Abstract
Human infection with Leishmania braziliensis leads to the establishment of cutaneous leishmaniasis (CL), characterized by the appearance of skin lesions that progress from non-ulcerated to ulcerated forms. Our goal was to characterize the immunological kinetics associated with this progression, comparing the cellular composition, cytokines and granzyme expression between lesions of patients with early (E-CL) and late stages (L-CL) of CL. Histopathological analysis showed that lesions from L-CL had more exuberant inflammatory infiltrate as compared to E-CL. Although E-CL and L-CL lesions were predominantly mononuclear, lesions from E-CL patients presented higher neutrophil and eosinophil counts than L-CL. While percentages of CD4+ and of CD68+ cells were slightly higher in L-CL, a five-fold increase of CD8+ cells was observed in L-CL, as compared to E-CL. Moreover, CD8+ T-cells from L-CL expressed significantly higher levels of granzymeA than E-CL. Interestingly, granzymeA expression was positively correlated with intensity of the inflammatory infiltrate in L-CL but not E-CL. Lastly, percentages of IFN-γ+ and IL-10+ cells were higher in L-CL as compared to E-CL, with CD4+ T-cells and CD68+ monocytes as the main sources of these cytokines, respectively. These results suggest that recruitment of CD8+granzymeA+ T-cells is involved in lesion progression in human CL.
Keywords: leishmaniasis, lesion, progression, CD8+ T cells, granzyme
Introduction
Leishmaniasis, caused by infection with parasites of the Leishmania genus, affects millions of individuals worldwide causing serious morbidity and mortality. Individuals infected with Leishmania present with different clinical forms, depending on the species/strain of the parasite, as well as characteristics of the host’s immune response1. In Brazil, L. braziliensis is the most prevalent species, and can cause at least two major clinical forms: cutaneous leishmaniasis (CL) and mucosal leishmaniasis (ML). CL is the most frequent form and is clinically characterized by the presence of a single skin ulcer with elevated borders that can progress to spontaneous healing2. While the self-healing of CL lesions appears to be associated with natural resistance, the immunological mechanisms of resistance have not been clearly defined. Non-self healing lesions usually respond well to antimonial administration, although this treatment is toxic to patients3. We have previously shown that early treatment fails to prevent ulcer formation in CL4. Thus, understanding the immunological kinetics of lesion progression in CL is critical for better understanding the establishment of pathology and how it can be prevented.
Different patterns of cytokine expression by T cells are related with severity of L. braziliensis infections in humans. It has been demonstrated that circulating CD4+ T cells of cutaneous leishmaniasis patients, stimulated in vitro with soluble Leishmania antigen (SLA), express IFN-gamma, but not IL-4 and IL-5, indicating the presence of specific Th1 cells in this form of the illness5. Moreover, analysis of intralesional cytokine gene expression showed that the Th1 cytokine mRNAs (IL-2, IFN-gamma, and lymphotoxin) were present in CL6,7. Coutinho et al.8 have demonstrated a predominant production of IFN-gamma by T cells from cured CL patients, suggesting a protective role for this cytokine in CL9. However, mucosal leishmaniasis, a more aggressive clinical form that can also arise from L. braziliensis infection, is associated with exuberant production of IFN-gamma and TNF-alpha10 and a deficient control of inflammation due to low expression of IL-10 receptor11. Moreover, we have shown that expression of granzyme A is evident in lesions from CL and is even more abundant in the severe ML form, suggesting a role for this molecule in tissue destruction11. It is clear that a balance between immunoregulatory mechanisms that lead to parasite control versus tissue destruction is critical for determining pathology or protection in human leishmaniasis.
With regards to the clinical evolution of CL, previous studies have shown that circulating T cells from patients with early L. braziliensis infection, characterized by approximately 15 days of illness and the presence of non-ulcerated lesion, displayed a down-modulated Th1-type response as compared to cells from patients with late CL, characterized by approximately 60 days of illness and the presence of ulcerated lesion12. Understanding the kinetics of establishment of the cutaneous lesion, through the determination of which cells are recruited to the lesion site, what is the level of expression of immunomondulatory cytokines and cytotoxic molecules will provide new insight towards the understanding of the mechanism involved with the pathology associated with human CL. Thus, the aim of this study was to compare the cellular composition, cytokine and granzyme expression in lesions of patients with early CL (E-CL) and late CL (L-CL), to better understand the immunological mechanisms involved in the clinical evolution of CL. Our data show that E-CL and L-CL are predominantly composed of mononuclear cells, although E-CL has a higher frequency of polymorphonuclear cells. Moreover, we demonstrated that the more exuberant inflammatory infiltrate observed in L-CL is consistent with lesion ulceration and is associated to higher IFN-gamma expression and the recruitment of CD8+ T cells expressing granzyme A. Importantly, an ongoing immunoregulation is present, characterized by the increased frequency of IL-10 in L-CL, consistent with further lesion resolution usually observed in CL patients.
