Abstract
Previous studies have suggested a role for cytosolic Ca2+-independent phospholipase A2 (PLA2) activity in the formation of endosome membrane tubules that participate in the export of transferrin (Tf) and transferrin receptors (TfR) from sorting endosomes (SEs) and the endocytic recycling compartment (ERC). Here we show that the PLA2 requirement is a general feature of endocytic trafficking. The reversible cytoplasmic PLA2-antagonist ONO-RS-082 (ONO) produced a concentration-dependent, differential block in the endocytic recycling of both low-density lipoprotein receptor (LDLR) and TfRs, and in the degradative pathways of LDL and epidermal growth factor (EGF). These results are consistent with the model that a cytoplasmic PLA2 plays a general role in the export of cargo from multiple endocytic compartments by mediating the formation of membrane tubules.
Keywords: Endosomes, membrane tubules, transferrin receptor, LDL receptor, EGF receptor, recycling, PLA2, phospholipase A2
Introduction
Endocytosis in mammalian cells involves multiple organelles and trafficking events. Molecules brought into the cell by receptor-mediated endocytosis must be sorted and delivered to the appropriate destinations [1]. In electron microscopy studies, membrane tubules (60–80 nm in diameter) have been observed extending from various endosomes [2; 3; 4], and various endocytic recycling receptors accumulate in these tubules [5; 6; 7; 8; 9; 10]. It has been suggested that the role of membrane tubules is to help sort membrane-bound recycling receptors away form soluble cargo that is destined for degradation [7; 11].
A variety of studies have led to the surprising conclusion that cytoplasmic phospholipase A2 (PLA2) activities are intimately involved in the formation of membrane tubules from both the Golgi complex and various endosomal compartments, and membrane trafficking pathways leading from these organelles [12]. In the case of endosomes, recent studies have revealed a concentration-dependent effect on the recycling of transferrin (Tf) and its receptor (TfR) when cells are treated with various cytoplasmic PLA2 antagonists such as ONO-RS-082 (ONO) or bromoenol lactone (BEL) [13]. High concentrations (~10 µM) block export of Tf/TfR from both peripheral sorting endosomes (SEs) and the central endocytic recycling compartment (ERC), whereas low concentrations (~1 µM) allow transport to, but not out of the central ERC. It is not known if these effects are limited to the trafficking of Tf/TfR or the compartments through which Tf/TfR recycle.
To determine if cytoplasmic PLA2 enzymes have a more general role in endocytic trafficking, we have examined the effects of PLA2 antagonists on the trafficking of various ligands and receptors through the recycling and degradative endocytic pathways. We find that the cytoplasmic PLA2-antagonists ONO and BEL inhibit the export of soluble and membrane-bound cargo from various endocytic compartments, in addition to inhibiting tubule formation from endosomes containing these cargos. These results provide evidence that cytoplasmic PLA2 activity is important for the trafficking of multiple soluble and membrane-bound cargos.
Material and Methods
Reagents and Antibodies
BFA and ONO were obtained from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA) and prepared as described [13]. Monoclonal anti-human low-density lipoprotein receptor (LDLR) IgG-C7 was from Research Diagnostics, Inc. (Flanders, NJ). DiI conjugated low-density lipoprotein (LDL-DiI) from human plasma, Tf-fluorescein (Tf-FITC), and Alexa Fluor 488 streptavidin-conjugated biotinylated epidermal growth factor (EGF-Alexa488) were from Molecular Probes (Eugene, OR). The secondary fluorescent antibodies goat anti-mouse FITC and goat anti-mouse TRITC were from Jackson ImmunoResearch Laboratories (West Grove, PA). The expression vector encoding a low-density lipoprotein receptor-green fluorescent protein chimera (LDLR-GFP) was provided by Dr. Enrique Rodriguez-Boulan (Weill Medical College of Cornell University), and one for Rab7-green fluorescent protein chimera (Rab7-GFP) was provided by Dr. Craig Roy (Yale University, New Haven, CT).
Cell Culture and Immunocytochemistry
HeLa cells were grown in modified Eagle’s minimal essential medium (MEM) with 10% NuSerum, and 1% penicillin/streptomycin from Life Technologies (Grand Island, NY). HeLa cells stably expressing LDLR-GFP (HeLa-LDLR-GFP) were grown in modified Eagle’s minimal essential medium (MEM) with 10% NuSerum, and 500 µg/ml G418 from Sigma. All cells were maintained at 37°C in a humidified atmosphere of 95% air, 5% CO2. For uptake experiments, the following concentrations were used: Tf-FITC (40 µg/ml), LDL-DiI (5 µg/ml), and EGF-Alexa488 (2 µg/ml). Following treatment with endocytic tracers and PLA2 antagonists, cells were fixed and processed for immunofluorescence as described [13], and analyzed with a Zeiss Axioscope II equipped with a Hamamatsu Orca II digital camera and Openlab software by Improvision, or a Perkin-Elmer UltraVIEW spinning disc confocal microscope and software.
