.g. HUVEC.
Human cerebral microvascular endothelial cells (HCMEC/D3) were grown in complete EMB-2 medium (Cambrex Bioscience, Wokingham, UK) as described [20]. After immortalization, these cells maintained normal endothelial cell markers including CD31, VE cadherin and vWF as well as blood brain barrier characteristics, among them the expression of tight junctional markers like PECAM-1, ZO-1, claudin 5, JAM-A, and the junction associated proteins β- and γ-catenin.
Microcarrier cell culture
Gelatine-coated cytodex microcarriers (MCs) (Sigma-Aldrich, Italy) were prepared and embedded in a fibrin gel as described [21]. Cells were stimulated with FGF-2 (20 ng/ml, R&D Systems, Minneapolis, MN) either alone or together with the various Aβ peptides (5 µM). At day 2 the area occupied by capillary-like formations was quantified in an inverted microscope at a magnification of 200×, using an ocular grid. The sprouting area was expressed as the number of grid units required to cover the entire pseudo-capillary surface. Results are reported as mean area units ± SEM.
BrdU labeling assay
Cell proliferation was determined by 5-bromo-2′-deoxy-uridine (BrdU) incorporation using a colorimetric ELISA according to the manufacturer's instructions (Roche Applied Science, Indianapolis, IN). Briefly, 1.5 × 103 cells were seeded in 96 multiplate in 10% FBS. After adherence (6 h), the supernatant was replaced with medium containing 0.1% FBS. After 24 h cells were incubated with 1–25 µM of either WT, E22Q or E22G variants, or reverse Aβ40–1 as control in the presence/absence of FGF-2 (20 ng/ml) for 48 h. BrdU was added during the late stage (6 h) of incubation. Cells were washed, fixed in ethanol and incubated with a monoclonal antibody directed against BrdU, followed by an alkaline phosphatase-conjugated secondary antibody. Stained cells were counted with a light microscope (Nikon Eclipse E400) at 200× magnification. Data are reported as cell number counted/well.
Western blotting
HBMEC (3 × 105) were seeded in 60-mm-diameter dishes in 10% FBS and starved in 0.1% serum for 24 h. To assess the effect of the Aβ peptides on FGF-2 production, cells were treated with WT, E22Q or E22G (18 h, 5 µM). For caspase-3 activation and Akt phosphorylation, cells were treated as above in the presence/absence of FGF-2 (20 ng/ml). In all cases, cells were lysed and subjected to Western blot as described [22].
Immunoprecipitation
Cells (8 × 105) were seeded for FGFR-1 analysis in 60-mm-diameter dishes in 10% FBS and starved overnight in 0.1% FBS. Cultures were pre-stimulated for 30 min with the Aβ peptides, treated with FGF-2 (20 ng/ml, 15 min), scraped from the dishes in a lysis buffer, and pre-cleared by centrifugation at 10,000× g (20 min 4 °C). An anti-FGFR-1 antibody (Upstate-Millipore, Billerica, MA; 10 µg) was added to the pre-cleared lysates (300 µg total protein) and incubated for 18 h at 4 °C, followed by addition of protein G (Sigma-Aldrich, Italy, 100 µl), and further incubation for 2 h at 4 °C. Immunoprecipitates were solubilized in sample buffer, boiled (10 min), separated in SDS/8% polyacrylamide gel and electro-blotted onto nitrocellulose membranes. Membranes were incubated overnight with an antibody anti p-Tyr (Cell Signaling, Danvers, MA; 0.3 µg/ml), followed by a secondary antibody HRP-conjugated (Promega, Italy) and enhanced chemiluminescence detection system (BIO-RAD, Italy).
Quantitation of apoptosis induction: cell death ELISA
HCMEC/D3 cells were seeded in 24 well plates in complete EBM-2 medium (Cambrex BioScience, Wokingham, UK) and after 1 day in culture were challenged for 1–3 days with 50 µM of WT, E22Q, and E22G peptides in EBM-2/1% FBS, prepared as described above. Apoptosis was quantitated by Cell Death ELISA plus (Roche) following the manufacturer's recommendations. Apoptotic cell death is expressed as fold of increase, compared to controls treated under identical conditions in the absence of amyloid peptides, as we previously described [23].
Statistical analysis
Statistical analysis was performed using Student's t-test for unpaired data or by an analysis performed by GraphPad-InStat® 3.05. Comparison between treatments were made by one way ANOVA (Dunnet Multiple Comparison test). Probability p < 0.05 was considered significant.