Materials and Methods
Patients
The patients analyzed in this study were from Corte de Pedra, an endemic area for Leishmania braziliensis, located 280 km southwest of Salvador, in the state of Bahia, Brazil. All patients were volunteers, and informed consent was obtained from all individuals prior to collection of lesion material. Diagnosis of leishmaniasis was performed based on clinical and laboratory criteria. Detection of suggestive popular or ulcerated cutaneous lesions was associated with a positive skin Montenegro test, parasite isolation and/or histopathological analysis to confirm a diagnosis of CL. Patients were classified as early-stage cutaneous leishmaniasis (E-CL – approximately 15 days of illness, non-ulcerated lesion) or late-stage cutaneous leishmaniasis (L-CL – approximately 60 days of illness, ulcerated lesion), as previously established by us4. For all cases, parasite species were typed to confirm that the disease was due to L. braziliensis infection. E-CL patients enrolled in this study (n= 6) or L-CL patients (n = 9) presented with a single non-ulcerated or ulcerated lesion, respectively, and had not been previously diagnosed with or treated for leishmaniasis. At the time of sample collection, the time of the active lesions were estimated among 15 (E-CL) or 30 to 60 days (L-CL), as reported by the patients themselves. Treatment was offered to all patients as needed despite their enrollment in this project and was administered after sample collection. E-CL and L-CL patients were not under treatment when samples were collected. Lesions were collected at the Corte de Pedra health care facility. The Ethical Committees of Universidade Federal de Minas Gerais and Universidade Federal da Bahia approved all procedures involved in this study.
Sample obtention
Skin biopsy specimens were taken from the borders of active lesions using a 4-mm-diameter punch, as routinely done by us and others, since the central area of the lesion comprises necrotic and hemorrhagic areas. In the case of early lesions, biopsies were collected from the papule of the non-ulcerated lesions, after the application of a local anesthetic. Lesions were maintained in a 30% sucrose solution for 30 min at 4°C and then transferred to OCT Tissue Tek freezing medium and immediately placed in dry ice. The material was stored at −70°C until analysis.
Histological and immunofluorescence staining
Individual 4 to 5 µm cryosections were placed in silane-precoated slides and fixed for 10 min with acetone. Slides were incubated with phosphate-buffered saline for 30 min and subjected either to hematoxylin-eosin staining or to immunofluorescence staining using specific monoclonal antibodies. Standard hematoxylin-eosin staining was performed to ensure tissue integrity as well as for evaluation of the intensity and composition of the inflammatory infiltrate. Immunofluorescence reactions involved incubation with fluorescein isothiocyanate (FITC)-and phycoerythrin (PE)-labeled monoclonal antibodies directed to surface receptors (CD4 clone S3.5, CD8 clone 3B5 or CD68 clone Ki-M7) and intracellular molecules (granzyme A clone CLB-GA28, IFN-γ clone or B27, IL-10 clone 9D7), respectively. Sections were incubated with antibodies mixture overnight at 4°C. After staining, preparations were extensively washed with phosphate-buffered saline, counterstained with 4’,6’-diamidino-2-phenylindole (DAPI), and mounted using Antifade mounting medium (Molecular Probes). Slides were kept at 4°C, protected from light, until acquisition in a laser scanning confocal microscope (Zeiss). Isotype controls were analyzed separately to confirm the lack of nonspecific staining. Monoclonal antibodies were purchased from Caltag (Burlingame, CA).
Light microscopy and confocal analysis
Hematoxylin-eosin stained sections were analysed using a light microscopy (Axiovert, Zeiss). We acquired the data using a power magnification of 400x, and the frequencies of neutrophils, eosinophils, and mononuclear cells were expressed as absolute numbers or percentage of the total cell count. A total of 16 fields/sample were acquired for the histological analysis.