Results and Discussion
ONO Inhibits the Normal Trafficking Pathways of Both Tf and LDLR
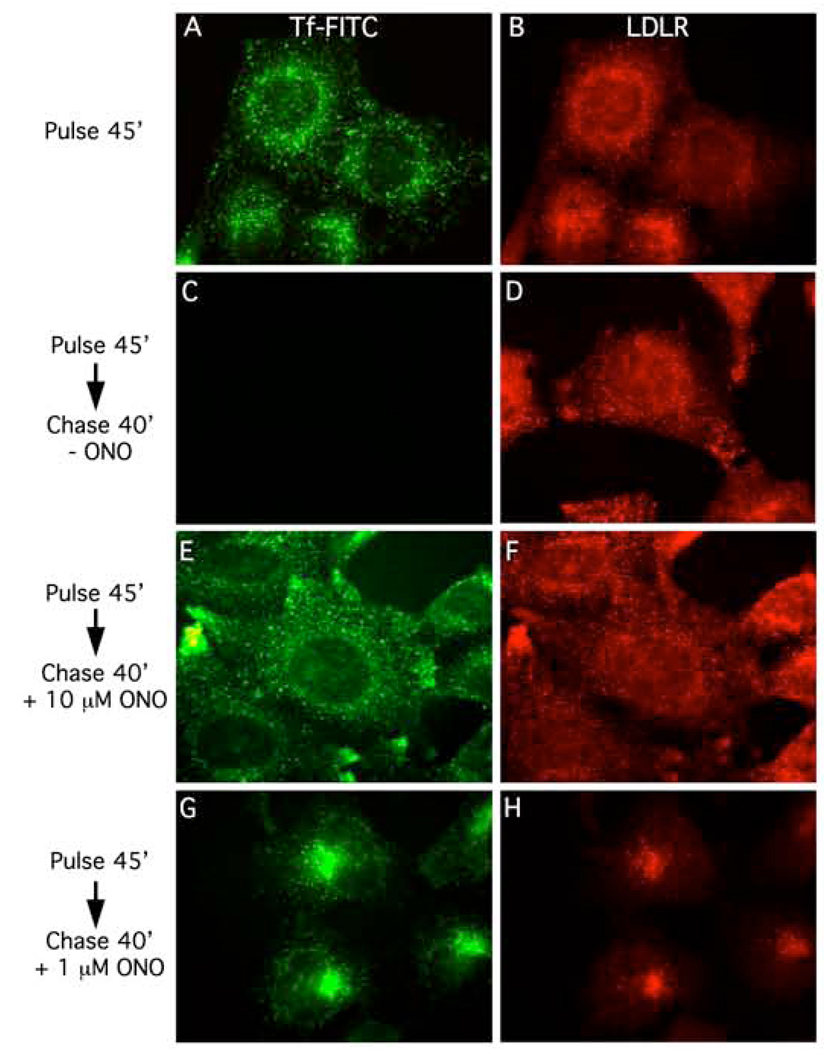
To determine if there exists a general role for PLA2 enzymes in endocytic trafficking, various pulse chase experiments were conducted with multiple ligands and receptors in the presence or absence of ONO. In control cells incubated continuously with Tf-FITC in MEM for 45 min at 37°C and then fixed and processed for immunofluorescence, endocytosed Tf and endogenous LDLRs were located in overlapping puncta throughout the cytoplasm (Fig. 1 A, B). These puncta correspond to peripheral SEs and the central ERC [13]. As expected, when cells were pulse-labeled for 45 min followed by a chase for 40 min, Tf-FITC was recycled to the cell surface and lost into the media; however, the steady-state distribution of LDLR remained unchanged (Fig. 1 C, D). In contrast, when cells were pulse-labeled with Tf-FITC in MEM for 45 min followed by a chase in MEM containing 10 µM ONO for 40 min, both Tf and LDLR were retained in more peripheral puncta throughout the cytoplasm (Fig. 1 E, F). However, when the chase was done in 1 µM ONO, both Tf and LDLR moved to a centrally located compartment corresponding to the central ERC (Fig. 1 G, H). Similar to previous studies on Tf and TfR trafficking [13], a concentration-dependent inhibition of LDLR trafficking was also obtained with a structurally different PLA2 antagonist, the irreversible suicide substrate BEL (data not shown) [14; 15].
Figure 1.
The reversible cytoplasmic PLA2 antagonist ONO-RS-082 (ONO) inhibits the normal trafficking pathways of both Tf-FITC and LDLR at two discrete steps in a concentration-dependent manner. In all experiments, HeLa cells were pulse-labeled with Tf-FITC for 45 min to label all endocytic compartments (left panels), subjected to various chase protocols in the presence or absence of ONO, and then processed for immunofluorescence to visualize LDLR (right panels). A and B, cells pulse-labeled for 45 min. C and D, cells pulse-labeled for 45 min and then chased in Tf-free medium for 40 min. E and F, cells pulse-labeled for 45 min and then chased in the presence of 10 µM ONO for 40 min. G and H, cells pulse-labeled for 45 min and then chased in the presence of 1 µM ONO for 40 min.
