Abstract
According to behavioral genetics research, the intergenerational correlation in weight derives solely from shared genetic predispositions, but complete genetic determinism contradicts the scientific consensus that social and behavioral change underlies the modern obesity epidemic. To address this conundrum, this article utilizes sibling data from the National Longitudinal Study of Adolescent Health and extends structural equation sibling models to incorporate siblings’ genetic relationships to explore the role of families’ social characteristics for adolescent weight. The article is the first to demonstrate that the association between parents’ obesity and adolescent weight is both social and genetic. Furthermore, by incorporating genetic information, the shared and social origins of the correlation between inactivity and weight are better revealed.
Intuitively, we know that there is an intergenerational correlation in weight. We expect thin parents to have thin children and overweight parents to have overweight children. But what does it mean that being thin or overweight “runs in the family?” What is the source of that similarity? The current assessment from behavioral genetics research – the customary arbiter of how genetic or social a particular trait is – is that the intergenerational correlation in weight is entirely due to shared genetic predispositions and that the shared social characteristics of families are insignificant (for a review, see Maes, Neale and Eaves 1997).
The assertion of exclusive genetic determinism, however, does not fit within the larger scientific discourse about the importance of social and behavioral change for the recent obesity epidemic (Kumanyika 2001). Specifically, genetic variation cannot explain the changes in obesity prevalence over the last 20 years (French, Story and Jeffery 2001; Kumanyika 2001). To better appreciate the social origins of this epidemic, we must think about both within- and between- population differences. Let us consider the distribution of weight prior to the obesity epidemic using data on adults from the 1890s. At that time, the distribution was reasonably close to a normal curve (Helmchen and Henderson 2004) and each person’s weight reflected a variety of risk factors, including his or her genetic predispositions. Within this population, only 4% of adults were obese (Helmchen and Henderson 2004). Since then, the entire weight distribution has shifted upward (Friedman 2003; Helmchen and Henderson 2004) and now nearly one-third of the U.S. adult population is obese (Ogden et al. 2006). Underlying genetic predispositions did not change in such a microscopic slice of evolutionary time, though they remain an important factor for locating a person within the population’s weight distribution. Instead, social, economic, and technological changes generated the upward shift in the population’s weight. This population shift means that many of today’s parents were thin during their primary school years but are now at greater risk of being overweight, as are their children. Their personal genetic profiles did not change.
The question remains whether the social and behavioral shifts important for population trends are reflected in and contribute to the intergenerational correlation in weight. Do families contribute more than their genes to children’s weight and, if so, how do familial social characteristics and processes matter? It is likely that genetic predispositions account for a large proportion of the intergenerational correlation in weight, but in the current context, familial social characteristics likely also play a role.
To challenge the claims of behavioral genetics research, we must adopt a different theoretical perspective and methodological approach. One key task is to query the theoretical association between familial social characteristics and their genetic predispositions. We generally assume that there is a positive association between social and genetic factors that, if ignored, inflates the estimated importance of families’ social characteristics. But genes, like any other omitted variable, could theoretically be uncorrelated or even negatively correlated with familial social characteristics. If there were indeed a negative correlation, we would actually underestimate the “true” role of social influences if genetic predispositions were ignored. In the following analyses, I find examples of all three patterns: positive correlation, no correlation, and negative correlation between genes and familial social characteristics. Furthermore, the associations between adolescent weight and both family inactivity and their physiological development are actually stronger in models that incorporate genetic information than in those that do not. Thus, the incorporation of genetic predispositions helps to reveal the social role of these family characteristics.
I also adopt a different methodological approach to demonstrate the social inheritance of weight and uncover the familial social factors involved. Behavioral genetics models provide reasonable estimates of genetic heritability, but are limited in their ability to estimate the role of shared environmental effects. The current analyses are an adaption of structural equation sibling models that incorporate the degree of genetic resemblance between siblings. This model accounts for gene-environment correlations and estimates the role of specific familial social characteristics for adolescent weight. This approach can be generalized to study other intergenerational correlations hypothesized to have both social and genetic underpinnings. Together, this theoretical articulation and methodological innovation provide a means to incorporate genetic information, while retaining a resolute focus on the sociological roots of intergenerational correlations.
THE SOCIOLOGICAL ASSUMPTION: FAMILIAL SOCIAL PROCESSES ARE SIGNIFICANT FOR WEIGHT
A variety of social, economic and technological shifts are hypothesized to underlie the upward shift in population weight, including increased use of labor-saving devices for work and leisure, sugar consumption, and reliance on automobiles for transportation (French, Story and Jeffery 2001). Although there is not a definitive explanation (or explanations) for the population shift in weight, one can ask whether these hypothesized factors are so universal as to overwhelm the patterning of family characteristics and processes or whether families are integral to their proliferation. Given that the layering-on of excess weight generally takes years of small, cumulative caloric imbalances that emerge through the daily patterning of our lives, it is likely that micro interactions and experiences, including those operating within families, position individuals relative to obesity-promoting risks.
Through their status characteristics, families bridge macro-level structural factors and micro-level processes of individual behavior as they reflect and funnel larger social processes and provide a setting within which individual habits develop and are deployed. Specific status characteristics salient for adolescent weight are parents’ socioeconomic status (SES) and whether or not they are immigrants. Given that parents and children share socioeconomic resources and that many children of immigrants are immigrants themselves, correlations in these status characteristics could foster a correlation in weight.
Parents’ SES is hypothesized to indirectly influence weight through the proximate determinants of nutrition and activity because of the relatively low prices for calorie-dense, nutritient-poor foods (Drewnowski and Specter 2004) and the stratified availability of physical activity options (Gordon-Larsen et al. 2006). At a more distal level, SES is associated with other health risks related to obesity, such as acute and chronic stress (Björntorp 2001; McLeod and Kessler 1990), depressive mood and psychosocial strain (Goodman, Slap and Huang 2003), access to quality medical care (Haas et al. 2003), neighborhood quality (Brooks-Gunn et al. 1993), and health-related knowledge (Link et al. 1998). Furthermore, as Link and Phelan (1995) note, the true value of socioeconomic resources is that they can be deployed instrumentally to combat an array of health risks. This being the case, family SES should create unequal exposure to the obesiogenic structural factors and, for those of privilege, mediate their effects. Recent research documents a significant association between parents’ SES, measured as either family income or parents’ educational attainment, and overweight during childhood and adolescence (Goodman, Slap and Huang 2003; Haas et al. 2003; Strauss and Knight 1999).
A family’s immigrant status in the United States is also correlated with weight, such that immigrants are leaner than their native counterparts (Popkin and Udry 1998). Differences in lifestyle modes, specifically immigrants’ healthier diet and greater physical activity, account for this observed difference given that weight increases across generations as immigrants’ children acculturate to U.S. lifestyle patterns (Gordon-Larsen et al. 2003; Popkin and Udry 1998). Immigrants’ relative thinness is particularly noteworthy given their typically low SES (Gordon-Larsen et al. 2003; Popkin and Udry 1998).
Correlated with these status characteristics is another familial structural attribute that could be important for adolescent weight: the distribution of resources within a family. In particular, the number of siblings within a family could be inversely related to adolescent weight. Just as having more siblings dilutes family resources for children’s intellectual development (Zajonc 1976), children in larger sibships may be leaner than their peers with few siblings: with more mouths to feed, there are fewer calories available per child, controlling for SES.
Such stratified families, in turn, shape members’ daily activities, especially for children and adolescents. Through overt control and subtle role modeling, parents likely influence their children’s weight by affecting children’s weight-related behaviors and beliefs. Parental control likely plays a key role as parents organize and structure the two health behaviors implicated in the obesity epidemic – eating and physical activity (Dietz and Gortmaker 2001; French, Story and Jeffery 2001). Parents control their children’s nutrition and physical activity across the children’s development. Parents purchase food and prepare meals, monitor children’s time use, and enroll them in after-school activities. Parents’ overt and covert control can significantly impact adolescents’ weight through the regulation of nutrition and physical and sedentary behavior (Arluk et al. 2003; Ogden, Reynolds and Smith 2006). In addition, parents socialize their children to share similar values, attitudes and behaviors related to eating, physical activity, and weight concerns (Bruss et al. 2005; Ogden and Chanana 1998). From an early age, parents model their weight-related preferences and orientations, as well as their level of self-restraint (Birch and Fisher 1998; Fogelholm et al. 1999; Wardle et al. 2001).
Familial patterns in weight likely emerge over many years with the accumulation of numerous interactions. Thus, the three familial dimensions just discussed (i.e., status characteristics, parental control, and socialization) likely intersect to condition a child’s developmental life course. Intergenerational processes and inequalities begin very early, even influencing children’s prenatal experiences (e.g., Barker et al. 1993), as evidenced by the similarities in birth weight amongst siblings (Conley, Strully and Bennett 2003). These initial life experiences set in motion important weight trajectories, such that heavier babies tend to become heavier adolescents (Dietz and Gortmaker 2001; Oken and Gillman 2003).
Parental control, socialization and status characteristics also produce a shared lifestyle, which then organizes family members’ future interactions and weight-related behavior. Although health behaviors or “lifestyles” are typically conceptualized as individual characteristics, theorists such as Weber, Bourdieu, and Giddens recognize that lifestyles are chosen from among limited possibilities defined by a person’s social constraints (Cockerham, Rutten and Abel 1997). Therefore, children from the same family, facing similar social constraints within and outside the family, are likely to have similar lifestyles. Prior research demonstrates that family members share lifestyle patterns and that these collective lifestyles are significant for adolescent weight. Adolescents who eat dinner with their family are less likely to be overweight (Taveras et al. 2005), as are adolescents who consistently eat breakfast (Affenito et al. 2005; Berkey et al. 2003). In addition, changes in the father’s energy intake and enjoyment of activity are associated with changes in the child’s body mass index (Davison and Birch 2001). Therefore, a family’s shared lifestyle could contribute to the intergenerational association of weight.
The repeated interactions within stratified families generate numerous avenues for influencing adolescents’ weight. Reflecting these accumulated exchanges, research demonstrates that the social and behavioral risk factors for obesity cluster within families (Davison and Birch 2001; Guillaume et al. 1995). There are strong correlations between parents’ and children’s nutritional intake (Mitchell et al. 2003; Oliveria et al. 1992), eating practices (Francis and Birch 2005), physical activity (Fogelholm et al. 1999; Mitchell et al. 2003), and television viewing (Davison, Francis and Birch 2005). Therefore, one would expect that intergenerational correlations in these proximate determinants of weight undergird the intergenerational correlation in weight. In the current analysis, I directly examine familial status characteristics and indirectly investigate parental control and children’s socialization. The specific mechanisms tested are shared familial lifestyles (with indicators of the similarity in siblings’ meal patterns and physical activity), initial developmental inequalities (with an indicator of birth weight), and familial status characteristics (with indicators of parents’ education, family income, sibship size, and immigrant status).