Result
Aβ peptides structural studies
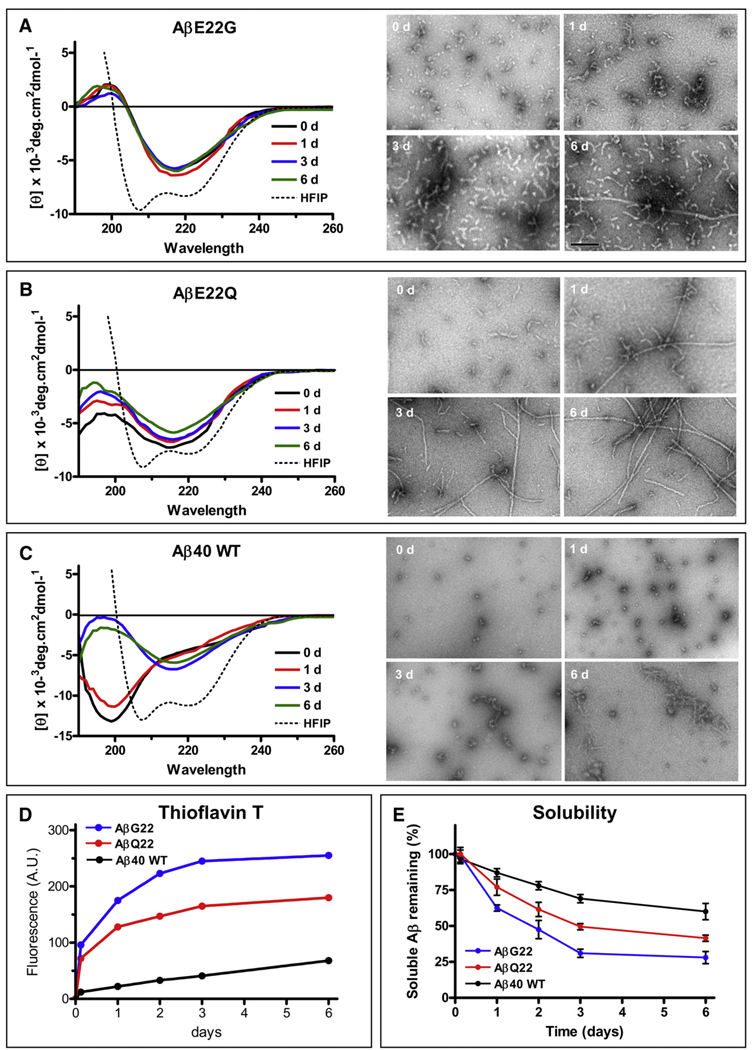
Aβ peptides displayed a high propensity to aggregate when aged at 37 °C under physiological salt concentrations. WT as well as E22Q and E22G variants all underwent oligomerization/fibrillization, albeit to completely different extents in spite of their homogeneous state at the starting point after HFIP pre-treatment, as indicated by their α-helical structure when evaluated by CD spectrometry (Figs. 1A–C) and elution as single peaks from Superdex G75 size exclusion chromatography columns (previously described for WT and E22Q [23], data not shown for E22G) and consistent with the known effect of HFIP in breaking down β-sheet structures, and leading to monodisperse Aβ preparations [24].
Fig. 1.
Fibrillization and structural studies of Aβ40 and mutants E22Q and E22G. (A) Changes in secondary structure of the Aβ E22G variant during the 6 days duration of the aggregation experiments in saline solution (left panels). The broken line indicates the α-helical structure of the peptide when solubilized in HFIP. Right panels illustrate EM images of the same samples at different time points (0, 1, 3 and 6 days). (B) Changes in secondary structure of the Aβ E22Q variant during the 6 days duration of the experiments (left panels); the broken line also indicates the α-helical structure of the peptide when solubilized in HFIP [note the similarities with (A)]. The right panels show electron microscopical images of the same samples at 0,1, 3 and 6 days. (C) Changes in secondary structure of Aβ40 wild type during the 6 days duration of the experiments in PBS (left panels). The broken line indicates the α-helical structure of the peptide when solubilized in HFIP [note the similarities with (A) and (B)]. The right panels show electron microscopical images of the same samples at 0, 1, 3 and 6 days. (D) Fluorescence values of Thioflavin T binding assay for samples collected at the different time points during the 6-day duration of the experiments. The data is representative of three independent experiments. (E) Peptide solubility at each time point estimated by O.D. 280 nm following 1 h centrifugation at 14,000× g as described in Materials and methods. Results are expressed in percentage of the peptide remaining in solution; mean ± SD of triplicate experiments.
Secondary structure analysis by CD spectroscopy (Figs. 1A–C, left panels) illustrates the different structural characteristics of the peptides. E22G, which in HFIP presents the double minimum at 208 and 222 nm characteristic of α-helical structures, immediately adopted a β-sheet rich structure with the typical minimum at 218 nm after addition of physiological solution (Fig. 1A), consistent with previous reports [25]. This conformation remained mostly unchanged up to day six. EM studies (right panel) illustrate that this variant developed the typical protofibril-like structures consistent of short flexible fibrils, generally 4–10 nm in diameter and up to 200 nm in length [17,26]. These protofibrillar components increased overtime to render an overwhelming number by day 3 and started assembling into longer, although scarce, more mature fibrils at day six. E22Q rapidly evolved from an initial α-helix conformation to a mixture of random-coil and β-sheet components when freshly solubilized in saline solution. Further incubation at 37 °C, the β-sheet content increased (Fig. 1B), although not achieving the extent observed with E22G. As illustrated in the EM images, E22Q rapidly assembled into heterogeneous protofibrillar structures that progressed with time to longer fibrillar components reminiscent to the typical amyloid fibrils evident at day one, becoming the predominant structures at day 6. The WT Aβ40 peptide, also in α-helical conformation when pre-treated with HFIP, adopted a typical unordered conformation under physiological saline, exhibiting a minimum at 198 nm (Fig. 1C). After 1-day incubation, a β-sheet component became evident (shoulder at ~ 218 nm). By day 3, the peptide acquired a β-sheet-rich conformation (minimum at 218 nm), while still exhibiting some random-coil structural components as shown by the still negative values below 200 nm, which remained unchanged up to day six. EM revealed the presence of small globular components and short fibrillar structures, which elongated with time, but without achieving the state of mature fibrils.
Thioflavin T binding, a property associated with the presence of β-sheet structures and typically correlating with the existence of fibrillar and/or protofibrillar components [17] also varied among the different peptides. Consistent with its striking tendency to form protofibrillar assemblies [8,25] E22G displayed the highest fluorescence signal, even at very short incubation times, followed by E22Q which, however, showed lower intensity signals than the former (Fig. 1D). In agreement with its low content in β-sheet components and its poor fibrillization illustrated in the EM, WT Aβ40 Thioflavin T binding levels were negligible at short incubation times and, although steadily increasing, remained low throughout the duration of the experiments.