ONO Inhibits the Export of LDLR and Tf from Sorting Endosomes and the Endocytic Recycling Compartment
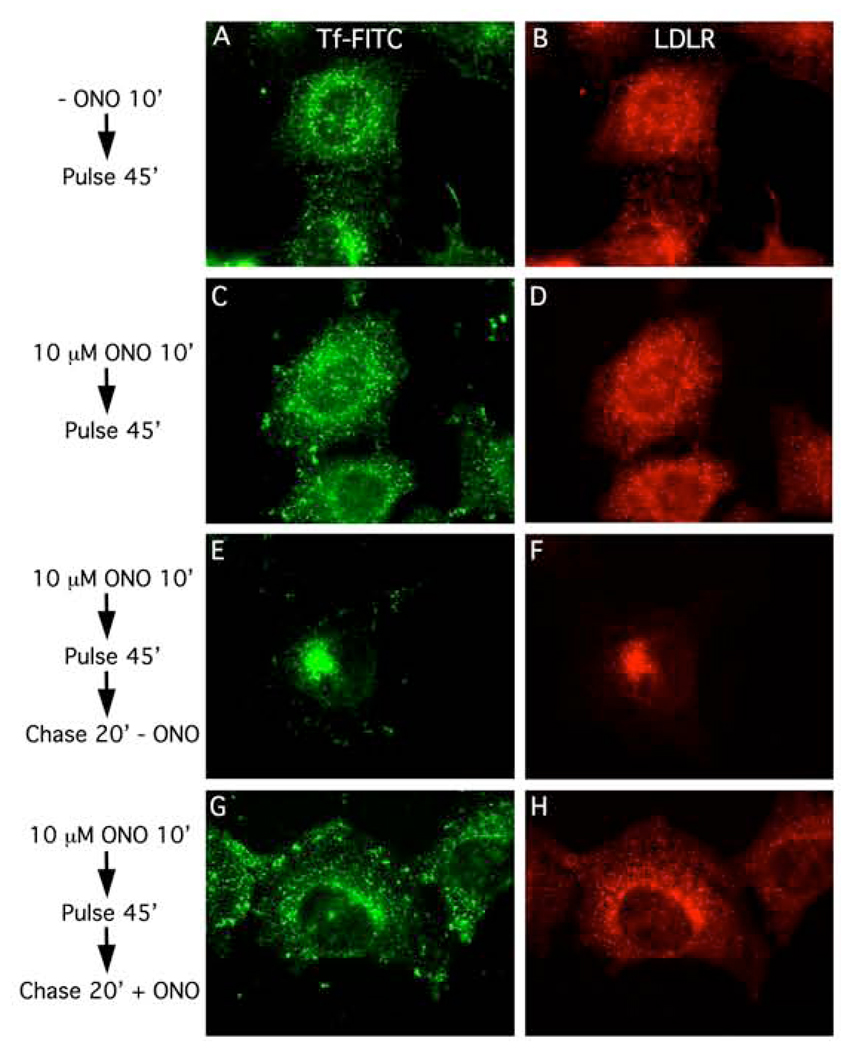
To determine if a portion of the Tf-FITC and LDLR pools were indeed trapped in peripheral SEs with high (10 µM) ONO, HeLa cells were pre-treated with or without 10 µM ONO for 10 min, then pulse-labeled with Tf-FITC for 45 min at 37°C, and subjected to various chase protocols. In cells pre-treated with 10 µM ONO for 10 min followed by continuous uptake of Tf-FITC for 45 min, Tf and LDLR were located in puncta throughout the cytoplasm corresponding to SEs (Fig. 2 A, B). Additional evidence for this conclusion was obtained by showing that both Tf-FITC and LDLR redistributed to the central ERC when cells were washed free of ONO and then chased for just 20 min (Fig. 2 C, D). However, if cells were kept in 10 µM ONO during the chase, then Tf and LDLR remained present in the more peripheral SEs (Fig. 2 E and F). These results show that 10 µM ONO causes a reversible block in the export of both Tf and LDLR out of peripherally located SEs.
Figure 2.
Export from early sorting endosomes is inhibited by 10 µM ONO. In all experiments, HeLa cells were pre-treated in the presence of the reversible PLA2 antagonist, ONO, pulse-labeled in the continued presence of ONO with Tf-FITC for 45 min to label all endocytic compartments (left panels), subjected to various chase protocols in the presence or absence of ONO, and then processed for immunofluorescence to visualize LDLR (right panels). A and B, cells pre-treated in the presence of 10 µM ONO for 10 min and then pulse-labeled for 45 min. C and D, cells pre-treated in the presence of 10 µM ONO for 10 min, pulse-labeled for 45 min, and then chased in the absence of ONO for 20 min. E and F, cells pre-treated in the presence of 10 µM ONO for 10 min, pulse-labeled for 45 min, and then chased in the absence of ONO for 20 min.