COUNTERFACTUAL EVIDENCE: FAMILY RESEMBLANCE IN WEIGHT IS ONLY GENETIC
Although sociologists expect families’ social and behavioral characteristics to be important for adolescent weight, behavioral geneticists consistently find that the shared social characteristics of families make no contribution to individual weight (Carmichael and McGue 1995; Comuzzie and Allison 1998; Jacobson and Rowe 1998; Maes, Neale and Eaves 1997; Sorensen, Holst and Stunkard 1992).1 Instead, they find that there is only a genetic basis for observed family similarities in weight and that environmental influences, when they exist, are unique to each sibling (Hetherington, Reiss and Plomin 1994). Although studies of young, co-residential siblings find a greater role for siblings’ shared environments and lower estimates of genetic heritability than do studies of non-residential, adult siblings (Carmichael and McGue 1995; Knuiman et al. 1996; Maes, Neale and Eaves 1997), the bulk of the evidence indicates that parent-child correlations in weight derive from their shared genetic profiles, not from their shared experiences.
In addition, behavioral geneticists caution that the documented correlations in weight-related behaviors among family members, such as the correlations in eating behaviors and television viewing noted above, could result from genetic predispositions, not from familial social processes. These shared behaviors, they argue, reflect gene-environment correlations, namely passive or evocative gene-environment correlations (Scarr and McCartney 1983). In passive gene-environment correlations, parents’ genetic predispositions influence the environments they provide for their children, which in turn influence their children’s development (Plomin, DeFries and Loehlin 1977; Scarr and McCartney 1983). In evocative gene-environment correlations, one person’s genetic predispositions determine the feedback that she receives from her social and physical environments and thereby elicit change in their environments (Scarr and McCartney 1983). For example, if a young child is genetically predisposed to desire physical activity, then she may respond emotionally to physical activity or its absence. As a result, her responsive parents may spend more time with her in more physical activities. Thus, what looks like a shared family lifestyle may emanate from the child’s genetic predispositions. With either type of gene-environment correlation, genetic predispositions, not social characteristics, generate the family correlations in weight-related behaviors. In support of this argument, behavioral genetics research finds a strong genetic basis for nutritional preferences and practices (Keski-Rahkonen et al. 2004; Reed et al. 1997; Tholin et al. 2005) and birth weight and weight trajectories (Parsons, Power and Manor 2001; Pietilainen et al. 2001), but only weak to modest genetic heritability (with a greater role for shared environment) in physical activity and inactivity (Franks et al. 2005; Goran 1997; Nelson et al. 2006; Plomin et al. 1990)
To arrive at these heritability conclusions, behavioral geneticists examine estimates from a biometrical model fitted to data from twin, adoption or family studies to estimate what proportion of the variance in weight can be attributed to genetic effects, environmental factors shared within a family, or environmental factors that are distinct to each sibling (Neale and Cardon 1992). Several strong assumptions are made to calculate these models (Maes, Neale and Eaves 1997; Parens 2004), many of which maximize the genetic heritability estimates relative to the variance attributed to the environment (Goldberger 1979). Significant for the current analysis are two assumptions that limit the examination of shared environmental effects. First, the models are unable to investigate the role of measured, shared family mechanisms (Parens 2004) because of the emphasis placed on portioning the variance into its genetic and environmental components (Turkheimer et al. 2005). Second, behavioral genetics models place greater emphasis on individual experience within the family than the family setting itself, reflecting the fact that thee models’ disciplinary home is psychology. The variance attributed to nonshared environment in these models thus captures any and all differences between siblings’ perception and reaction to environmental experiences, even for structurally shared experiences (Turkheimer et al. 2005). With limited information to adjudicate between shared experiences and siblings’ differential reactions and perceptions, the model’s assumptions privilege individual experiences and therefore have limited capacity to uncover the role of shared environmental exposures. In sum, behavioral genetics models are robust, but they are not conclusive about the role of shared environmental characteristics operating within families, given their core assumptions. Therefore, the current analysis takes a different methodological approach to address the relative contribution of familial processes.
CONCEPTUAL FRAMEWORK
Both behavioral geneticists and sociologists typically assume that there is a positive correlation between social factors and genetic predispositions and that ignoring this correlation leads to inflated estimates of the role of social factors for a given trait. Yet, as noted previously, the fundamental relationship between a particular social characteristic and a specific genetic allele or the sum total of a person’s genetic make-up could be positive, zero, or even negative. Recognizing the possible variation in these unobserved relationships can stimulate new hypotheses and new ways of thinking about how genetic information may (or may not) change our current understanding of sociologically-relevant traits.
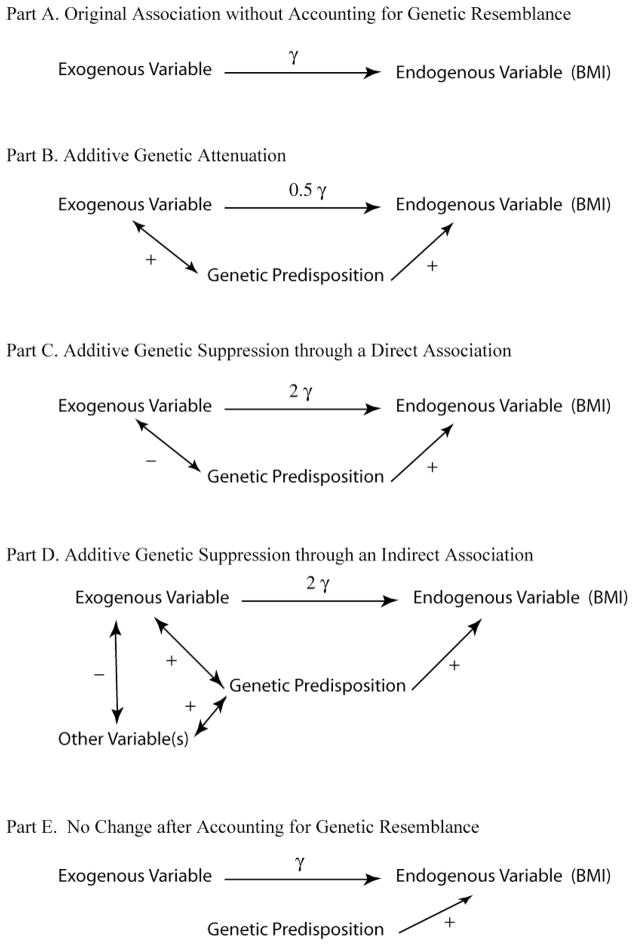
Figure 1 demonstrates each theoretical option, generically referring to exogenous and endogenous characteristics and genetic predispositions. Panel A in Figure 1 demonstrates our standard, sociological model of the effect of an exogenous characteristic on an endogenous characteristic, weight (measured as BMI). The estimated coefficient should decline (as shown in part B, relative to part A of figure 1) if genetic predispositions are positively correlated with both weight and the exogenous characteristic. This is the typical theoretical expectation for the direction of change in genetically informed models given our assumptions.
Figure 1.
Attenuation and Suppression in Genetically-Informed Models
If the estimated coefficient increases with the inclusion of genetic information, however, then there is a suppression effect in the standard sociological model generated by a positive correlation between genetic predispositions and weight and a negative correlation between genetic predispositions and the exogenous variable. This negative correlation could arise through either a direct association (part C of fig. 1) or an indirect association with another variable (part D of figure 1). The existence of a suppressor effect is less intuitive, but it could arise through the patterning of social responses to our traits with genetic underpinnings. In the simplest scenario, a person’s genetic predispositions for a given trait could be at odds with her social environments to the point where others directly act to counter her genetic predispositions, such as when parents overtly try to help underweight children gain weight. The suppression could also arise through more complex dynamics, involving “other variables,” referenced in partl D of figure 1, that unfold over the life course, akin to the processes described by Freese (2008 [in this issue]). For example, the family’s SES or other family processes could be both positively correlated with genetic predispositions and negatively correlated with the exogenous variable, which would then strengthen the association between the exogenous and endogenous variables once genetic predispositions were included. This pattern is particularly likely for characteristics that mediate the effects of structural inequalities.
If little to no change is exhibited, then the association between weight and the exogenous characteristic is unrelated to genetic predispositions (part E of fig 1). The lack of an association could arise relative to a specific allele or with regard to the sum total of a person’s genetic makeup in analyses that examine differences across siblings with different genetic resemblances. In the latter case, a zero correlation could exist either because either there is no significant association between the social characteristic and any alleles or because various associations across alleles cancel each other out.
We can hypothesize how the family characteristics investigated in the current analysis are likely to change with the incorporation of genetic information. First, estimated coefficients for parental obesity should decline, given that the correlation between parents’ and children’s weight is partially due to the transmission of parents’ genes to their children (a passive gene-environment correlation). Second, immigrant status should not change because immigrant status should be orthogonal to genetic predispositions given that the immigrant advantage has been linked to behavioral differences, particularly better nutrition and physical activity (Gordon-Larsen et al. 2003; Popkin and Udry 1998), and to differences in economic development between the United States and typical immigrant-sending countries (Monteiro et al. 2004). Third, the association with family SES should likely diminish in a genetically informed model, given that parents’ socioeconomic attainment could be hindered if they are overweight (Cawley 2004; Crosnoe 2007; Gortmaker et al. 1993; Pagan and Davila 1997) and that parents transmit genes relevant for both weight and socioeconomic attainment to their children (a passive gene-environment correlation). Fourth, the pattern for sibship size is more difficult to predict, but this association could also decline, given that parents’ fertility is expected to be under greater genetic control with the loosening of social mores following the second demographic transition (Udry 1996). Fifth, the coefficients for lifestyle variables could either decrease or increase. On the one hand, they should decrease if there is a strong genetic basis underlying these behavioral patterns. Given previous behavioral genetics estimates of heritability, this is more likely for nutrition-related indicators than for physical activity and inactivity. On the other hand, these associations should increase because of the negative association between weight-increasing lifestyle patterns and family SES (Drewnowski and Specter 2004; Gordon-Larsen et al. 2006) and the anticipated positive association between genetic predispositions and SES. Finally, the estimates for birth weight could either increase or decrease, for reasons identical to those for lifestyle patterns.
In sum, sociologists expect parents’ social characteristics to be significant for adolescent weight, but behavioral genetics models conclude otherwise. This conclusion, however, is at least partially due to the assumptions embedded within the standard behavioral genetics model. With a new approach, I seek to provide demonstrable evidence of familial social contributions. Furthermore, my analysis proposes that the association between specific family characteristics could increase, decrease or remain the same in this genetically-informed model, depending on the underlying processes and relationships heretofore unobserved. The incorporation of genetic resemblance in the models should allow for greater confidence in a social and behavioral interpretation of these mechanisms.
DATA AND METHODS
I utilize data from the National Longitudinal Study of Adolescent Health (Add Health), a nationally representative school-based sample of adolescents in grades 7–12 in 1994 (Udry 2003). After an in-school interview in 1994, a stratified sample of adolescents was chosen for an in-home interview and parent interview in 1995 (wave 1) and a follow-up interview in 1996 (wave 2). For my purposes, the tremendous asset of the data is the special oversample of adolescent pairs living in the same household during wave 1 – specifically, twins, other full siblings, and half siblings (N = 2,477 pairs in waves 1 and 2).2 Sibling pairs were identified in the in-school survey, but previously unreported twins discovered during the in-home interview were also interviewed. Because discovered twins did not attend sampled schools, probability sample weights cannot be used (Chantala and Tabor 1999; Harris et al. 2003).