To estimate the decrease in solubility of the different peptides upon incubation in physiologic buffer for up to 6-days, we evaluated the changes in absorbance at 280 nm at each one of the aggregation time points and after 1 h centrifugation at 14,000× g. As illustrated in Fig. 1E, WT Aβ40 exhibited the highest solubility, correlating with its lowest propensity to form fibrillar/protofibrillar assemblies (Figs. 1C and D). Consistent with the transition from random-coil to β-sheet conformations observed after day 3, soluble Aβ40 continued to decrease, reaching a minimum of 65% by day 6 under the experimental conditions tested. E22Q showed a parallel decrease in solubility with aging at 37 °C, but in agreement with its higher content of β-sheet rich components and higher tendency to fibrillize (Figs. 1B and D), the remnant soluble peptide reached lower values than those of WT (~45% by day 6). E22G, the most aggressive of the peptides studied herein showed the lowest solubility of the three. Soluble E22G decreased by ~50% after 2 days and only 25–30% of the original material remained soluble after 6-day incubation, consistent also with the peptide conformation and fibrillogenic propensity illustrated in Figs. 1A and D.
Influence of Aβ peptides on endothelial proliferation
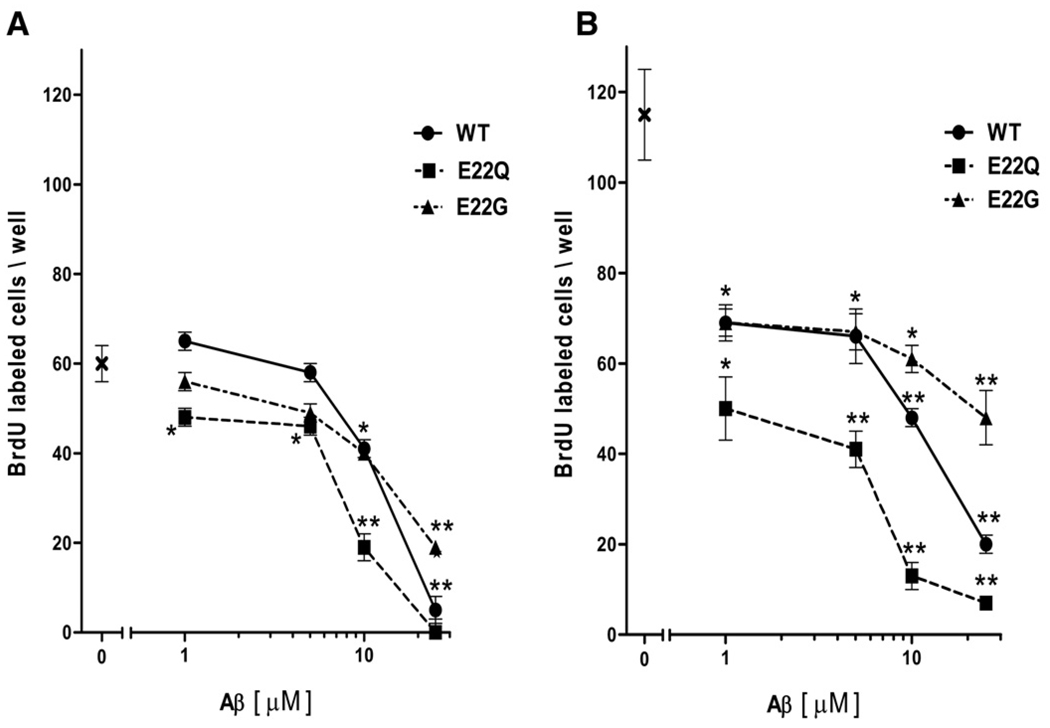
We assessed the influence of Aβ peptides on brain endothelial cells (HBMEC) by determining their ability to incorporate bromodeoxy-uridine (BrdU) during the late stage (6 h) of incubation (48 h). Perhaps as a result of their structural differences, the various Aβ peptides (1 to 25 µM) produced a distinct pattern of BrdU incorporation. While all peptides exhibited negligible effects on proliferation up to 5 µM, differences were observed at the highest concentrations (Fig. 2A). Both, WT and E22Q had a significant effect on cell proliferation at 25 µM, achieving 90% (p < 0.05) and total inhibition (p < 0.01), respectively. In contrast, the E22G peptide exhibited milder effects on proliferation, producing significant outcome only at 25 µM with 60% inhibition (p < 0.01, vs. control, respectively, Fig. 2A). The reverse Aβ sequence used as control exerted no effect even at 25 µM (data not shown).
Fig. 2.
Aβ peptides (WT, E22Q, E22G) affect HBMEC proliferation. (A) Cells were exposed to Aβs (1–25 µM) for 48 h and growth was evaluated by BrdU incorporation. Data are reported as cell number counted/well. Numbers represent mean ± SEM. *P < 0.05 and *P < 0.01 versus controls by Student's t-test. (B) Aβ effects on proliferation induced by FGF-2 (20 ng/ml). X: denotes initial cell counts.
We further examined whether the peptides affected the endothelial cells' responses to angiogenic stimuli, and therefore to express their angiogenic phenotype, following challenge with FGF-2 or VEGF at competent doses (20 and 50 ng/ml, respectively). FGF-2, as VEGF (not shown), nearly doubled BrdU incorporation, taken as a measure of phenotype expression (Fig. 2B). Co-incubation with the Aβ peptides drastically prevented, although up to different extent, the formation of the angiogenic phenotype. In fact, while the exposure to peptide E22Q abruptly reduced BrdU incorporation at concentrations as low as 1 µM (60% inhibition, p < 0.01 vs. control), with > 90% inhibition at higher concentrations (Fig. 2B), the effects exerted by WT and particularly by E22G were different. Both were characterized by a slower decline in BrdU incorporation following the initial fast decrease noted at 1 µM, but at higher concentration (25 µM) the WT induced marked inhibition (~80%), although not as potent as E22Q, whereas E22G only reached about 50% inhibition (Fig. 2B). Responses to VEGF, as well as their inhibition exerted by Aβ peptides, reproduced those observed with FGF-2 (data not shown).