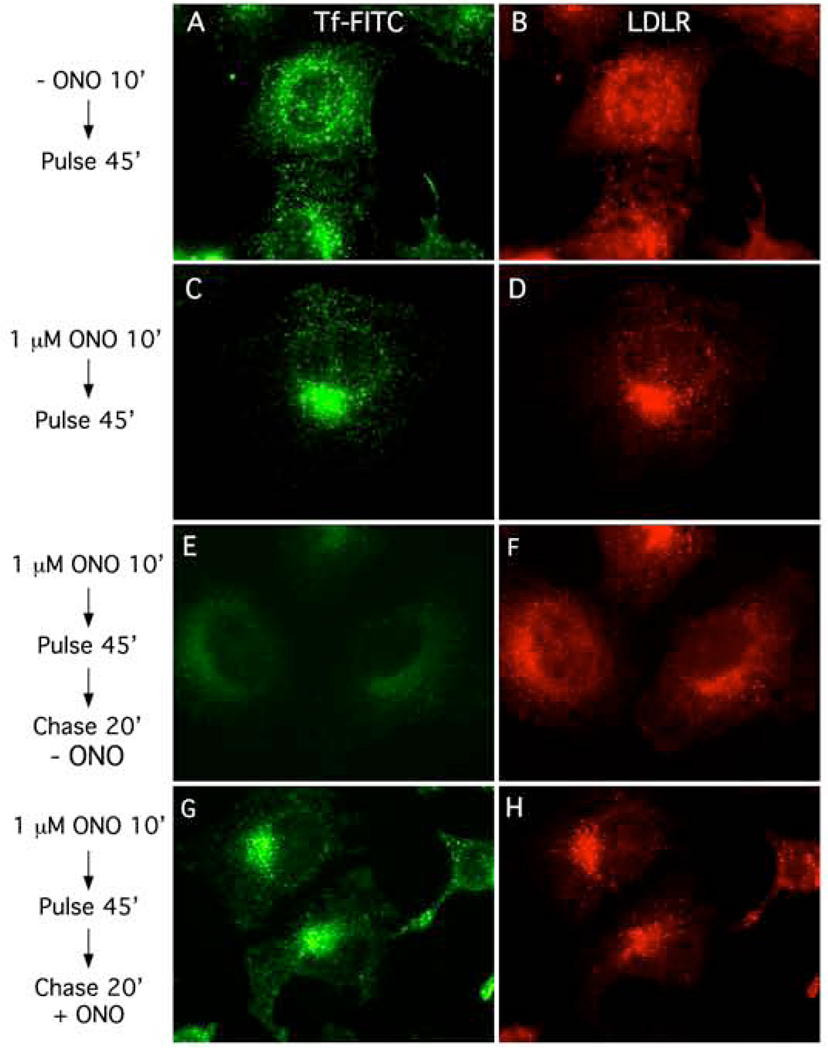
Similar experiments were done to see if 1 µM ONO inhibited export from the central ERC. In control cells incubated in MEM alone for 10 min followed by continuous uptake of Tf-FITC for 45 min, Tf and LDLR were located in peripheral SEs throughout the cytoplasm (Fig. 3 A, B). However, when cells were pre-treated with 1 µM ONO for 10 min followed by continuous uptake of Tf-FITC for 45 min, Tf and LDLR accumulated in the centrally located ERC (Fig. 3 C, D). This accumulation was reversible because following washout of ONO and chase for 20 min, Tf was recycled from the central compartment into the media and lost from cells (Fig. 3 E). In addition, LDLRs were once again found in peripheral compartments indicating that they too had recycled to the cell surface for another round of endocytic uptake (Fig. 3 H). In contrast, cells treated identically but then chased in 1 µM ONO showed that Tf and LDLR were retained in the central ERC (Fig. 3 G and H). These results show that 1 µM ONO allows transport of both Tf and LDLR to, but not export from, the centrally located ERC.
Figure 3.
Export from the central recycling compartment is inhibited by 1 µM ONO. In all experiments, HeLa cells were pre-treated in the presence or absence of the reversible PLA2 antagonist, ONO, pulse-labeled in the continued presence of ONO with Tf-FITC for 45 min to label all endocytic compartments (left panels), subjected to various chase protocols in the presence or absence of ONO, and then processed for immunofluorescence to visualize LDLR (right panels). A and B, cells pre-treated in the absence of ONO for 10 min and then pulse-labeled for 45 min. C and D, cells pre-treated in the presence of 1 µM ONO for 10 min and then pulse-labeled for 45 min. E and F, cells pre-treated in the presence of 1 µM ONO for 10 min, pulse-labeled for 45 min, and then chased in the absence of ONO for 20 min. G and H, cells pre-treated in the presence of 1 µM ONO for 10 min, pulse-labeled for 45 min, and then chased in the presence of 1 µM ONO for 20 min.