The final sample excludes pairs in which either sibling was pregnant or had an unknown pregnancy status between 1994 and 1996 (n = 197), because pregnancy is associated with weight changes. Next, I use a random number generator in Stata to randomly select one sibling pair per family for families with three or more surveyed adolescents, in order to obtain unbiased standard errors in the models.3 The final sample comprises 1,704 sibling pairs from 1,704 families. Twins of undetermined zygosity (i.e., those for whom it is unclear whether they are identical or fraternal twins) are included in the general models, but not in models that incorporate siblings’ genetic resemblance.
I utilize multiple imputation to address missing data, which replaces missing values with predictions based on information observed in the sample (Rubin 1987). I multiply impute the full Add Health sample before adolescents are matched to their siblings using the freely-available ICE software within Stata (Royston 2005a; 2005b). ICE uses a different estimation equation for each variable depending on the variable’s properties (using OLS, logistic, ordinal or multinomial logistic regression as appropriate) and performs well with interactions among variables (i.e., not imputing Z from the interaction term ZxW; Acock 2005; Royston 2005b). In the imputations, I interact all variables with the adolescent’s sex to investigate these interactions in supplementary empirical models. I create five imputed data sets and combine empirical results across all imputed samples, accounting for the variation within and between imputed data sets (Acock 2005; Rubin 1987). Appendix Table A1 presents the sample sizes across the imputed data sets for the sibling pairs analyzed.
Appendix Table A1.
Number of sibling pairs by sex and relationship across multiply imputed data sets
| Imputed Data Set #1 | ||||||
|---|---|---|---|---|---|---|
| MZ Twins | DZ Twins | Full Siblings | Half Siblings | UD Twins | N | |
| Male-Male | 115 | 108 | 270 | 79 | 14 | 586 |
| Female-Female | 106 | 83 | 230 | 62 | 23 | 504 |
| Female-Male | 0 | 141 | 351 | 122 | 0 | 614 |
| N | 221 | 332 | 851 | 263 | 37 | 1,704 |
| Imputed Data Set #2 | ||||||
| MZ Twins | DZ Twins | Full Siblings | Half Siblings | UD Twins | N | |
| Male-Male | 119 | 105 | 271 | 80 | 15 | 590 |
| Female-Female | 108 | 84 | 226 | 63 | 23 | 504 |
| Female-Male | 0 | 143 | 342 | 125 | 0 | 610 |
| N | 227 | 332 | 839 | 268 | 38 | 1,704 |
| Imputed Data Set #3 | ||||||
| MZ Twins | DZ Twins | Full Siblings | Half Siblings | UD Twins | N | |
| Male-Male | 119 | 107 | 268 | 80 | 14 | 588 |
| Female-Female | 104 | 82 | 232 | 60 | 23 | 501 |
| Female-Male | 0 | 144 | 346 | 125 | 0 | 615 |
| N | 223 | 333 | 846 | 265 | 37 | 1,704 |
| Imputed Data Set #4 | ||||||
| MZ Twins | DZ Twins | Full Siblings | Half Siblings | UD Twins | N | |
| Male-Male | 118 | 104 | 269 | 80 | 14 | 585 |
| Female-Female | 105 | 83 | 232 | 60 | 23 | 503 |
| Female-Male | 0 | 142 | 346 | 128 | 0 | 616 |
| N | 223 | 329 | 847 | 268 | 37 | 1,704 |
| Imputed Data Set #5 | ||||||
| MZ Twins | DZ Twins | Full Siblings | Half Siblings | UD Twins | N | |
| Male-Male | 114 | 106 | 267 | 82 | 15 | 584 |
| Female-Female | 104 | 84 | 227 | 61 | 23 | 499 |
| Female-Male | 0 | 141 | 352 | 128 | 0 | 621 |
| N | 218 | 331 | 846 | 271 | 38 | 1,704 |
Source: Random sample of sibling pairs per family in the National Longitudinal Study of Adolescent Health, Waves 1 and 2
Note: UD Twins refers to twins of unidentified zygosity.
Measures
Adolescent weight
Weight is measured as BMI (weight in kilograms/[height in meters]2). In wave 2, adolescents reported their weight and height and Add Health interviewers also weighed and measured them. To improve the reliability of the dependent variable, I create two measures: self-reported BMI and interviewer-measured BMI. Although interviewer-measured BMI is more accurate (Strauss 1999), self-reported BMI correctly classifies 96% of adolescents according to obesity status and has been validated for research use (Goodman, Hinden and Khandelwal 2000). Because of adolescents’ rapid physical development (Ogden et al. 2002b), it is important to account for developmental stage in modeling adolescent weight. To model BMI net of developmental stage, I regress BMI on two wave 1 indicators of the adolescent’s personal physiological development and then use the residual from that regression in all analyses.4 The two indicators of physical development are (1) adolescents’ self-report of their overall physical development relative to same-sex peers their age and (2) a principal components factor score of their physical development across specific domains. The factor score for boys is based on their self-reported amount of facial hair, underarm hair, and the depth of their voice relative to boys their same age. The factor score for girls is based on their self-reported breast development and overall curvature relative to girls their same age.
Parental obesity
At wave 1, the parental respondent reported whether the adolescent’s biological mother and biological father were “obese.” These two items are combined to create the following three dichotomous variables, with a reference category of those without any obese parents: (1) both biological parents are obese, (2) only the biological mother is obese, and (3) only the biological father is obese. Given that parental obesity is reported, it is subject to measurement error and likely captures those who are severely overweight (Greenleaf et al. 2004).
Familial status characteristics
Parents’ education, measured in years and averaged in two-parent families, is obtained from the parents’ report, but supplemented with the adolescent’s report when parents’ data are missing. The family’s income-to-needs ratio is measured as the parents’ report of family income divided by the poverty threshold appropriate for the family size and number of children in the household.5 Values equal to or below 1.0 reflect living in poverty while values above 1.0 equals the multiple by which family income exceeds the poverty line. The size of adolescents’ sibships is based on their wave 1 report of their household composition. Adolescent’s immigration status is based on their report of being born abroad.
Familial social mechanisms
Several measures are used to create lifestyle indicators. An adolescent’s physical activity is measured as spells of “roller-blading, roller-skating, skate-boarding, or bicycling” activity in wave 1, based on a standard seven-day recall questionnaire on physical activity that has been validated in other studies (Baranowski 1988; Sallis et al. 1993).6 Response options were “not at all,” “1 or 2 times,” “3 or 4 times,” and “5 or more times” per week. Inactivity is a principal components factor score based on three items in wave 1: the usual number of hours per week spent watching television, watching videos, and playing video or computer games, respectively. The measure of frequency of eating meals is a principal components factor score of the number of days within the last seven that the adolescent reported eating breakfast, lunch, and dinner at wave 2.
Birth weight
Parental respondents reported adolescents’ birth weight in pounds and ounces, which I convert into decimals of pounds.
Age
Adolescents’ age (in years) at wave 1 is an individual-level control variable to account for the social development of weight across ages.
Methods
Quantitative behavioral genetics models
For comparability with prior research and the structural equation sibling models, I estimate quantitative behavior genetics models separately by sex with data for monozygotic and same-sex dizygotic twins. The models estimate the role of additive genetic variation (A), dominance genetic variation (D), twins’ shared environment (C), and nonshared environment (E) (Neale and Cardon 1992).7 Because it is not possible to simultaneously estimate D and C with data from twins reared together, I estimate the following models: ACE, ADE, AE, and CE. I then determine the best fitting model using RMSEA and AIC (Neale and Cardon 1992) and calculate heritability (h2) from that model.
Structural equation sibling models
With two siblings per family and the siblings’ independent reports of their family characteristics, it is possible to estimate a structural equation sibling model (Teachman, Carver and Day 1995). The model has two levels: one level to evaluate the role of specific, shared family characteristics that lead to differences in weight between families and a second level to evaluate the role of specific individual characteristics for each sibling’s deviation from the family’s average weight (Hauser 1988). Within each level, there is a measurement model to assess how well the particular survey items capture these family and individual characteristics. Accounting for measurement error is particularly important in these analyses, because items are self-reported, and the bias generated by measurement error is exacerbated when examining relationships at two or more levels (i.e., families and individuals; Griliches 1979). In the current application, the measurement model also serves an important, substantive purpose for modeling siblings’ shared lifestyle. Family lifestyle characteristics are measured by both siblings’ reports and, therefore, reflect the average lifestyle of the two siblings or the collective dimension of family lifestyles. One final note about the current model: to better examine familiial social characteristics for adolescent weight I only include adolescents’ age as an individual characteristic to conserve degrees of freedom. Thus, I minimally model within-family differences in weight.
To control for genetic predispositions, I simultaneously estimate four separate sibling models – one for each type of genetic resemblance (i.e., monozygotic twins, dizygotic twins, full siblings, and half siblings). I then test what parameters are equal across the four genetic resemblance types. A key test for this analysis is to determine whether the family characteristics operate similarly across genetic resemblance types. If so, I can then compare the results from a simple sibling model that pools all pairs and the models that incorporate genetic resemblance to know how the parameter estimates change in magnitude and significance with the inclusion of genetic information. For more information on these models, see appendixes A and B.
Limitations
Structural equation sibling models are preferable to hierarchical linear models given the risks of measurement error, but the measurement model has two limitations. First, the dependent variable must have multiple measures (i.e., self-reported BMI and measured BMI) to arrive at reliable estimates. To minimize the potential bias that could result from using self-reported BMI, the dependent variable loads onto measured BMI. Second, it is only possible estimate a measurement model when there are multiple continuous indicators for a characteristic. Because of this, there are no estimated measurement models for parental obesity, nativity, and age.
The data also have limitations. First, the sibling subsample is not nationally representative and I cannot account for Add Health’s complex survey design. Second, the analysis only reflects families with more than one co-residential adolescent. Third, I must rely on self-reports of lifestyle and parents’ obesity, though the current analysis more thoroughly addresses measurement error relative to prior studies. Parents’ self-reported obesity introduces another limitation, however, because the measures of parents’ and adolescent weight are not parallel. Finally, the data are now over 10 years old and thus are less able to explain current associations.
RESULTS
Because this is a specialized sample, a few sample characteristics are worth noting. The proportion of adolescents who are overweight (BMI > 95th percentile for sex-specific age growth charts; Ogden et al. 2002b) at wave 2 (12%) is similar to national estimates of overweight prevalence for 1988–1994 (11%) and 1999–2000 (13%), the closest years for which data are available (Ogden et al. 2002a; Troiano and Flegal 1998). As for parents’ obesity, 6% of adolescents have two obese parents, 14% have only an obese mother, and 5% have only an obese father. The other sample characteristics fit well with national estimates and are presented in Table 1.
Table 1.