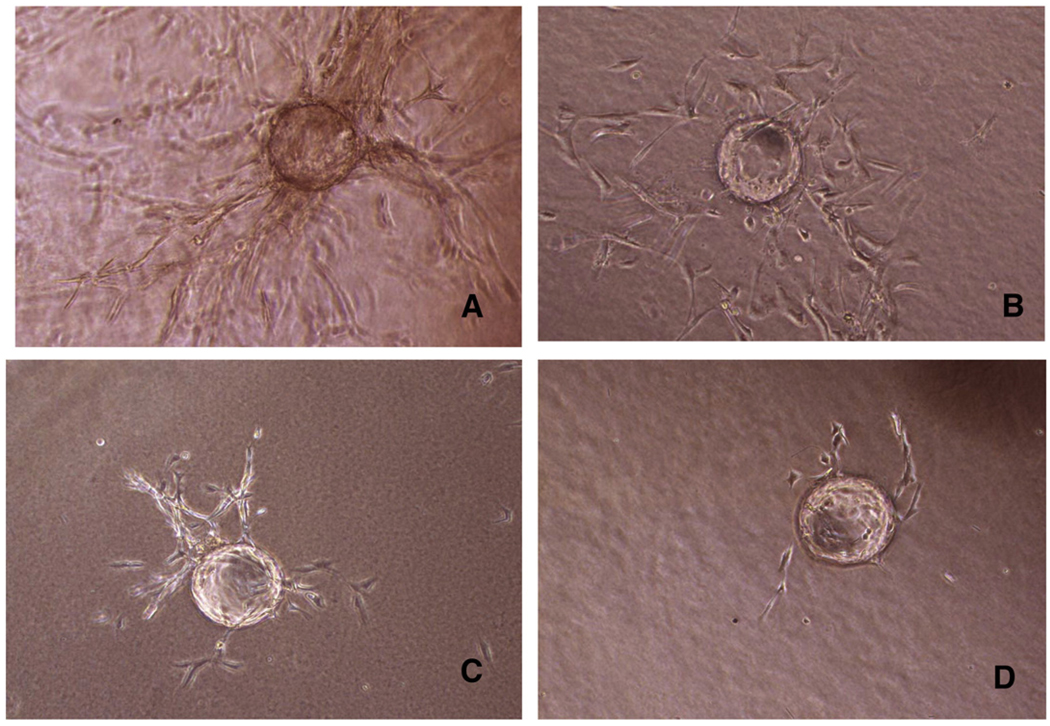
Aβ peptides differentially affect the formation of pseudo-capillaries
Pseudo-capillaries produced by HBMEC on microcarriers, spreading in the fibrin gel, provide a measure of the cell angiogenic potential. Challenge with FGF-2 (20 ng/ml) induced the formation of a flourishing network of pseudo-capillaries (Fig. 3A), which was influenced at a variable extent by the co-incubation with the Aβ peptides. Morphologically, E22Q nearly suppressed the pseudo-capillary sprouting (Fig. 3D), while peptide E22G only reduced the sprouting area by ~30% (Fig. 3B). WT exerted an intermediate effect on pseudo-capillaries formation, relative to the former peptides (Fig. 3C). Quantitation of the area covered by pseudo-capillaries indicates a very significant effect of E22Q and WT, with the former achieving the highest inhibition, while the effect of E22G failed to reach significance, in agreement with the cell proliferation data illustrated in Fig. 2.
Fig. 3.
Formation of pseudo-capillaries from HBMEC. Cytodex microcarriers were coated with HBMEC, and then embedded in fibrin gel. Incubation was carried out for 2 days. Pseudo-capillaries sprouting were monitored by an inverted microscope at 20× magnification, using an ocular grid. Representative pictures of capillaries formation after FGF-2 stimulation (A) or after co-incubation with Aβ peptides (E22G, WT and E22Q; B, C, D, respectively) are shown.
Influence of Aβ peptides on the FGF-2 pathway
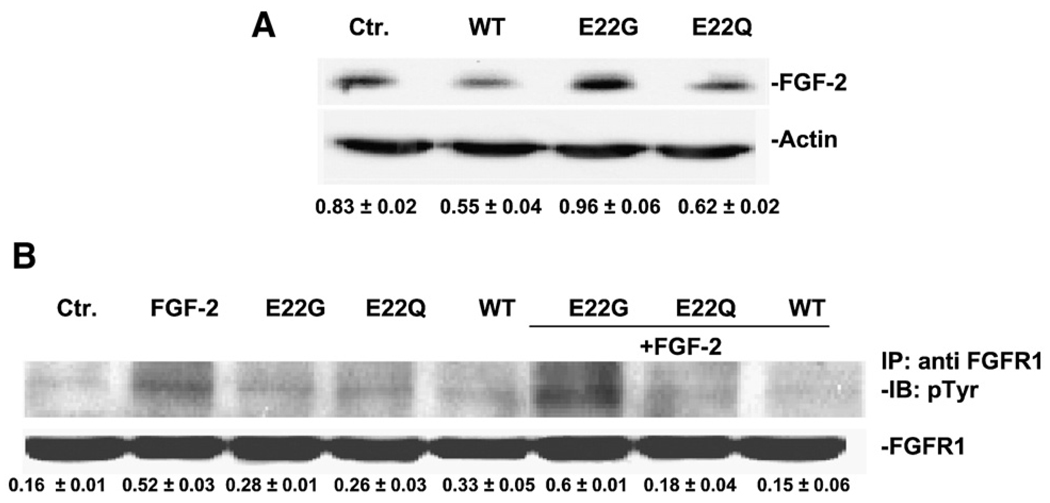
In a previous report we have documented the relationship between endogenous FGF-2 and Aβ40 ability to cause endothelial injuries [13]. We extended those observations and compared the effect of mutated peptides on FGF-2 production by measuring its expression in HBMEC following incubation (18 h) with the peptides under study at a concentration (5 µM), level that would not impair endothelial cell survival (see Fig. 2). The robust FGF-2 signal detected in HBMEC, compared to other endothelial cell lines routinely used in our laboratory, e.g. HUVEC, was fully maintained following peptide E22G exposure (Fig. 4A, lane 3). Conversely, both the WT and E22Q peptides negatively affected FGF-2 production, leading to its decreased expression (Fig. 4A, lanes 2 and 4). Values underneath bands, representing Optical Densities obtained by scanning gels from this and subsequent experiments provided values of signals which were subjected to ANOVA analysis (reported in individual legends).