ONO Inhibits the Normal Trafficking Pathways of Both LDLR and LDL
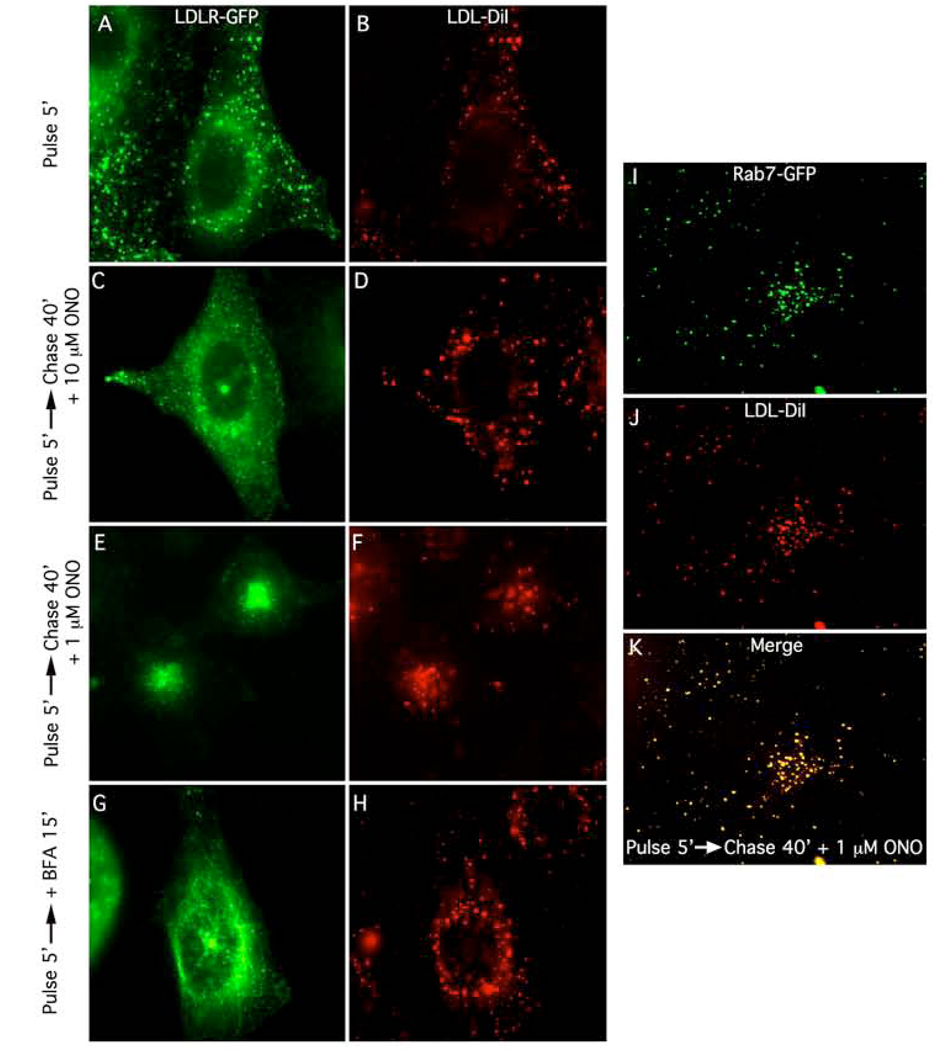
LDLRs and LDL dissociate in SEs, with LDLR recycling to the plasma membrane either directly or indirectly through the ERC, whereas LDL is transported through late endosomes (LEs) to lysosomes [1]. Therefore, we wanted to see if the trafficking of LDL were influenced by ONO treatment. In HeLa cells stably expressing an LDLR-GFP construct and incubated with LDL-DiI for 5 min at 37°C, both LDLR and LDL co-localized in puncta representing peripheral SEs (Fig. 4 A, B). As expected, under these conditions of 5 min uptake, LDLRs were also found in later endocytic compartments that did not contain LDL-DiI. When HeLa-LDLR-GFP cells were pulsed with LDL-DiI for 5 min followed by a 40 min chase in 10 µM ONO, both LDLR and LDL remained localized to these peripheral SEs (Fig. 4 C, D). However, when these cells were pulsed with LDL-DiI for 5 min followed by a 40 min chase in 1 µM ONO, LDLR and LDL localized in the juxtanuclear region, but they were found in different populations of vesicles (Fig. 4 E, F). Additional evidence that LDLR and LDL-DiI are in different compartments was obtained by showing that BFA treatment caused the LDLR-positive compartments to form tubules, but LDL was still localized in puncta throughout the cytoplasm (Fig. 4 G, H). These results show that ONO also caused a concentration-dependent block in the normal trafficking of both LDLR and LDL: first at high concentrations at a common step in peripheral SEs; and, second, at low concentrations in separate compartments that are downstream from SEs. Similar results were obtained with the PLA2 antagonist BEL (data not shown).