Unweighted descriptive statistics of variables for full sample, Averaged across multiply imputed data sets
| Sibling 1 |
Sibling 2 |
|||
|---|---|---|---|---|
| Mean, % | S.D. | Mean, % | S.D. | |
| Adolescent Body Mass Index | ||||
| Original BMI, reported | 22.89 | (4.64) | 22.92 | (4.67) |
| Original BMI, measured | 22.90 | (4.90) | 22.94 | (4.93) |
| Residualized BMI, reported | 10.16 | (0.34) | 10.16 | (0.35) |
| Residualized BMI, measured | 13.39 | (0.49) | 13.40 | (0.50) |
| Parental Obesity | ||||
| Both obese | 5.6% | 5.7% | ||
| Only mom obese | 14.2% | 14.0% | ||
| Only dad obese | 4.6% | 4.4% | ||
| Neither obese | 75.6% | 75.8% | ||
| Age, Wave 1 | 15.49 | (1.72) | 15.48 | (1.72) |
| Family Status Characteristics | ||||
| Parents education, avg. years | 13.19 | (2.50) | 13.20 | (2.50) |
| Income-to-needs ratio | 2.45 | (2.84) | 2.47 | (2.85) |
| Sibship size | 2.07 | (1.23) | 2.06 | (1.23) |
| Immigrant status, adolescent | 7.8% | 8.2% | ||
| Family Social Mechanisms | ||||
| Birth weight | 6.77 | (1.61) | 6.79 | (1.61) |
| Ride bikes/skate, times/week | 0.66 | (0.95) | 0.64 | (0.95) |
| Factor score: meals, Wave 2 | −0.02 | (1.04) | −0.03 | (1.05) |
| Breakfasts, days last week | 4.23 | (2.57) | 4.25 | (2.57) |
| Lunch, days last week | 5.52 | (2.14) | 5.52 | (2.15) |
| Dinner, days last week | 6.26 | (1.57) | 6.23 | (1.57) |
| Factor score: inactivity, Wave 1 | 0.30 | (1.27) | 0.00 | (1.02) |
| Television, usual hours/week | 16.49 | (15.26) | 16.50 | (15.37) |
| Videos, usual hours/week | 4.62 | (7.04) | 4.77 | (7.14) |
| Video/comp. games, usual hours/week | 3.05 | (6.06) | 3.11 | (6.18) |
| Physical Development for Residualizing BMI | ||||
| General assessment, scale: 1–5 | 3.12 | (1.12) | 3.13 | (1.12) |
| Factor score: development | −0.02 | (1.03) | −0.02 | (1.03) |
Source: Multiply imputed, random sample of sibling pairs per family in Add Health, Waves 1 & 2
Note: Standard errors in parentheses.
The sample reveals the clustering of weight among family members across generations and siblings. To examine parent-child associations, I regress individual adolescents’ residualized, interviewer-measured BMI on parental obesity. Adolescents with two obese parents are 0.48 BMI points heavier than adolescents whose parents are not obese (P < .0001), which is an increase of approximately one standard deviation. Adolescents with only an obese mother are 0.24 BMI points heavier (p < .0001) than adolescents without obese parents, and adolescents with only an obese father are 0.14 BMI points heavier (P < .001). This difference between the associations of adolescent BMI and mother’s obesity and of adolescent BMI and father’s obesity is statistically significant (P < .05) and does not vary according to the child’s sex or when additional individual characteristics are controlled (all individual weighted analyses are not displayed but are available upon request).
Sibling correlations also reveal the clustering of weight within families. For these analyses, I examine the sibling pair data and calculate the correlation in siblings’ BMI for the full pairs sample and separately for each genetic relationship. Overall, the correlation in residualized interviewer-measured BMI is 0.42, but the correlations are greatest for monozygotic twins (0.73) and smallest for half siblings (0.22), with dizygotic and full siblings falling in between (0.33 and 0.45, respectively) (all correlations are significant at P < .001). These correlation differences indicate the genetic basis for weight, because they increase as the level of genetic similarity increases.
Behavioral Genetics Models
Given these genetic differences in sibling correlations, I estimate a series of behavioral genetics models separately by sex to uncover the heritability of weight (calculated as residualized measured BMI) in these data. The models provide a comparison to prior research and complement the structural equation sibling models. Across the multiply imputed data sets, a model identifying additive genetic and unshared environmental factors (the AE model) fits best for females and a model identifying additive genetic, nonadditive genetic and unshared environment factors (the ADE model) fits best for males (see appendix table B1). Although different models are preferred for girls and boys, the heritability estimates are equal. Seventy-four percent of the variation in BMI in this sample can be attributed to genetic predispositions. Regardless of sex, all environmental effects operate as non-shared effects and account for 26% of the variation in BMI. Like previous research, these results imply that the social dimension of families is negligible for weight.
Appendix Table B1.
Results from behavior genetics models for residualized measured BMI, Averaged across multiply imputed data sets
| Female Pairs |
Male Pairs |
|||||||
|---|---|---|---|---|---|---|---|---|
| ACE | ADE | AE | CE | ACE | ADE | AE | CE | |
| χ2 | 15.54 | 17.6279 | 15.20 | 25.0954 | 15.54 | 17.6279 | 15.20 | 25.0954 |
| df | 3 | 3 | 3 | 4 | 3 | 3 | 3 | 4 |
| AIC | 20.84 | 22.22 | 20.35 | 29.69 | 20.84 | 22.22 | 20.35 | 29.69 |
| RMSEA | 0.205 | 0.216 | 0.201 | 0.241 | 0.205 | 0.216 | 0.201 | 0.241 |
| a2 | - | - | 0.73 | - | - | 0.09 | - | - |
| d2 | - | - | - | - | - | 0.65 | - | - |
| e2 | - | - | 0.26 | - | - | 0.26 | - | - |
| h2 | - | - | 0.73 | - | - | 0.74 | - | - |
Source: Monozygotic and same-sex dizygotic twin pairs in the study sample from the National Study of Adolescent Health, Waves 1 and 2
Note: Best fitting model in bold; AIC, Akaike’s information criterion; RMSEA, root mean square error approximation.
Structural Equation Sibling Models for all Sibling Pairs
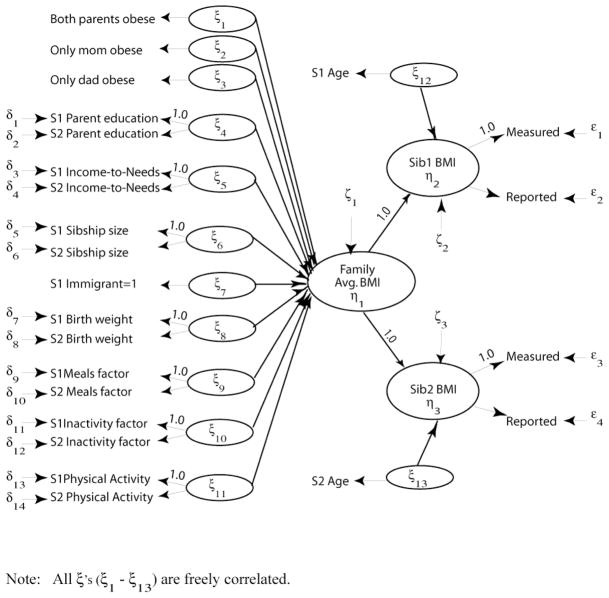
Structural equation sibling models take a different approach to account for genetic resemblance, focusing instead on estimating the role of specific familial social characteristics. But before modeling differences across genetic pairs, I examine patterns for all siblings. I estimate two models: the first only includes parental obesity and adolescent age; the second adds all family characteristics. A graphical representation of the full structural equation sibling model is presented in figure 2, wherein the latent constructs for the structural model are represented by ovals and the observed indicators are represented by text. Observed indicators with an estimated measurement model are indicated by an arrow from either δx or εy. The left side of the figure depicts the between-family regression predicting differences across families in “family average BMI.” The top- and bottom-right parts of figure 2 detail the two within-family regressions predicting sibling 1’s and sibling 2’s respective BMI. The disturbance term capturing all unobserved characteristics of families is labeled ζ1 and the disturbance terms for the unobserved characteristics of Sibling 1 and Sibling 2 are labeled ζ2 and ζ3, respectively.
Figure 2.
Basic Structural Equation Sibling Model with Controls
Table 2 presents the estimated structural path coefficients (γ, represented by arrows to “family BMI,” “sibling1 BMI,” and “sibling 2 BMI” in figure 2) for the best fitting reduced-form and full models. The standardized estimates can be interpreted like correlation coefficients to compare estimates across variables with different metrics. As expected, older adolescents and children of obese parents are heavier. Parental obesity is, of course, a strong predictor of adolescent weight. If both parents are obese, siblings’ average BMI increases by 0.41 points and if only the mother is obese, average BMI increases by 0.21. In this reduced-form model, father’s obesity is not statistically significant. When the additional family characteristics are included, the coefficients for having two obese parents or an obese mother decline slightly, suggesting that these other characteristics partially explain the associations between parents’ and children’s weight. The path for father’s obesity actually increases modestly and becomes statistically significant, implying a suppressor effect in the model excluding additional family controls. As in the individual correlations above, mother’s obesity is more strongly correlated with adolescent weight than is father’s obesity, but model fit tests reveal that these apparent differences are not statistically significant. Mothers’ and fathers’ obesity place adolescent boys and girls at equal risk for increased weight.8 The difference between these sibling results and the initial individual models in the significance of mothers’ and fathers’ obesity could be due to the inability of individual models to adequately model family-level characteristics (Teachman 1995; Teachman, Carver and Day 1995); sibling models better control for unobserved interfamilial differences that affect the study outcome (Hauser and Mossel 1985)
Table 2.
Structural path coefficients for final sibling models by sex, With and without family control variables and averaged across multiply imputed data sets. N = 1,090
| Reduced-Form Model (Model F) |
Full Model (Model N) |
|||||
|---|---|---|---|---|---|---|
| γ | S.E. | Standardized | γ | S.E. | Standardized | |
| Parental Obesity (relative to neither parent obese) | ||||||
| Both parents obese | 0.408 | 0.067 ** | 0.256 | 0.377 | 0.068 ** | 0.236 |
| Only mom obese | 0.214 | 0.043 ** | 0.210 | 0.185 | 0.043 ** | 0.182 |
| Only dad obese | 0.120 | 0.070 | 0.076 | 0.137 | 0.067 * | 0.088 |
| Individual Characteristics | ||||||
| S1 Age, Wave 1 | 0.030 | 0.011 ** | 0.104 | 0.028 | 0.012 * | 0.097 |
| S2 Age, Wave 1 | 0.028 | 0.010 ** | 0.097 | 0.025 | 0.010 * | 0.087 |
| Family Characteristics | ||||||
| Parents’ educationa | - | - | - | −0.012 | 0.005 * | −0.116 |
| Income-to-needs ratioa | - | - | - | −0.022 | 0.037 | −0.038 |
| Sibship size | - | - | - | −0.019 | 0.012 | −0.063 |
| Immigrant | - | - | - | −0.102 | 0.049 * | −0.078 |
| Birth weighta | - | - | - | 0.009 | 0.005 | 0.093 |
| Meals factor | - | - | - | −0.114 | 0.032 ** | −0.214 |
| Inactivity factor | - | - | - | 0.073 | 0.037 * | 0.123 |
| Bike, skate per week | - | - | - | −0.028 | 0.037 | −0.038 |
Note: Standardized coefficients are akin to correlations for comparison across measures.
Two-tailed tests of significance for model coefficients:
p < .05,
p < .01
These variables have been transformed for inclusion in the models. See Appendix A.
Next, I ask which familial characteristics help explain the association between parental obesity and children’s weight. The primary contributors are family lifestyle characteristics. Adolescents in families where siblings regularly eat three meals a day are leaner than those where siblings miss meals. Also, adolescents are heavier when families spend more hours engaged in inactivity. A secondary contributor is parents’ education. Adolescents’ weight declines as parents’ education increases, suggesting that socioeconomic disparities confound the social inheritance of weight. Finally, immigrant adolescents are leaner than their native-born peers. The family’s income-to-needs ratio, number of siblings, birth weight, and physical activity (measured as biking and skating) are not statistically significant.