Fig. 4.
FGF-2 expression and FGFR-1 phosphorylation response to Aβ peptides. (A) The effect of WT, E22Q and E22G on FGF-2 production was assessed by Western blotting. A representative gel out of three with similar results is shown. Normalization of optical densities (OD, FGF-2/Actin) is indicated below each lane. ANOVA test showed significant difference between control and treated group. P < 0.01 for WT or E22Q vs. Ctr. (B) FGFR-1 phosphorylation in cells pre-treated with Aβs (5 µM), in response to FGF-2 (20 ng/ml, 15 min). FGFR-1 was immunoprecipitated (IP), its activation was investigated by anti-Tyr antibody and the results were normalized with total FGFR-1. The gel shown is representative of three experiments exhibiting similar results. P < 0.01 for FGF-2+WT or FGF-2+E22Q vs. FGF-2.
Given the key role of FGFR-1 stimulation as a signal that initiates the autocrine FGF-2 loop, we investigated its phosphorylation in cells exposed to the various Aβ peptides. The phosphorylation, detected by an antibody recognizing phosphorylated tyrosines, was evaluated in immunoprecipitates with an anti-FGFR-1 antibody, in membranes isolated from cells exposed to Aβ peptides (5 µM, 15 min) in the presence/absence of FGF-2. While, in quiescent cells the phosphorylation signal was barely detectable (Fig. 4B, lane 1), exposure to the natural ligand FGF-2 (20 ng/ml) produced marked FGFR-1 phosphorylation (lane 2). Application of E22G peptide left the FGFR-1 phosphorylation unchanged, whereas WT or E22Q peptides reduced phosphorylation to levels observed in quiescent cells (Fig. 4B; compare lanes 6–8 with lane 2).
PI3K/Akt, as a component of the phosphorylation cascade ensuing FGFR-1 stimulation, is a known pro-survival signal in the endothelium. We measured Akt phosphorylation following short exposure (30 min) of cells to the Aβ peptides (5 µM); slight changes relative to control (0.1% FBS) occurred in quiescent cells. However, as expected, upon challenge with FGF-2, Akt phosphorylation was highly increased (Fig. 5A, lane 2). Combined challenge with FGF-2 and Aβ peptides resulted in a marked reduction of Akt phosphorylation WT and E22Q, whereas the influence of E22G was weak (Fig. 5A; compare lanes 6–8 with lane 2).
Fig. 5.
Effect of Aβ on FGF-2-induced Akt phosphorylation and caspase-3 activation. (A) Akt phosphorylation was evaluated by Western blotting as described in Materials and methods. The gel shown is representative of three exhibiting similar results. Results were normalized versus total Akt and the respective pAkt/total-Akt optical densities are indicated below each lane. ANOVA test showed significant difference between control and treated group. P < 0.01 for FGF-2 + WT or FGF-2 + E22Q or FGF-2 + E22G vs. FGF-2. (B) For Caspase-3 activation, HBMEC were pre-treated with Aβs (5 µM, 18 h) in the presence/absence of FGF-2 (20 ng/ml). Western blotting analysis measured cleaved caspase-3. Results were normalized versus Actin. The gel shown was representative of three experiments exhibiting similar results. P < 0.01 for WT or E22Q Ctr. vs. and FGF-2 + WT vs. FGF-2; P < 0.05 for FGF-2 + E22Q vs. FGF-2.
For further downstream signal analysis we measured the cleaved caspase-3 expression. In control conditions (0.1% FBS) or following FGF-2 stimulation for 18 h, the cleaved caspase-3 expression was negligible, whereas WT peptide and E22Q produce a marked increase (8–9 times relative to control). In contrast, peptide E22G produced negligible effects on enzyme activation (Fig. 5B). FGF-2 administration, concomitantly with Aβ peptides, failed to prevent caspase-3 activation by WT and E22Q peptides (compare lanes 6 and 7 vs. lane 2).
Influence of Aβ peptides in the induction of apoptosis
Based on the ability of E22Q and WT peptides to inhibit phosphorylation of the Akt cascade (Fig. 5A), a well known pro-survival signal in the endothelium, together with their capability to activate caspase-3 (Fig. 5B), we measured the peptides' effect in inducing apoptosis. Human microvascular endothelial cells were challenged with the different Aβ peptides for 1–3 days as described in Materials and methods, and apoptosis evaluated by the formation of nucleosomes (DNA–histone complexes) by ELISA. E22Q exerted the most potent apoptotic effect, being the only one capable of inducing nucleosome formation (1.5-fold respect to control) at day 1 (not shown). After 3-days challenge, and as illustrated in Fig. 6, E22Q continued to be the most potent apoptosis inducer (almost 2.5-fold increase respect to control), followed by WT (1.3-fold), while E22G had no effect under the conditions tested, in agreement with its negligible effect in other EC functions like cell proliferation and caspase-3 activation.