Figure 4.
ONO inhibits the normal trafficking pathways of both LDLR-GFP and LDL-DiI at two discrete steps in a concentration-dependent manner. In all experiments, HeLa cells expressing LDLR-GFP (right panels) were pulse-labeled with LDL-DiI (left panels) for 5 min, and then subjected to various chase protocols in the presence or absence of the PLA2 inhibitor, ONO, or the fungal metabolite, BFA. A and B, cells pulse-labeled for 5 min. C and D, cells pulse-labeled for 5 min and then chased in the presence of 10 µM ONO for 40 min. E and F, cells pulse-labeled for 5 min and then chased in the presence of 1 µM ONO for 40 min. G and H, cells pulse-labeled for 5 min and then chased in the presence of 5 µg/ml BFA for 15 min. (I–K) LDL-DiI in the presence of 1 µM ONO accumulates in Rab7-positive late endosomes. In this experiment, HeLa cells were transfected (48 h) with Rab7-GFP, pulse-labeled with LDL-DiI for 5 min, and then subjected a chase in the presence of 1 µM ONO for 40 min. I, Rab7-GFP. J, LDL-DiI. K, Merge.
The organelles that accumulated LDL in the presence of 1 µM ONO are reminiscent of LEs. To determine if the LDL-containing compartments are in fact LEs, cells were transfected to express Rab7-GFP, a LE marker [16]. After transfection (48 h), cells were pulse-labeled with LDL-DiI for 5 min at 37°C, and then chased for 40 min with 1 µM ONO. The results showed that in the presence of 1 µM ONO, LDL accumulated in centrally located compartments that are positive for Rab7 (Fig. 4 I–J). Thus, 1 µM ONO allows transport of LDL to, but not out of, LEs.
Recycling of Fluid-Phase Marker is Inhibited by ONO
Up to 50% of endocytosed fluid-phase markers can also be rapidly recycled from endocytic compartments [1], therefore we examined if PLA2 antagonists also inhibit recycling of a fluid-phase marker, fluorescent Lucifer yellow (LY). When cells were pulse-labeled by the endocytic uptake of LY and chased for various periods of time, ONO slowed the loss of LY from cells (Supplemental Fig. 1A). LY recycling was presumably slowed because the membrane tubules mediating recycling of TfR and LDLR also carry significant amounts of fluid phase.
ONO Inhibits the Normal Trafficking Pathway of EGF
The studies above show that a low concentration of ONO inhibits the export of LDL out of LEs. EGF binds to EGFR at the cell surface, is internalized, and remains bound to its receptor as it traffics from SEs through LEs to lysosomes [17]. Therefore, we wanted to see if the trafficking of EGF were influenced by treatment with ONO. In HeLa cells pulsed with EGF-Alexa488 for 5 min at 37°C, EGF was present in puncta throughout the cytoplasm representing peripheral SEs (Supplemental Fig. 1B). As expected in control cells, EGF was trafficked through the degradative pathway and mostly degraded when cells were pulse-labeled for 5 min followed by a chase for 40 min. When HeLa cells were pulsed with EGF-Alexa488 for 5 min followed by a 40 min chase in 10 µM ONO, EGF remained localized primarily to these peripheral SEs. However, when these cells were pulsed with EGF-Alexa488 for 5 min followed by a 40 min chase in 1 µM ONO, EGF was localized to fewer, larger vesicles in the juxtanuclear region corresponding to LEs. These results show that, similar to LDL trafficking, ONO also caused a concentration-dependent block in the normal trafficking of EGF through the degradative pathway. Similar results were obtained with the irreversible suicide substrate BEL (data not shown).
Tubulation of LDLR- and TfR-enriched Endosomes is Inhibited by ONO
The above results demonstrate that PLA2 antagonists produce concentration-dependent blocks in the trafficking of multiple ligands and receptors at several steps along the endocytic recycling and degradative pathways. It has been suggested that these transport steps may involve membrane tubules because PLA2 antagonists inhibit brefeldin A (BFA)-stimulated tubulation of endosomes that contained Tf/TfR [13]. If a PLA2-dependent, tubule-mediated mechanism were involved in general trafficking events, then ONO should also prevent the formation of BFA-induced, LDLR-enriched membrane tubules. Indeed, treatment of cells with 5 µg/ml BFA for 15 min induced an LDLR-containing tubular network, which was inhibited by ONO (Supplemental Fig. 1C). These LDLR-enriched tubules also contained Tf-FITC showing that the tubules were derived from compartments through which both molecules pass, i.e., SEs and the ERC.