I next examine the model’s ability to explain the variance in BMI both within and between families. Note that the variance in BMI within families (0.232 for sibling 1 and 0.243 for sibling 2) is greater than the variance between families (0.121), such that 66% of the total variation in BMI lies within families. The greater within-family variation may help to explain why behavioral genetics models identify such a large role for non-shared environments. The final model explains 22% of the between-family variance in BMI (= 1−[Ψ11/0.121 = 1 − [0.094/0.121]) and 52% of the within-family variance in BMI (sibling 1 = 1−[Ψ22/0.232] = 1 − [0.108/0.232]; sibling 2 = 1−[Ψ33/0.242] = 1 − [0.119/0.242]). Incorporating the genetic resemblance of siblings should further explain the within-family variance in weight, but not the between-family variance.
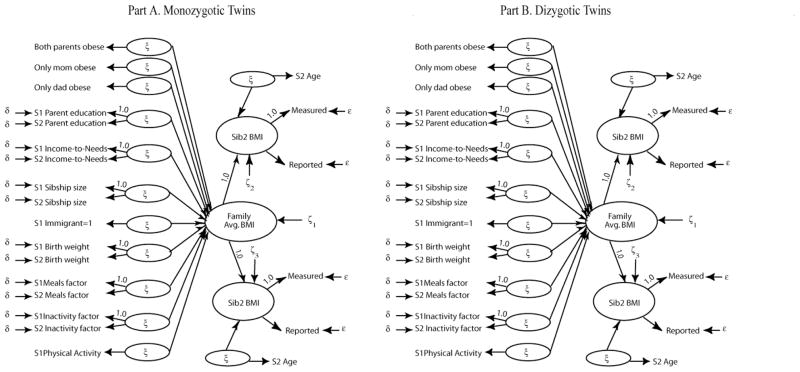
Structural Equation Sibling Models by Genetic Resemblance
I next estimate multiple groups models across different sibling relationships to analyze the patterning and similarity of BMI across genetic resemblances. The only difference between the genetic resemblance models and the models for all siblings is that I cannot estimate a measurement model for physical activity. Thus, the latent factor is set to equal sibling 1’s measure of physical activity.9 To incorporate genetic resemblance, I simultaneously estimate a separate sibling model for each genetic relationship, with all parameters freely estimated, as pictured in Figure 3. Then, I test which model parameters are equal across groups, beginning with whether the measurement properties of the model are similar across pairs and then evaluating the structural component of the model. For the model fit tests, see appendix table B2. Model fit improves dramatically when siblings’ genetic resemblance is accounted for, suggesting a significant genetic basis for weight. The best fitting models suggest that the measurement models and the structural coefficients for the effects of age and the family characteristics are equal across genetic pairs. The final model finds all parameters equal except for some differences in the variance of BMI across families (“family BMI”) and differences in the variance in BMI within families (“siblings’ BMI), such that the variance is smaller for monozygotic twins than for all other pairs. Given that the paths from the family characteristics to family BMI are equal, we can compare how the path coefficients change relative to table 2 once genetic information is incorporated.
Figure 3.
Structural Sibling Model to Include Genetic Predispositions: All Parameters Freely Estimated for All Genetic Resemblance Groups (Appendix Table B2, Model A)
Appendix Table B2.
Goodness-of-fit tests for sibling model differences across genetic resemblance, Averaged across multiply imputed data sets. N = 1,667
| Model Comparison | ||||||
|---|---|---|---|---|---|---|
| Averaged L2 | df | Averaged BIC | Contrast | BIC | ||
| Full Model | ||||||
| A | All parameters freely estimated across genetic resemblance groups | 433.8 | 584 | −3898.8 | ||
| Tests of equality in measurement model | ||||||
| B | A + λy’s invariant across all groups | 436.2 | 590 | −3940.9 | B - A | −42 |
| C | B + θε’s invariant across all groups | 469.9 | 602 | −3996.2 | C - B | −55 |
| D | C + λx’s invariant across all groups | 471.5 | 620 | −4128.1 | D - C | −132 |
| E | D + θδ’s invariant across all groups | 738.6 | 656 | −4128.2 | E - D | 0 |
| Tests of equality in structural model | ||||||
| F | E + Φ’s invariant across all groups, except for covariance of siblings’ age: FS = HS & DZ = MZ | 1371.0 | 928 | −5513.7 | F - E | −1517 |
| G | F + γ’s invariant across sex | 1299.8 | 967 | −5874.2 | G - F | −360a |
| H | G + φ1 (Family BMI) invariant across all groups | 1343.8 | 970 | −6121.2 | H - G | −247a |
| I | G + φ1 (Family BMI) invariant for all but MZ | 1310.5 | 969 | −6140.4 | I - G | −266a |
| J | G + φ2 & φ3 (Siblings’ BMI) equal for FS & DZ | 1313.9 | 970 | −6145.1 | J - I | −5a |
| K | J + φ2 & φ3 (Siblings’ BMI) equal for FS, DZ & HS | 1330.7 | 971 | −6139.1 | K - J | 6a |
| L | J + only mom obese γ = only dad obese γ | 1314.6 | 971 | −6151.9 | L - J | −7a |
Note: BIC is used to test for model fit because not all models are nested within prior models. Differences between model BICs greater than −10 is “very strong” or “definitive” evidence, as implied by very high posterior odds, in favor of the later model (Raftery 1995).
One of the four imputed data sets could not converge with Model G. Thus it is omitted from the analysis for Models G – L.
Table 3 presents the structural path coefficients (γ) for the final multiple groups model. Before comparing across models, note that the relative ranking in the magnitudes of the family coefficients and their statistical significance remains unchanged after accounting for genetic resemblance, with two exceptions. First, immigrant status is not statistically significant in the genetic models. Second, birth weight becomes relatively more important for adolescent weight, in terms of both magnitude and statistical significance, in the genetic models.
Table 3.
Structural path coefficients for final sibling models by genetic resemblance, Averaged across multiply imputed data sets. N = 1,667
| % Change from Table 2a |
|||||
|---|---|---|---|---|---|
| γ | S.E. | Standardized | γ | Standardized | |
| Parental Obesity (relative to neither parent obese) | |||||
| Both parents obese | 0.337 | 0.045 ** | 0.265 | −10.5% | 12.0% |
| Only mom obese | 0.189 | 0.029 ** | 0.223 | 2.4% | 22.7% |
| Only dad obese | 0.148 | 0.052 ** | 0.103 | 8.0% | 17.3% |
| Individual Characteristics | |||||
| S1 Age, Wave 1 | 0.031 | 0.006 ** | 0.105 | 10.6% | 8.5% |
| S2 Age, Wave 1 | 0.032 | 0.006 ** | 0.108 | 25.1% | 24.1% |
| Family Characteristics | |||||
| Parents’ educationb | −0.011 | 0.004 ** | −0.118 | −12.5% | 1.3% |
| Income-to-needs ratiob | −0.020 | 0.026 | −0.041 | - | - |
| Sibship size | − 0.006 | 0.010 | −0.022 | - | - |
| Immigrant | −0.069 | 0.036 | −0.064 | - | - |
| Birth weightb | 0.017 | 0.006 ** | 0.135 | 81.2% | 45.6% |
| Meals factor | −0.115 | 0.029 ** | − 0.228 | 1.7% | 6.3% |
| Inactivity factor | 0.099 | 0.031 ** | 0.187 | 35.9% | 52.1% |
| Bike, skate per week | −0.014 | 0.010 | −0.044 | - | - |
Note: Standardized coefficients are akin to correlations for comparison across measures.
Two-tailed tests of significance for model coefficients:
p < .05,
p < .01.
Changes for nonsignficant factors are not displayed.
These variables have been transformed for inclusion in the models. See Appendix A.
Many of the parameter estimates do change in magnitude. Recall that whether estimates increase or decrease depends on the underlying relationships between genetic predispositions, weight, and exogenous family characteristics. As figure 1 demonstrated, the structural path coefficient should decline if genetic predispositions are positively correlated with both weight and the exogenous characteristic, increase if there is a suppressor effect operating in the simple sibling model, or remain the same if genetic predispositions are uncorrelated with the exogenous characteristic. Given these possibilities, we would expect a dramatic drop in the coefficients for parental obesity given the genetic underpinnings of weight and the genetic relationship between parents and their children. The estimated coefficient for having two obese parents does attenuate in the genetics models, but the magnitude of the change is only moderate. Surprisingly, the change in the nonstandardized coefficient between models in Tables 2 and 3 is only a 10.5% reduction. In addition, the coefficient for having only an obese mother changes very little and the coefficient for having only an obese father actually increases, suggesting a prior suppression effect. Even if these latter two changes are interpreted conservatively to suggest no change across models (as opposed to an increase), this is unexpected. If the association between parental obesity and adolescent weight operated primarily through parents’ and children’s genetic makeup, then one would expect not only attenuation in the coefficient, but a relatively large attenuation in the genetics models. The findings imply an important, and typically underestimated, role of social and behavioral factors for parent-child weight similarities. Given that one would expect a more dramatic drop in these estimates, the results also hint at the existence of an important, but unexamined, gene-environment interaction in the data.
The other coefficient showing a modest attenuation is parents’ education. This suggests that the association between parents’ education and adolescent obesity partially reflects a positive correlation of each characteristic with genetic predispositions. The coefficient demonstrating the smallest change is that for meal frequency. The estimate remains virtually unchanged across models, suggesting that the association between meal frequency and adolescent weight does not reflect genetic predispositions.
The coefficients for the remaining family characteristics, birth weight and inactivity, actually increase notably in the genetics models. This suggests that a suppression effect is operating in the models for all sibling pairs. The association between birth weight and adolescent weight increases by 81%, indicating that the social patterning of weight trajectories, and not just underlying genetic predispositions, contributes to heavier babies becoming heavier adolescents. Inactivity also increases considerably in the genetics models. The 36% increase in the coefficient for inactivity suggests that genetic predispositions suppress the social links between the behavioral patterning of a sedentary lifestyle and weight. To put it differently, we underestimate the role of social processes when we do not incorporate genetic resemblance in the standard models. A possible explanation for this finding is the negative relationship between SES and both birth weight and inactivity. Children from advantaged families are less likely to have low birth weight (Conley and Bennett 2001) and are less likely to be inactive (Gordon-Larsen, McMurray and Popkin 2000; Janssen et al. 2006), which is also true in the current sample. Given the positive correlation between genetic predispositions and parents’ SES, particularly education, socioeconomic inequalities in both birth weight and inactivity could be key to these suppression patterns. Family SES, as an important mediator of larger structural patterns, could be particularly important within the current obesiogenic environment, exacerbating the risks for adolescents in families with more sedentary hobbies and heavier birth weights. In sum, both genetic and social factors within families are significant in the determination of adolescent weight and the role of familial social characteristics is better revealed with an incorporation of genetic information.