Fig. 6.
Apoptotic response of Aβ peptides. Quantitation of nucleosome formation by Cell death Elisa in human microvascular endothelial cells after challenge with WT, E22Q, and E22G peptides (50 µM; 3 days). Results are expressed as fold of increase, compared to the control without peptide.
Discussion
This study highlights novel biological properties of Aβ40 mutated peptides (E22Q and E22G), in comparison with the parent WT, in endothelial cells derived from human brain microvessels. First, all peptides negatively affected endothelial cell proliferation at low micromolar concentrations, although important differences were noted in their responses to angiogenic stimuli (FGF-2 and VEGF). Second, the ability to down-regulate signals of the FGF-2 pathway (FGF-2 expression, FGFR-1 activation and Akt phosphorylation) revealed even greater differences among the peptides studied. While the WT and E22Q peptides severely suppressed the FGF-2 system, the E22G peptide exhibited negligible effects, at least at sub-toxic concentrations. Third, the peptides differed also in the apoptotic effects elicited in brain microvascular endothelial cells with E22Q exerting the most potent response correlating with the peptide's powerful ability to inhibit proliferation, angiogenesis and phosphorylation of the Akt survival signaling. Consistent with its aggressive behavior as a toxicity inducer [9,10] the E22Q peptide emerged as a potent insult to the brain endothelium, preventing the expression of the angiogenic phenotype promoted by FGF-2 or VEGF more intensely and at lower concentration than the other variants. In addition, the expression of the angiogenic phenotype as revealed by their ability to form pseudo-capillaries in a 3D-fibrin gel, an in vitro assay that surrogates the in vivo situation, provided results consistent with the conclusions described above.
The mechanism underlying the Aβ-induced endothelial dysfunction lies, as indicated by the present evidence, in the downregulation of the FGF-2 signaling cascade which appears to be related to the peptides' effects on the acquisition of the angiogenic phenotype. Both WT and E22Q strongly suppressed the expression/production of the 18 kDa isoform of FGF-2, while E22G application left it unchanged. Similarly, both WT and E22Q – but not E22G – severely reduced phosphorylation of the FGF-2 receptor, FGFR-1, the initial crucial step in the activation of endogenous FGF-2 synthesis, which maintains endothelial integrity. Signals downstream of FGFR-1 (e.g. PI3K/Akt and cleaved caspase-3 expression) changed accordingly. These data indicate that the decrease in Akt phosphorylation, enhanced caspase activation, and subsequent apoptosis induction produced by E22Q and to a minor extent by WT reflect their ability to interfere with the FGF-2 ligand interaction with its receptor.
The notion that Aβ peptides, beside the cytotoxic effect for vessel wall cells, induce impairment of angiogenesis, was first proposed by Mullan et al. [12]. In a previous report from our laboratory, we have illustrated the downregulation of FGF-2 pathway as a causative mechanism for endothelial injuries and impairment of angiogenesis produced by the WT peptide [13]. This work presents evidences showing that mutated peptides, particularly E22Q, characterized by strong vascular tropism, also target FGF-2 pathway preventing the evolution of the angiogenic phenotype.
Aggregation/fibrillization of Aβ plays a critical role in neuro-degeneration and it is now considered that the transition from the soluble circulating monomeric species to the oligomeric, protofibrillar and end-point fibrillar species plays a significant role in disease pathogenesis. In particular, oligomeric and protofibrillar forms seem to display the most potent effect in neuronal cells inducing synaptic disruption and neurotoxicity [27]. Mutants with accelerated formation of these intermediate species as well as increased stability of such structures, particularly the Arctic variant, elicit an early and more dramatic effect than the wild type peptide [8,24,28]. The effect of different Aβ genetic variants on cerebral vascular cells has in contrast not been so well characterized. However, in the case of E22Q, the most studied variant, the exacerbated peptide toxicity for endothelial and smooth muscle cells [10,11] in comparison with the WT is also mediated by oligomeric/aggregated states [29]. To our knowledge no studies have been performed in cerebral vessel wall cells with the Arctic variant.
The E22G peptide rapidly forms protofibrillar structures in the time frame of our experimental paradigm, but these assemblies, notable for their neurotoxic potential, do not influence endothelial cells' angiogenic functions or induce dramatic apoptotic responses. Whether this relates to a specificity of action of the different mutants for different cell types remain to be elucidated. The formation of oligomers/protofibrils shared by the E22Q and E22G variants may elicit contrasting cell responses in vessel wall and neuronal cells, perhaps correlating with the different clinical phenotypes associated with the respective mutants. While patients with the E22Q mutation, highly active in endothelial cells in vitro, show striking cerebral hemorrhage episodes in the virtual absence of cognitive impairment (which only develops after multiple strokes), patients with the E22G variant, showing limited activity for endothelial cells in our studies, exhibit total absence of hemorrhagic episodes in the presence of severe dementia and neurodegeneration.
What drives this dichotomy still remains to be clarified although a number of factors including differences in the diversity of the species composing the deposits within the various pathological entities, as well as in the brain clearance mechanisms of the different genetic variants may play a significant part. The heterogeneity of the deposits is also a likely differential contributor to the severity of CAA. In the case of the Dutch variant, the vascular amyloid, largely constituted by 40-residues-long peptide as in the case of sporadic and other familial AD cases [10,30–34], contained a mixture of wild type and mutated species at a 1:1 stoichiometric ratio [35,36]. Recently, the additional presence of Aβ42 species carrying the E22Q mutation was demonstrated as minor components of the vascular lesions [37]. It is likely that the heterogeneity of the deposits containing species with high tendency to fibrillize may exert a seeding effect or induce conformational mimicry as demonstrated in vitro with synthetic homologues [9], and thereby contribute to the aggressiveness of the Dutch phenotype. Whether heterogeneity exists in the Arctic deposits or play any role in the disease clinical phenotype remains to be clarified since to the moment no biochemical studies have been performed in these cases.