Conclusions
Here we provide the first evidence that cytoplasmic PLA2 activity is involved in the trafficking of multiple endocytic cargoes. Reversible and irreversible PLA2 antagonists inhibited the trafficking of TfR, Tf, LDLR, LDL, and EGF in a concentration-dependent manner (Supplemental Fig. 2). It has been suggested that membrane tubules facilitate the sorting of membrane-bound cargo destined for recycling away from soluble cargo [18; 19; 20], and previous studies have shown that PLA2 antagonists inhibit the formation of membrane tubules containing Tf/TfR [13]. The accumulation of Tf and LDLR in SEs in the presence of 10 µM antagonist is consistent with the idea that membrane tubules are involved in trafficking from SEs to the ERC. The inhibition of Tf/TfR and LDLR export from the ERC by a low concentration of antagonist suggests that PLA2-dependent membrane tubules are involved in this export step. In addition, the fact that export from SE and ERC is sensitive to different concentrations of antagonists suggests that different PLA2 enzymes may operate at each location. Future studies are aimed at identifying specific cytoplasmic PLA2 enzymes involved in each of these steps.
The molecular mechanism by which a cytoplasmic PLA2 may affect membrane trafficking is unknown. PLA2 enzymes hydrolyze phospholipids to generate a positive curve inducing lysophospholipid and a free fatty acid. Localized accumulation of lysophospholipids could affect membrane curvature and recruit effector proteins that function in membrane bending and fission [21; 22]. Moreover, our studies here are consistent with the growing realization that a number of membrane trafficking events are facilitated by the continual remodeling of the phospholipid:lysophospholipid content [23; 24].
Supplementary Material
(A) ONO inhibits the recycling of the fluid-phase marker LY. Cells were pulse labeled by endocytic uptake of LY for 45 min, washed, and then chases in the presence or absence of 10 µM ONO. Total pixel intensity was quantified using NIH ImageJ software. (B) ONO inhibits the normal trafficking pathways of EGF at two discrete steps in a concentration-dependent manner. In all experiments, HeLa cells were pulse-labeled with EGF-Alexa488 for 5 min to label endocytic compartments, subjected to various chase protocols in the presence of the PLA2 inhibitor, ONO, and then processed for immunofluorescence. A, cells pulse-labeled for 5 min. B, cells pulse-labeled for 5 min and then chased in medium for 40 min. C, cells pulse-labeled for 5 min and then chased in the presence of 10 µM ONO for 40 min. D, cells pulse-labeled for 5 min and then chased in the presence of 1 µM ONO for 40 min. (C) ONO inhibits BFA-stimulated tubulation of endocytic compartments. In all experiments, HeLa cells were pulse-labeled with Tf-FITC for 45 min to label all endocytic compartments (left panels), subjected to various chase protocols in the presence of the fungal metabolite BFA and/or the PLA2 inhibitor, ONO, and then processed for immunofluorescence to visualize LDLR (right panels). A and B, cells pulse-labeled for 45 min and then chased in the presence of 5 µg/ml BFA for 15 min. C and D, cells pulse-labeled for 45 min, chased in the presence of 10 µM ONO for 10 min, and then treated with 5 µg/ml BFA in the continued presence of ONO for 15 min.
A model of the effect of PLA2-antagonists on endocytic membrane trafficking in mammalian cells.⊥ indicates differential PLA2-antagonist-induced block of endocytic trafficking events. Following receptor-mediated endocytosis, high concentrations (10 µM) of PLA2 antagonists are required to inhibit the export of both membrane bound and soluble molecules from early sorting endosomes. In contrast, lower concentrations of PLA2 antagonists inhibit export of membrane-associated molecules from the endocytic recycling center and soluble molecules from late endosomes. Tf, transferrin; TfR, transferrin receptor; LDL, low density lipoprotein; LDLR, LDL receptor; EGF, epidermal growth factor; EGFR, EGF receptor.