DISCUSSION
Although the obesity epidemic reflects social and behavioral shifts, behavioral genetics research has concluded that these macro-level contextual patterns are not mirrored in the micro context of families. Instead, behavioral geneticists conclude that any social and behavioral processes significant for weight are unique to each sibling and do not reflect families’ shared experiences. To challenge this conclusion, the current analysis has taken a different methodological approach, adapting structural equation sibling models to incorporate genetic resemblance and investigate specific social mechanisms operating within families. The present study also challenges the traditional sociological and behavioral genetics assumption that standard sociological models overestimate the importance of familial social characteristics because they do not account for genetic predisposition. I argue and find evidence that, while this is the case for some characteristics, others do not change with the incorporation of genetic information, and still others actually reveal that the associated effects were previously underestimated. Genetics plays a significant role in determining an adolescent’s weight, but families’ social and behavioral characteristics are also important. Furthermore, incorporating genetic information not only reveals that familial social patterns compound biological weight trajectories; it also illuminates the fact that the association between inactivity and adolescent weight is embedded within a family’s collective lifestyle.
Traditional behavioral genetics models are robust in the calculation of genetic heritability but are poorly suited to investigating family processes. The disparate findings between this approach and my own derive from the models’ respective goals. In particular, behavioral genetics models aim to parse the variance of any characteristic into its genetic and environmental components, which makes it difficult to incorporate measured shared family characteristics (Turkheimer et al. 2005). The genetically informed structural equation sibling models do not seek to segregate the variance into its genetic and environmental components and instead allow the variance across pairs and their associations with familial characteristics to be freely estimated.10 In addition, behavioral genetics models attribute all differences between siblings’ exposure, perception, and reaction to structurally shared environmental experiences to each sibling’s unique experience (Turkheimer et al. 2005). The genetically informed structural equation sibling models investigate the shared environmental exposures and allocate differences in each sibling’s reaction and perception as measurement error of these structural patterns. Neither specification is ideal; it would be preferable to model both the structurally shared environmental exposures and sibling’s differential experiences of them, but currently available data are not sufficiently rich to do so. To capture both, one would need multiple measures of these characteristics from multiple perspectives (e.g., for each child and parent) and extremely large samples to have sufficient power to estimate the within- and between-family variances. An even better design would utilize data on children of twins to fully model both the genetic and environmental transmission of traits from parents to children (D’Onofrio et al. 2003).
Given the ability of genetically informed structural equation sibling models to identify significant associations between familial characteristics and differences in weight across families, the current analysis helps reveal the functioning of families within the current obesiogenic environment. The standardized coefficients for familial lifestyle, particularly meal frequency and inactivity, are as large as the effects of having an obese parent. Because of this, families’ adoption, organization, and maintenance of sustainable, healthy lifestyles is quite important, especially given the challenges involved in doing so in our postindustrial, time-squeezed economy. Family practices are, thus, significant because they are critically located within social processes that predispose people to gaining weight.
Prior research demonstrates that other contexts also have implications for adolescent weight, such as peers, neighborhoods, schools, health care systems, and regions (see Crosnoe and Muller 2004; Davison and Birch 2001; Gordon-Larsen et al. 2006; Kumanyika et al. 2002). Future research should move beyond the current study’s indirect evidence of the important mediating role of families and, instead, directly examine the interplay of families and these other contexts. In particular, research should investigate how parents’ status characteristics help locate children within these contexts, how these contexts structure parents’ weight-related practices, and how parents moderate these contexts’ impact, either diminishing or exacerbating their risks. Because sociologists are theoretically and empirically adept at investigating the collective efficacy of complex social contexts, we are well-poised to enter conversations about the interaction of multi-level processes for weight.
The current approach could also be applied to the study of other intergenerational correlations. Previous sociological research has studied how parental investments structure children’s eventual attainments, engendering similarities between parents and their children across a variety of domains, such as values (Glass, Bengtson and Dunham 1986), occupational attainments (Blau and Duncan 1967), and family formation patterns (McLanahan and Bumpass 1988). Future research could adopt the approach developed here to incorporate genetic predispositions and determine whether prior studies overestimate or underestimate the role of parental investments in children’s attainments.
Despite making significant advances, the analysis here has several limitations. Meal frequency and weight are both measured in wave 2 of Add Health and thus the causality could work in the opposite direction. Heavier adolescents could eat fewer meals as a weight-loss strategy. Meal frequency, however, is associated with fewer parental resources, such that missing meals is more common among adolescents with less educated and single parents (thee results are not shown but are available upon request). In addition, the analysis also cannot explain trends in the prevalence of overweight over time, but instead documents the pattern of associations in the mid-1990s for one American cohort. Finally, the analysis relies on proxies for parental control and socialization.
Sociologists have long known that family relationships are more than their hereditary connections. The current analysis not only reflects this sociological perspective, but also demonstrates it. Family weight similarities are not solely genetic. Rather, complex social interactions, behavioral patterns, and environmental conditions influence intergenerational and sibling similarities in weight. Identifying the significant familial social factors in this process is made possible by an incorporation of genetic information, and it provides further evidence of the importance of social contexts for adolescent weight.
APPENDIX A. DETAILS ON THE STRUCTURAL EQUATION SIBLING MODELS
In structural equation sibling models, the between-family and within-family regressions each have a structural component and a measurement model component. The structural component of the between-family equation can be written in LISREL notation (Joreskog and Sorbom 2001) as
| (1) |
where ξ are the exogenous, family-level latent factors that are freely correlated with each other, but uncorrelated with the structural disturbance, ζ1. The disturbance captures all other causes of family-level differences in BMI that are not measured. The estimates for γ indicate the strength of the relationship between the family-level latent factors and the family average of BMI values. When exogenous latent factors (ξ) are measured with continuous variables, the latent factor has a measurement model estimated using indicators from both siblings’ reports, such that
| (2) |
| (3) |
where λx is the loading of sibling 2’s measure on the family-level latent factor ξk and δk is the random measurement error of xk. If, however, the exogenous family-level latent factor (ξk) is measured by a categorical variable, then a measurement model is not estimated and the latent factor is set equal to sibling 1’s report. The choice of which sibling’s information to use is inconsequential because I double-enter the data, switching which sibling is “sibling 1” and which is “sibling 2,” to make the data symmetric and the ordering of siblings random. I then adjust the degrees of freedom to reflect the true sample size for tests of statistical significance and model fit. Note that for the supplemental analyses estimating separate models by sex, the data are not symmetric, but the order of the siblings is random.
There are two structural equations to estimate the within-family regressions, one for each sibling. The structural component of the within-family regressions can be written as
| (4) |
| (5) |
such that each sibling’s BMI reflects the family’s average BMI (η1; predicted in equation 1) and their own individual characteristics. The structural disturbances (ζ2 and ζ3) are uncorrelated with the latent, individual-level factor for age and capture all sources of individual variation in BMI that do not derive from the family and that also differ across siblings. Because the data for sibling 1 and sibling 2 are symmetric in the genetic models, I constrain each sibling’s latent BMI to load equally on the family’s mean BMI (η1).
Because there is only one indicator for sibling’s age there is no estimated measurement model for this exogenous individual characteristic. In contrast, there is a measurement model for each sibling’s latent BMI, such that BMI loads onto the adolescent’s measured BMI. Self-reported BMI is expected to deviate from the measured BMI, but be highly correlated with it.
When genetic resemblance is incorporated into the analysis, I simultaneously estimate a separate sibling model for each genetic relationship, which is equivalent to estimating equations (1), (4), and (5) four separate times. I then test to see what parameters can be set equal across genetic pairs. If the pairs derive from the same population of families, then one would expect ζ1 to be equivalent across all pairs. In contrast,ζ2 and ζ3 should differ across pairs given their differences in genetic resemblance.
Transformations of Measures
Structural equation modeling requires normal distributions for continuous variables, especially for the endogenous variables. Residualized BMI scores are approximately normal. I transform parents’ education, the family’s income-to-needs ratio and birth weight using the transformation that best approximates normality. For the family’s income-to-needs ratio I use a started log with Stata’s “lnskew0” command. Parents’ education and birth weight are transformed using Box-Cox transformations with Stata’s “bcskew0.” The Stata-generated formulas for the transformations are as follows: tBIRTHWT = (BIRTHWT1.509984 − 1)/1.509984; tPARED = (PARED1.129795 − 1)/1.129795; tlsINCNEEDS = log(INCNEEDS + 0.3994804).
APPENDIX B. DETAILS ON MODEL FIT
The fit statistics used in these analyses are also used in the prior sociological and behavioral genetics literature. For the quantitative behavioral genetics models, I use RMSEA and AIC to evaluate model fit (Neale and Cardon 1992). A low RMSEA (0.05 or below) indicates that the models accurately and sufficiently represent the data. The model with the lowest AIC is the preferred model. Both RMSEA and AIC incorporate parsimony in their assessment of fit (Neale and Cardon 1992). The fit statistics and model results for the behavioral genetics models can be found in appendix table B1.
For the structural equation sibling models (SEM), I use the Bayesian information criterion, (BIC) statistic to assess model fit (Raftery 1995). BIC is a penalized χ2 statistic that adjusts for sample size, defined for model k as:
| (6) |
where Lk2 is the log likelihood, n is the sample size and dfk is the degrees of freedom for the model. A smaller BIC (i.e., more negative) indicate a better fitting model. To compare two models, regardless of whether they are nested, one takes the difference of their BICs. Differences in BIC of 2 or more provide positive evidence, of 6 or more provide strong evidence, and of 10 or more provide very strong evidence (Raftery 1995).
Appendix table B2 presents the goodness-of-fit statistics for the tests of equality across genetic resemblance groups. Model A begins with all parameters estimated separately for each group. Accounting for genetics dramatically improves model fit (BIC difference = −1454). According to models B through E, the measurement properties are equal across genetic resemblance groups and models F through K demonstrate that most of the structural properties are equal. With only one exception, the covariances (Φ) amongst the independent variables (ξ) are equal across groups (model F). The only aberration is that the covariance between siblings’ age differs, such that, as one would expect, the covariance in age is smaller for twins than for other siblings. The covariance in age is equal for monozygotic and dizygotic twins and it is equal for full siblings and half siblings. The structural path coefficients (γ) are also equal across all pairs (model G). Note that model G would not converge for one imputed data set (sample 4) and thus the sample was dropped for this and the following model tests. All statistics were adjusted appropriately. The variation in “family average BMI” (φ1) is equal across all but the monozygotic twins (model I), implying that the monozygotic twins do not derive from the same population of families as the other pairs. Given that I utilize multiple imputation to handle missing data and select a random sibling pair within each family, this likely reflects Add Health’s non-random inclusion of twins found during the in-home wave 1 interview. Last, the within-family variance in BMI is equal for dizygotic twins and full siblings, but significantly different for half siblings and monozygotic twins (model K), congruent with behavioral genetics expectations.11
Footnotes
Jacobson and Rowe Jacobson, K. C., and D. C. Rowe. 1998. “Genetic and shared environmental influences on adolescent BMI: interactions with race and sex.” Behavior Genetics 28:265–78. found a role for shared family environment for White adolescent girls’ BMI, but not for adolescent boys or Black adolescent girls.
Other pairs are available in Add Health (e.g., cousins, step-siblings, adopted siblings, co-residential romantic partners), but I restrict the data to twins, full siblings and half-siblings to better ensure that pairs have similar family experiences.
If the data included more than one sibling pair per family, then the standard errors would be downwardly biased and one would be more likely to erroneously conclude that a coefficient was statistically significant.
Age can serve as a loose proxy for physical development, but it is preferable to use direct measures of physical development to adjust BMI.