The present observations, showing a nearly comparable endothelial damage produced in vitro by the WT and E22Q peptides, pose the question of their relevance for the respective CAA pathologies, which differ substantially in their clinical manifestations. It is plausible that factors other than the endothelial injury may be operant in vivo to modulate the toxic damage of the peptides. For example, as mentioned above differences among the peptides on their clearance or uptake by the endothelium, in conjunction with their differential susceptibility to enzymatic degradation [38] may alter the final effect on the vasculature. In fact, the E22Q substitution significantly reduced the peptide's clearance from the brain, reducing its transport across the blood brain barrier and the vascular drainage pathways with subsequent accumulation of the peptide around the blood vessels and in brain [39]. This phenomenon was recapitulated in transgenic models carrying the E22Q mutation together with the D23N Iowa substitution [40]. Additional factors may contribute to the hemorrhagic phenotype expressed in vivo by E22Q in contrast to the WT counterpart and the E22G variant. A typical example is the differential secretion and activation of proteases (e. g. matrix metalloproteases) induced by the peptides which may be significant contributors to the disease symptoms. In this sense it has been demonstrated that E22Q, in difference to the WT counterpart, induces secretion and activation of MMP-2 by vessel wall cells [41] which may disrupt membrane permeability, contribute to the loss of vessel wall integrity and result in hemorrhagic stroke.
Overall, the data presented herein indicate that the presence of similar structures do not necessarily evoke the same cellular response and suggest that the toxic effect of the oligomeric Aβ assemblies goes beyond mere multimerization.
Supplementary Material
Acknowledgments
This publication was supported in part by Telethon-Project no. GGP06148, and Progetto Ordinario Regione (MZ), the NIH grants NS051715 (AR) and AG, 010491 (JG) and by the American Heart Association (JG and AR).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.yexcr.2008.11.002.
REFERENCES
- 1.Renskin AAM, de Waal RMW, Kremer B, Verbeek MM. Pathogenesis of cerebral amyloid angiopathy. Brain Res. Rev. 2003;43:207–223. doi: 10.1016/j.brainresrev.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Vinters HV. Cerebral amyloid angiopathy: a critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 3.Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, Frangione B, Holton JL. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J. Neuropathol. Exp. Neurol. 2003;62:885–898. doi: 10.1093/jnen/62.9.885. [DOI] [PubMed] [Google Scholar]
- 4.Maia LF, Mackenzie IRA, Feldman HH. Clinical phenotypes of cerebral amyloid angiopathy. J. Neurol. Sci. 2007;15:23–30. doi: 10.1016/j.jns.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 5.Yamada M. Cerebral amyloid angiopathy: an overview. Neuropathology. 2000;1:8–22. doi: 10.1046/j.1440-1789.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 6.Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GTAM, Luyendijk W, Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral haemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 7.Maat-Schieman M, Roos R, Van Duinen S. Hereditary cerebral hemorrhage with amyloidosis-Dutch type. Neuropathology. 2005;25:288–297. doi: 10.1111/j.1440-1789.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 8.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Näslund J, Lannfelt L. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat. Neurosci. 2001;4:293–887. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 9.Wisniewski T, Ghiso J, Frangione B , Peptides homologous to the amyloid protein of Alzheimer's disease containing a glutamine for glutamic acid substitution have accelerated amyloid fibril formation. Biochem. Biophys. Res. Commun. 1991;179:1247–1254. doi: 10.1016/0006-291x(91)91706-i. [DOI] [PubMed] [Google Scholar]
- 10.Miravalle L, Tokuda T, Chiarle R, Giaccone G, Bugiani O, Tagliavini F, Frangione B, Ghiso J. Substitutions at codon 22 of Alzheimer's abeta peptide induce diverse conformational changes and apoptotic effects in human cerebral endothelial cells. J. Biol. Chem. 2000;275:27110–27116. doi: 10.1074/jbc.M003154200. [DOI] [PubMed] [Google Scholar]
- 11.Paris D, Humphrey J, Quadros A, Patel N, Crescentini R, Crawford F, Mullan M. Vasoactive effects of A beta in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer's disease: role of inflammation. Neurol. Res. 2003;25:642–651. doi: 10.1179/016164103101201940. [DOI] [PubMed] [Google Scholar]
- 12.Paris D, Townsend K, Quadros A, Humphrey J, Sun J, Brem S, Wotoczek-Obadia M, DelleDonne A, Pate N, Obregon DF, Crescentini R, Abdullah L, Coppola D, Rojiani AM, Crawford F, Sebti SM, Mullan M. Inhibition of angiogenesis by Ab peptides. Angiogenesis. 2004;7:75–85. doi: 10.1023/B:AGEN.0000037335.17717.bf. [DOI] [PubMed] [Google Scholar]
- 13.Donnini S, Cantara S, Morbidelli L, Giachetti A, Ziche M. FGF-2 overexpression opposes the beta amyloid toxic injuries to the vascular endothelium. Cell Death Differ. 2006;13:1088–1096. doi: 10.1038/sj.cdd.4401803. [DOI] [PubMed] [Google Scholar]