Acknowledgements
The authors would like to thank Enrique Rodriquez-Boulan for providing the LDLR-GFP construct and Craig Roy for the Rab7-GFP construct. This work was supported by NIH grants GM 60373 and DK 51596 (to WJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 2.Marsh M, Griffiths g, Dean GE, Mellman I, Helenius A. Three-Dimensional Structure of Endosomes in BHK-21 Cells. P.N.A.S. 1985;83:2899–2903. doi: 10.1073/pnas.83.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wall DA, Hubbard AL. Receptor-mediated endocytosis of asialoglycoproteins by rat liver hepatocytes: biochemical characterization of the endosomal compartments. J Cell Biol. 1985;101:2104–2112. doi: 10.1083/jcb.101.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willingham MC, Hanover JA, Dickson RB, Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984;81:175–169. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson JM, Whitney JA, Neutra MR. Identification of an endosomal antigen specific to absorptive cells of suckling rat ileum. J. Cell Biol. 1987;105:691–703. doi: 10.1083/jcb.105.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geuze HJ, Slot JW, Schwartz AL. Membranes of sorting organelles display lateral heterogeneity in receptor distribution. J Cell Biol. 1987;104:1715–1723. doi: 10.1083/jcb.104.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geuze HJ, Slot JW, Strous GJAM, Lodish H, Schwartz AL. Intracellular site of asiaologlycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- 8.Geuze HJ, Stoorvogel W, Strous GJ, Slot JW, Bleekemolen JE, Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988;107:2491–24501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klumperman J, Hille A, Veenendaal T, Oorschot V, Stoorvogel W, Vonfigura K, Geuze HJ. Differences in the endosomal distributions of the 2 mannose 6-phosphate receptors. J Cell Biol. 1993;121:997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoorvogel W, Geuze HJ, Strous GJ. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J Cell Biol. 1987;104:1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee S, Maxfield FR. Role of membrane organization and membrane domains in endocytic lipid trafficking. Traffic. 2000;1:203–211. doi: 10.1034/j.1600-0854.2000.010302.x. [DOI] [PubMed] [Google Scholar]
- 12.Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–221. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 13.de Figueiredo P, Doody A, Polizotto RS, Drecktrah D, Wood S, Banta M, Strang M, Brown WJ. Inhibition of transferrin recycling and endosome tubulation by phospholipase A2 antagonists. J. Biol. Chem. 2001;276:47361–47370. doi: 10.1074/jbc.M108508200. [DOI] [PubMed] [Google Scholar]
- 14.Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- 15.Hazen SL, Zupan LA, Weiss RH, Getman DP, Gross RW. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium- dependent and -independent phospholipases A2. J Biol Chem. 1991;266:7227–7232. [PubMed] [Google Scholar]
- 16.Pfeffer S. Membrane domains in the secretory and endocytic pathways. Cell. 2003;112:507–517. doi: 10.1016/s0092-8674(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 17.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 18.Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn KW, Maxfield FR. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J Cell Biol. 1992;117:301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallop JL, McMahon HT. BAR domains and membrane curvature: bringing your curves to the BAR. Biochem Soc Symp. 2005:223–231. doi: 10.1042/bss0720223. [DOI] [PubMed] [Google Scholar]
- 22.Kozlov MM. Fission of biological membranes: interplay between dynamin and lipids. Traffic. 2001;2:51–65. doi: 10.1034/j.1600-0854.2001.020107.x. [DOI] [PubMed] [Google Scholar]
- 23.Bankaitis VA. The Cirque du Soleil of Golgi membrane dynamics. J Cell Biol. 2009;186:169–171. doi: 10.1083/jcb.200907008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J Cell Biol. 2009;186:211–218. doi: 10.1083/jcb.200904147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) ONO inhibits the recycling of the fluid-phase marker LY. Cells were pulse labeled by endocytic uptake of LY for 45 min, washed, and then chases in the presence or absence of 10 µM ONO. Total pixel intensity was quantified using NIH ImageJ software. (B) ONO inhibits the normal trafficking pathways of EGF at two discrete steps in a concentration-dependent manner. In all experiments, HeLa cells were pulse-labeled with EGF-Alexa488 for 5 min to label endocytic compartments, subjected to various chase protocols in the presence of the PLA2 inhibitor, ONO, and then processed for immunofluorescence. A, cells pulse-labeled for 5 min. B, cells pulse-labeled for 5 min and then chased in medium for 40 min. C, cells pulse-labeled for 5 min and then chased in the presence of 10 µM ONO for 40 min. D, cells pulse-labeled for 5 min and then chased in the presence of 1 µM ONO for 40 min. (C) ONO inhibits BFA-stimulated tubulation of endocytic compartments. In all experiments, HeLa cells were pulse-labeled with Tf-FITC for 45 min to label all endocytic compartments (left panels), subjected to various chase protocols in the presence of the fungal metabolite BFA and/or the PLA2 inhibitor, ONO, and then processed for immunofluorescence to visualize LDLR (right panels). A and B, cells pulse-labeled for 45 min and then chased in the presence of 5 µg/ml BFA for 15 min. C and D, cells pulse-labeled for 45 min, chased in the presence of 10 µM ONO for 10 min, and then treated with 5 µg/ml BFA in the continued presence of ONO for 15 min.
A model of the effect of PLA2-antagonists on endocytic membrane trafficking in mammalian cells.⊥ indicates differential PLA2-antagonist-induced block of endocytic trafficking events. Following receptor-mediated endocytosis, high concentrations (10 µM) of PLA2 antagonists are required to inhibit the export of both membrane bound and soluble molecules from early sorting endosomes. In contrast, lower concentrations of PLA2 antagonists inhibit export of membrane-associated molecules from the endocytic recycling center and soluble molecules from late endosomes. Tf, transferrin; TfR, transferrin receptor; LDL, low density lipoprotein; LDLR, LDL receptor; EGF, epidermal growth factor; EGFR, EGF receptor.