United States Census Bureau, Housing and Household Economic Statistics Division. 2005. Poverty Thresholds in 1994, By Size of Family and Number of Related Children Under 18 years. (http://www.census.gov/hhes/www/poverty/threshld/thresh94.html). Retrieved June 16, 2006.
Preliminary analyses also investigated other physical activity indicators based on survey items inquiring about spells playing an “active sport, such as baseball, softball, basketball, soccer, swimming or football” or doing “exercise, such as jogging, walking, karate, jumping rope, gymnastics, dancing.” These indicators were not statistically significant and dropped for parsimony.
Dominance genetic variation occurs when there are gene-gene interactions, whereas additive genetic variation reflects the simple expression of different alleles (or coding values) across genes Neale, Michael C., and Lon R. Cardon. 1992. Methodology for Genetic Studies of Twins and Families. London: Kluwer Academic Publishers..
I tested whether there are gender interaction effects and found that the social inheritance of weight is not conditional on the sex of the child or the sex of the parent.
The choice of which sibling’s indicator to use is inconsequential because the data are symmetric, meaning that the data are double entered, switching which is sibling 1 and which is sibling 2. See appendix A for more information.
One could, however, apply Guo and Wang’s Guo, Guang, and Jianmin Wang. 2002. “The Mixed or Multilevel Model for Behavior Genetic Analysis.” Behavior Genetics 32:37. hierarchical linear modeling behavioral genetics approach to derive standard behavioral genetics models estimates of explained variance. I did not adopt their approach because it simply re-parameterizes the standard behavioral genetics model and, thus, is unable to estimate the role of specific familial characteristics.
This also implies that dizygotic twins do not have a special “twin environment” for BMI.
An earlier version of this project co-authored with Gary Sandefur was presented at the 2004 Annual Meetings of the Population Association of America. Funding for this research was provided by the University of Wisconsin-Madison Health & Society Research Competition, sponsored by the Robert Wood Johnson Health & Society Scholars Program and NICHD grant R01-HD050144 (PI: G.D. Sandefur). This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). I wish to thank anonymous reviewers, Michelle Frisco, Peter Bearman, Gary Sandefur, Alair MacLean, Jeremy Freese, Arthur Goldberger, Robert M. Hauser, and those affiliated with the Robert Wood Johnson Health & Society Scholars Program at Columbia University for their comments and suggestions. Kristin Burnett, Jason Houle and Krissy Zeiser provided excellent research assistance.
References
- Acock Alan C. Working With Missing Values. Journal of Marriage and Family. 2005;67:1012–1028. [Google Scholar]
- Affenito Sandra G, Thompson Douglas R, Barton Bruce A, Franko Debra L, Daniels Stephen R, Eva Obarzanek, Schreiber George B, Striegel-Moore Ruth H. Breakfast consumption by African-American and white adolescent girls correlates positively with calcium and fiber intake and negatively with body mass index. Journal of the American Dietetic Association. 2005;105:938–45. doi: 10.1016/j.jada.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Arluk Shaye L, David Branch J, Swain David P, Dowling Elizabeth A. Childhood obesity’s relationship to time spent in sedentary behavior. Military Medicine. 2003;168:583–6. [PubMed] [Google Scholar]
- Baranowski Tom. Validity and Reliability of Self Report Measures of Physical Activity: An Information-Processing Perspective. Research Quarterly for Exercise and Sport. 1988;59:314–327. [Google Scholar]
- Barker David JP, Gluckman PD, Godfrey KM, Harding JE, Owen JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Rockett HR, Gillman MW, Field AE, Colditz GA. Longitudinal study of skipping breakfast and weight change in adolescents. International Journal of Obesity & Related Metabolic Disorders. 2003;27:1258–66. doi: 10.1038/sj.ijo.0802402. [DOI] [PubMed] [Google Scholar]
- Birch Leann L, Fisher Jennifer O. Development of Eating Behaviors Among Children and Adolescents. Pediatrics. 1998;101:539–549. [PubMed] [Google Scholar]
- Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obesity Reviews. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- Blau Peter Michael, Otis Dudley Duncan. The American occupational structure. New York: Wiley; 1967. [Google Scholar]
- Brooks-Gunn Jeanne, Duncan Greg, Kato Klebanov Pamela, Sealand Naomi. Do Neighborhoods Influence Child and Adolescent Development? The American Journal of Sociology. 1993:99. [Google Scholar]
- Bruss MB, Morris JR, Dannison LL, Orbe MP, Quitugua JA, Palacios RT. Food, culture, and family: exploring the coordinated management of meaning regarding childhood obesity. Health Communication. 2005;18:155–75. doi: 10.1207/s15327027hc1802_4. [DOI] [PubMed] [Google Scholar]
- Carmichael CM, McGue M. A cross-sectional examination of height, weight, and body mass index in adult twins. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 1995;50:B237–44. doi: 10.1093/gerona/50a.4.b237. [DOI] [PubMed] [Google Scholar]
- Cawley John. The Impact of Obesity on Wages. Journal of Human Resources. 2004;39:451–474. [Google Scholar]
- Chantala Kim, Tabor Joyce. Strategies to perform a design-based analysis using the Add Health data. Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill; 1999. [Google Scholar]
- Cockerham William C, Rutten Alfred, Abel Thomas. Conceptualizing Contemporary Health Lifestyles: Moving Beyond Weber. Sociological Quarterly, The. 1997;38:321–342. [Google Scholar]
- Comuzzie Anthony G, Allison David B. The search for human obesity genes. Science. 1998;280:1374–1377. doi: 10.1126/science.280.5368.1374. [DOI] [PubMed] [Google Scholar]
- Conley Dalton, Bennett Neal G. Birth weight and income: Interactions across generations. Journal of Health & Social Behavior. 2001;42:450–465. [PubMed] [Google Scholar]
- Conley Dalton, Strully Kate W, Bennett Neil G. The Starting Gate: Birth Weight and Life Chances. Berkeley, CA: University of California Press; 2003 . [Google Scholar]
- Crosnoe Robert. Gender, Obesity, and Education. Sociology of Education. 2007;80:240–261. [Google Scholar]
- Crosnoe Robert, Muller Chandra. Body mass index, academic achievement, and school context: examining the educational experiences of adolescents at risk of obesity. Journal of Health & Social Behavior. 2004;45:393–407. doi: 10.1177/002214650404500403. [DOI] [PubMed] [Google Scholar]
- D’Onofrio Brian M, Turkheimer Eric N, Eaves Lindon J, Corey Linda A, Kare Berg, Solaas Marit H, Emery Robert E. The role of the Children of Twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- Davison KK, Birch LL. Childhood overweight: a contextual model and recommendations for future research. Obesity Reviews. 2001;2:159–71. doi: 10.1046/j.1467-789x.2001.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison Kirsten Krahnstoever, Francis Lori A, Birch Leann L. Links between Parents’ and Girls’ Television Viewing Behaviors: A Longitudinal Examination. The Journal of Pediatrics. 2005;147:436. doi: 10.1016/j.jpeds.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH, Gortmaker SL. Preventing obesity in children and adolescents. Annual Review of Public Health. 2001;22:337–53. doi: 10.1146/annurev.publhealth.22.1.337. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. American Journal of Clinical Nutrition. 2004;79:6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- Fogelholm M, Nuutinen O, Pasanen M, Myohanen E, Saatela T. Parent-child relationship of physical activity patterns and obesity. International Journal of Obesity & Related Metabolic Disorders. 1999;23:1262–8. doi: 10.1038/sj.ijo.0801061. [DOI] [PubMed] [Google Scholar]
- Francis Lori A, Birch Leann L. Maternal Influences on Daughters’ Restrained Eating Behavior. Health Psychology. 2005;24:548–554. doi: 10.1037/0278-6133.24.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PW, Ravussin E, Hanson RL, Harper IT, Allison DB, Knowler WC, Tataranni PA, Salbe AD. Habitual physical activity in children: the role of genes and the environment. Am J Clin Nutr. 2005;82:901–8. doi: 10.1093/ajcn/82.4.901. [DOI] [PubMed] [Google Scholar]
- French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annual Review of Public Health. 2001;22:309–35. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- Friedman Jeffrey M. A War on Obesity, Not the Obese. Science. 2003;299:856–858. doi: 10.1126/science.1079856. [DOI] [PubMed] [Google Scholar]
- Glass Jennifer, Bengtson Vern L, Chorn Dunham Charlotte. Attitude Similarity in Three-Generation Families: Socialization, Status Inheritance, or Reciprocal Influence? American Sociological Review. 1986;51:685–698. [Google Scholar]
- Goldberger Arthur S. Heritability. Economica. 1979;46:327–347. [Google Scholar]
- Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depression and obesity. American Journal of Public Health. 2003;93:1844–50. doi: 10.2105/ajph.93.11.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI. Genetic influences on human energy expenditure and substrate utilization. Behavior Genetics. 1997;27:389–99. doi: 10.1023/a:1025644215744. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:417–24. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen Penny, Harris Kathleen M, Ward Dianne S, Popkin Barry M. Acculturation and overweight-related behaviors among Hispanic immigrants to the US: the National Longitudinal Study of Adolescent Health. Social Science & Medicine. 2003;57:2023–34. doi: 10.1016/s0277-9536(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen Penny, McMurray Robert G, Popkin Barry M. Determinants of adolescent physical activity and inactivity patterns. Pediatrics. 2000;105:e83. doi: 10.1542/peds.105.6.e83. [DOI] [PubMed] [Google Scholar]
- Gortmaker Steven L, Aviva Must, Perrin James M, Sobal Arthur M, Dietz William H. Social and Economic Consequences of Overweight in Adolescence and Young Adulthood. The New England Journal of Medicine. 1993;329:1008–1012. doi: 10.1056/NEJM199309303291406. [DOI] [PubMed] [Google Scholar]
- Greenleaf Christy, Starks Misty, Gomez Laura, Chambliss Heather, Martin Scott. Weight-related words associated with figure silhouettes. Body Image. 2004;1:373. doi: 10.1016/j.bodyim.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Griliches Zvi. Sibling Models and Data in Economics: Beginnings of a Survey. The Journal of Political Economy. 1979;87:S37–S64. [Google Scholar]
- Guillaume M, Lapidus L, Beckers F, Lambert A, Bjorntorp P. Familial trends of obesity through three generations: the Belgian-Luxembourg child study. International Journal of Obesity & Related Metabolic Disorders. 1995;19:S5–9. [PubMed] [Google Scholar]
- Guo Guang, Wang Jianmin. The Mixed or Multilevel Model for Behavior Genetic Analysis. Behavior Genetics. 2002;32:37. doi: 10.1023/a:1014455812027. [DOI] [PubMed] [Google Scholar]