- 14.Paris D, Aitghezala G, Mathura VS, Patel N, Quadros A, Laporte V, Mullan M. Anti-angiogenic activity of the mutant Dutch Aβ peptide on human brain microvascular endothelial cells. Mol. Brain Res. 2005;136:212–230. doi: 10.1016/j.molbrainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, Rostagno A, Frangione B. Systemic catabolism of Alzheimer's Abeta40 and Abeta42. J. Biol. Chem. 2004;279:45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 16.Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochem. Biophys. Acta. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Walsh DM, Hartley DM, Kusumoto Y, Yezoui F, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 18.Levine H. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 19.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell. Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 20.Weksler BB, Subileau EA, Perriere N, Charmeau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turoski P, Male DK, Rouix F, Greenwood J, Romeo IA, Couraud PO. Blood–brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 21.Nehls V, Drenckhahn D. A novel microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvas. Res. 1995;50:311–322. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- 22.Cantara S, Donnini S, Morbidelli L, Giachetti A, Schulz R, Memo M, Ziche M. Physiological levels of amyloid peptides stimulate the angiogenic response through FGF-2. FASEB J. 2004;18:1943–1945. doi: 10.1096/fj.04-2114fje. [DOI] [PubMed] [Google Scholar]
- 23.Cam J, Meyerson J, Mezhericher E, Frangione B, Ghiso J, Rostagno A. Differential apoptotic response of primary human cerebral endothelial cells to oligomeric assemblies of amyloid beta genetic variants. In: Fisher A, Hanin I, Poewe W, Windisch M, editors. New Trends in Alzheimer and Parkinson Related Disorders. Bologna, Italy: Medimond International Proceedings; 2008. pp. 141–146. [Google Scholar]
- 24.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 25.Päiviö A, Jarvet J, Gräslund A, Lannfelt L, Westlind-Danielsson A. Unique physicochemical profile of beta-amyloid peptide variant Abeta1–40E22G protofibrils: conceivable neuropathogen in Arctic mutant carriers. J. Mol. Biol. 2004;339:145–159. doi: 10.1016/j.jmb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid beta-protein fibrillogenesis: detection of a protofibrillar intermediate. J. Biol. Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 27.Walsh DM, Selkoe DJ. Aβ oligomers — a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 28.Whalen BM, Selkoe DJ, Hartley DM. Small non-fibrillar assemblies of amyloid beta-protein bearing the Arctic mutation induce rapid neuritic degeneration. Neurobiol. Dis. 2005;20:254–266. doi: 10.1016/j.nbd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhauer PB, Johnson RJ, Wells JM, Davies TA, Fine RE. Toxicity of various amyloid beta peptide species in cultured human blood–brain barrier endothelial cells: increased toxicity of Dutch-type mutant. J. Neurosci. Res. 2000;60:804–810. doi: 10.1002/1097-4547(20000615)60:6<804::AID-JNR13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J. Biol. Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- 31.Miller DL, Papayannopoulos IA, Styles J, Bobin SA, Lin YY, Biemann K, Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch. Biochem. Biophys. 1993;301:41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- 32.Shin Y, Cho HS, Fukumoto H, Shimizu T, Shirasawa T, Greenberg SM, Rebeck GW. Abeta species, including IsoAsp23 Abeta, in Iowa-type familial cerebral amyloid angiopathy. Acta Neuropathol. 2003;105:252–258. doi: 10.1007/s00401-002-0639-0. [DOI] [PubMed] [Google Scholar]
- 33.Kumar-Singh S, Cras P, Wang R, Kros JM, van Swieten J, Lübke U, Ceuterick C, Serneels S, Vennekens K, Timmermans JP, Van Marck E, Martin JJ, van Duijn CM, Van Broeckhoven C. Dense-core senile plaques in the Flemish variant of Alzheimer's disease are vasocentric. Am. J. Pathol. 2002;161:507–720. doi: 10.1016/S0002-9440(10)64207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, Roos RA, Frosch MP, Greenberg SM. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prelli F, Levy E, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Expression of a normal and variant Alzheimer's beta-protein gene in amyloid of hereditary cerebral hemorrhage, Dutch type: DNA and protein diagnostic assays. Biochem. Biophys. Res. Commun. 1990;170:301–307. doi: 10.1016/0006-291x(90)91274-v. [DOI] [PubMed] [Google Scholar]
- 36.Castaño EM, Prelli F, Soto C, Beavis R, Matsubara E, Shoji M, Frangine B. The length of amyloid-beta in hereditary cerebral hemorrhage with amyloidosis, Dutch type. Implications for the role of amyloid-beta 1–42 in Alzheimer's disease. J. Biol. Chem. 1996;271:32185–32191. doi: 10.1074/jbc.271.50.32185. [DOI] [PubMed] [Google Scholar]
- 37.Nishitsuji K, Tomiyama T, Ishibashi K, Kametani F, Ozawa K, Okada R, Maat-Schieman ML, Roos RA, Iwai K, Mori H. Cerebral vascular accumulation of Dutch-type Abeta42, but not wild-type Abeta42, in hereditary cerebral hemorrhage with amyloidosis, Dutch type. J. Neurosci. Res. 2007;85:2917–2923. doi: 10.1002/jnr.21413. [DOI] [PubMed] [Google Scholar]
- 38.Morelli L, Llovera R, Gonzalez SA, Affranchino JL, Prelli F, Frangione B, Ghiso J, Castaño EM. Differential degradation of amyloid beta genetic variants associated with hereditary dementia or stroke by insulin-degrading enzyme. J. Biol. Chem. 2003;278:23221–23226. doi: 10.1074/jbc.M300276200. [DOI] [PubMed] [Google Scholar]
- 39.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer's amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol. Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 40.Davis J, Xu F, Miao J, Previti ML, Romanov G, Ziegler K, Van Nostrand WE. Deficient cerebral clearance of vasculotropic mutant Dutch/Iowa Double A beta in human A betaPP transgenic mice. Neurobiol. Aging. 2006;27:946–954. doi: 10.1016/j.neurobiolaging.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Jung SS, Zhang W, Van Nostrand WE. Pathogenic A beta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J. Neurochem. 2003;85:1208–1215. doi: 10.1046/j.1471-4159.2003.01745.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.