- Haas JS, Lee LB, Kaplan CP, Sonneborn D, Phillips KA, Liang SY. The association of race, socioeconomic status, and health insurance status with the prevalence of overweight among children and adolescents. American Journal of Public Health. 2003;93:2105–10. doi: 10.2105/ajph.93.12.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Kathleen Mullan, Florey Francesca, Tabor Joyce, Bearman Peter S, Jones Jo, Udry J Richard. The National Longitudinal Study of Adolescent Health: Research Design. Chapel Hill, NC: Carolina Population Center, University of North Carolina; 2003. [Google Scholar]
- Hauser Robert M. A note on two models of sibling resemblance. American Journal of Sociology. 1988;93:1401–1423. [Google Scholar]
- Hauser Robert M, Mossel Peter A. Fraternal Resemblance in Educational Attainment and Occupational Status. American Journal of Sociology. 1985;91:650–673. [Google Scholar]
- Helmchen Lorens A, Max Henderson R. Changes in the distribution of body mass index of white U.S. men, 1890–2000. Annals of Human Biology. 2004;31:174–181. doi: 10.1080/03014460410001663434. [DOI] [PubMed] [Google Scholar]
- Hetherington E Mavis, Reiss David, Plomin Robert., editors. Separate social worlds of siblings: the impact of nonshared environment on development. Hillsdale, N.J: L. Erlbaum; 1994. [Google Scholar]
- Jacobson KC, Rowe DC. Genetic and shared environmental influences on adolescent BMI: interactions with race and sex. Behavior Genetics. 1998;28:265–78. doi: 10.1023/a:1021619329904. [DOI] [PubMed] [Google Scholar]
- Janssen Ian, Boyce WF, Simpson K, Pickett W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. The American Journal of Clinical Nutrition. 2006;83:139–45. doi: 10.1093/ajcn/83.1.139. [DOI] [PubMed] [Google Scholar]
- Jöreskog Karl, Sörbom Dag. LISREL 8: User’s Reference Guide. Lincolnwood, IL: Scientific Software International; 2001. [Google Scholar]
- Keski-Rahkonen Anna, Viken Richard J, Jaakko Kaprio, Rissanen Aila, Rose Richard J. Genetic and Environmental Factors in Breakfast Eating Patterns. Behavior Genetics. 2004;34:503. doi: 10.1023/B:BEGE.0000038488.22974.b1. [DOI] [PubMed] [Google Scholar]
- Knuiman MW, Divitini ML, Welborn TA, Bartholomew HC. Familial correlations, cohabitation effects, and heritability for cardiovascular risk factors. Annals of Epidemiology. 1996;6:188–94. doi: 10.1016/1047-2797(96)00004-x. [DOI] [PubMed] [Google Scholar]
- Kumanyika S, Jeffery RW, Morabia A, Ritenbaugh C, Antipatis VJ Force Public Health Approaches to the Prevention of Obesity Working Group of the International Obesity Task. Obesity prevention: the case for action. International Journal of Obesity & Related Metabolic Disorders. 2002;26:425–36. doi: 10.1038/sj.ijo.0801938. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK. Minisymposium on obesity: overview and some strategic considerations. Annual Review of Public Health. 2001;22:293–308. doi: 10.1146/annurev.publhealth.22.1.293. [DOI] [PubMed] [Google Scholar]
- Link Bruce G, Northridge Mary E, Phelan Jo C, Ganz Michael L. Social Epidemiology and the Fundamental Cause Concept: On the Structuring of Effective Cancer Screens by Socioeconomic Status. The Millbank Quarterly. 1998;73:375–402. doi: 10.1111/1468-0009.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link Bruce G, Phelan Jo. Social Conditions as Fundamental Causes of Disease. Journal of Health and Social Behavior. 1995:80–94. [PubMed] [Google Scholar]
- Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behavior Genetics. 1997;27:325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- McLanahan Sara, Bumpass Larry. Intergenerational Consequences of Family Disruption. American Journal of Sociology. 1988;94:130–152. [Google Scholar]
- McLeod Jane D, Kessler Ronald C. Socioeconomic status differences in vulnerability to undesirable life events. Journal of Health & Social Behavior. 1990;31:162–172. [PubMed] [Google Scholar]
- Mitchell Braxton D, Rainwater David L, Hsueh Wen-Chi, Kennedy Amy J, Stern Michael P, MacCluer Jean W. Famiial Aggregation of Nutrient Intake and Physical Activity: Results from the San Antonio Family Heart Study. Annals of Epidemiology. 2003;13:128–35. doi: 10.1016/s1047-2797(02)00255-7. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Conde WL, Lu B, Popkin Barry M. Obesity and inequities in health in the developing world. International Journal of Obesity and Related Metabolic Disorders. 2004;28:1181–1186. doi: 10.1038/sj.ijo.0802716. [DOI] [PubMed] [Google Scholar]
- Neale Michael C, Cardon Lon R. Methodology for Genetic Studies of Twins and Families. London: Kluwer Academic Publishers; 1992. [Google Scholar]
- Nelson Melissa C, Penny Gordon-Larsen, North Kari E, Adair Linda S. Body Mass Index Gain, Fast Food, and Physical Activity: Effects of Shared Environments over Time. Obesity. 2006;14:701–709. doi: 10.1038/oby.2006.80. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002a;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002b;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Ogden Cynthia L, Carroll Margaret D, Curtin Lester R, McDowell Margaret A, Tabak Carolyn J, Flegal Katherine M. Prevalence of Overweight and Obesity in the United States 1999–2004. JAMA. 2006:295. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden J, Chanana A. Explaining the effect of ethnic group on weight concern: finding a role for family values. International Journal of Obesity & Related Metabolic Disorders. 1998;22:641–7. doi: 10.1038/sj.ijo.0800641. [DOI] [PubMed] [Google Scholar]
- Ogden Jane, Reynolds Rebecca, Smith Andrea. Expanding the concept of parental control: A role for overt and covert control in children’s snacking behaviour? Appetite. 2006;47:100–6. doi: 10.1016/j.appet.2006.03.330. [DOI] [PubMed] [Google Scholar]
- Oken E, Gillman MW. Fetal origins of obesity. Obesity Research. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Oliveria SA, Ellison RC, Moore LL, Gillman MW, Garrahie EJ, Singer MR. Parent-child relationships in nutrient intake: the Framingham Children’s Study. Am J Clin Nutr. 1992;56:593–598. doi: 10.1093/ajcn/56.3.593. [DOI] [PubMed] [Google Scholar]
- Pagan Jose A, Davila Alberto. Obesity, Occupational Attainment, and Earnings. Social Science Quarterly. 1997;78:756–770. [Google Scholar]
- Parens Erik. Genetic Differences and Human Identities: On Why Talking about Behavioral Genetics is Important and Difficult. Hastings Center Report Special Supplement. 2004;34:S1–S36. [PubMed] [Google Scholar]
- Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. British Medical Journal. 2001;323:1331–5. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietilainen KH, Kaprio J, Rasanen M, Winter T, Rissanen A, Rose RJ. Tracking of body size from birth to late adolescence: contributions of birth length, birth weight, duration of gestation, parents’ body size, and twinship. American Journal of Epidemiology. 2001;154:21–9. doi: 10.1093/aje/154.1.21. [DOI] [PubMed] [Google Scholar]
- Plomin Robert, Corley Robin, DeFries JC, Fulker DW. Individual Differences in Television Viewing in Early Childhood: Nature as well as Nurture. Psychological Science. 1990;1:371–377. [Google Scholar]
- Plomin Robert, DeFries JC, Loehlin John C. Genotype-Enviornment Interaction and Correlation in the Analysis of Human Behavior. Psychological Bulletin. 1977;84:309–312. [PubMed] [Google Scholar]
- Popkin Barry M, Udry J Richard. Adolescent obesity increases significantly in second and third generation U.S. immigrants: The National Longitudinal Study of Adolescent Health. Journal of Nutrition. 1998;128:701–706. doi: 10.1093/jn/128.4.701. [DOI] [PubMed] [Google Scholar]
- Raftery Adrian E. Bayesian model selection in social research. In: Marsden Peter V., editor. Sociological Methodology. Cambridge: Basil Blackwell; 1995. pp. 111–173. [Google Scholar]
- Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA. Heritable variation in food preferences and their contribution to obesity. Behavior Genetics. 1997;27:373–87. doi: 10.1023/a:1025692031673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston Patrick. Multiple imputation of missing values: update. Stata Journal. 2005a;5:1–14. [Google Scholar]
- Royston Patrick. Multiple imputation of missing values: Update of ice. Stata Journal. 2005b;5:527–536. [Google Scholar]
- Rubin Donald. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- Sallis James F, Buono Michael J, Roby Julia J, Micale Frank G, Nelson Julie A. Seven-day recall and other physical activity self-reports in children and adolescents. Medicine and Science in Sports and Exercise. 1993;25:99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Scarr Sandra, McCartney Kathleen. How People Make their Own Environments: A Theory of Genotype→Environment Effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Sorensen TI, Holst C, Stunkard AJ. Childhood body mass index--genetic and familial environmental influences assessed in a longitudinal adoption study. International Journal of Obesity & Related Metabolic Disorders. 1992;16:705–14. [PubMed] [Google Scholar]
- Strauss RS. Self-reported weight status and dieting in a cross-sectional sample of young adolescents: National Health and Nutrition Examination Survey III. Archives of Pediatrics & Adolescent Medicine. 1999;153:741–7. doi: 10.1001/archpedi.153.7.741. [DOI] [PubMed] [Google Scholar]
- Strauss Richard S, Knight Judith. Influence of the Home Environment on the Development of Obesity in Children. Pediatrics. 1999;103:e85. doi: 10.1542/peds.103.6.e85. [DOI] [PubMed] [Google Scholar]
- Taveras Elsie M, Rifas-Shiman Sheryl L, Berkey Catherine S, Rockett Helaine RH, Field Alison E, Frazier A Lindsay, Colditz Graham A, Gillman Matthew W. Family Dinner and Adolescent Overweight. Obesity. 2005;13:900–906. doi: 10.1038/oby.2005.104. [DOI] [PubMed] [Google Scholar]
- Teachman Jay D. Families as Natural Experiments: A Procedure for Estimating the Potentially Biasing Influence of Families on Relationships Between Variables. Journal of Family Issues. 1995;16:519–537. [Google Scholar]
- Teachman Jay D, Carver Karen, Day Randal. A Model for the Analysis of Paired Data. Journal of Marriage and the Family. 1995;57:1011–1024. [Google Scholar]
- Tholin Sanna, Rasmussen Finn, Tynelius Per, Karlsson Jan. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. American Journal of Clinical Nutrition. 2005;81:564–569. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101:497–504. [PubMed] [Google Scholar]
- Turkheimer Eric, D’Onofrio Brian M, Maes Hermine H, Eaves Lindon J. Analysis and Interpretation of Twin Studies Including Measures of the Shared Environment. Child Development. 2005;76:1217–1233. doi: 10.1111/j.1467-8624.2005.00846.x. [DOI] [PubMed] [Google Scholar]
- Udry J Richard. Biosocial Models of Low-Fertility Societies. Population and Development Review. 1996;22:325–333. [Google Scholar]
- Udry J Richard. The National Longitudinal Study of Adolescent Health (Add Health), Waves I & II, 1994–1996; Wave III, 2001–2002 [MRDF]”. Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill [producer and distributor]; 2003. [Google Scholar]
- Wardle J, Guthrie C, Sanderson S, Birch L, Plomin R. Food and activity preferences in children of lean and obese parents. International Journal of Obesity. 2001;25:971–977. doi: 10.1038/sj.ijo.0801661. [DOI] [PubMed] [Google Scholar]
- Zajonc RB. Family Configuration and Intelligence. Science. 1976;192:227–236. doi: 10.1126/science.192.4236.227. [DOI] [PubMed] [Google Scholar]