Confocal analysis were performed using a Meta-510 Zeiss laser scanning confocal system running LSMix software coupled to a Zeiss microscope (Axiovert 100) with an oil immersion Plan-Apochromat objective (63X, 1.2 numerical aperture) and Bio-Rad MRC 1024 laser scanning confocal system running LaserSharp 3.0 software coupled to a Zeiss microscope (Axiovert 100) with a water immersion objective (40X, 1.2 numerical aperture). A water-cooled argon UV laser (488 nm) or a krypton/argon laser was used to excite the preparation (through its 363-nm, 488-nm, or 568-nm line), and light emitted was selected with band-pass filters (522/35 for FITC or 598/40 for PE). For DAPI visualization a mercury lamp were used to excite the preparation (through its 20/80 nm line), and light emitted was selected with band-pass filters (363/90 for DAPI). For each section, the inflammatory infiltrate present in the connective tissue adjacent to the epithelia was located and an area presenting with an uniform infiltrate was selected for analysis. Within this inflammatory area, a minimum of six images (fields) were collected. Image analysis and processing were performed with LSMix (Zeiss) or LaserSharp (Bio-Rad), Confocal Assistant, Adobe Photoshop, and Image Tool software. Analyses were performed by counting the total number of cells in six to nine fields acquired and calculating the average of cells number per field for each patient. This procedure was performed for each parameter analyzed, allowing determination of the total number of inflammatory cells (total number of DAPI+ cells within the inflammatory infiltrate), the number of FITC or PE single-positive cells, and the number of double positive cells. The counts were performed blindly, the results were expressed as the average of cells number per field for each parameter for each patient, and then the values were averaged for each group. The results are representative of two experiments per patient.
Statistical analysis
Statistical analysis of the data was performed using JMP statistical software from SAS and BioEstat 3.0 statistical software. The comparisons of percentage for a given parameter between the groups were performed using the nonparametric t test or Mann-Whitney test. Spearmann correlation analysis test was also performed. Results were considered statistically different when the analysis returned a P value of <0.05.
Results
Distinct inflammatory profiles in E-CL and L-CL lesions
The composition of the inflammatory infiltrate in lesions from E-CL and L-CL patients was determined using conventional histological analysis as described in Materials and Methods. Sections stained with hematoxylin-eosin (HE) from E-CL patients were compared to L-CL patients. Histopathological analysis of lesions from patients with both stages of CL showed a keratinized stratified squamous epithelium, presenting hyperkeratosis, parakeratosis, acanthosis and a large number of cells with hydropic degeneration in the prickly layer (not shown). Diffuse chronic inflammation with predominantly mononuclear cell infiltration was observed in the dense connective tissue (Table 1) classified as productive and exudative type. Quantitative analysis showed that lesions from patients with E-CL presented higher percentage of polymorphonuclear cells (p = 0.02), neutrophils (p = 0.04), and eosinophils (p = 0.01) when compared to lesions from L-CL (Table 1). However, the frequency of polymorphonuclear cells in both groups was very low in relation to the total inflammatory infiltrate (less than 2%). It was observed that the intensity of the inflammatory infiltrate (number of inflammatory cells per field) was higher in lesions from patients with L-CL than E-CL (p = 0.004) (Table 1).
Table 1.
Total number of inflammatory cells per area and frequencies of mononuclear and polymorphonuclear (neutrophil and eosinophil) cells in lesions from patients with early cutaneous leishmaniasis (E-CL) and late cutaneous leishmaniasis (L-CL).
| Parameter | E-CL (n=6) | L-CL (n=12) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | Mean | Median | Minimum | Maximum | P value | |
| Number of total cells/field | 200.96 | 188.87 | 118.44 | 302.34 | 388.74 | 395.63 | 233.75 | 551.09 | 0.0037 |
| % mononuclear cells | 97.92 | 97.97 | 97.13 | 98.45 | 98.66 | 98.95 | 96.83 | 99.45 | 0.0492 |
| % polymorphonuclear cells | 2.73 | 2.83 | 2.17 | 3.25 | 1.61 | 1.17 | 0.66 | 3.80 | 0.0192 |
| % neutrophils | 2.08 | 2.03 | 1.55 | 2.88 | 1.35 | 1.05 | 0.55 | 3.17 | 0.0492 |
| % eosinophils | 0.65 | 0.58 | 0.34 | 1.22 | 0.26 | 0.18 | 0.07 | 0.62 | 0.0131 |
Hematoxilin-eosin stained sections were analyzed using light microscopy. All connective tissue from each lesion was evaluated in a power magnification of 400X. The comparisons of percentage for a given parameter between the groups were performed using the nonparametric Mann-Whitney test.
Lesions from L-CL display a higher proportion of CD8+ cells than E-CL lesions
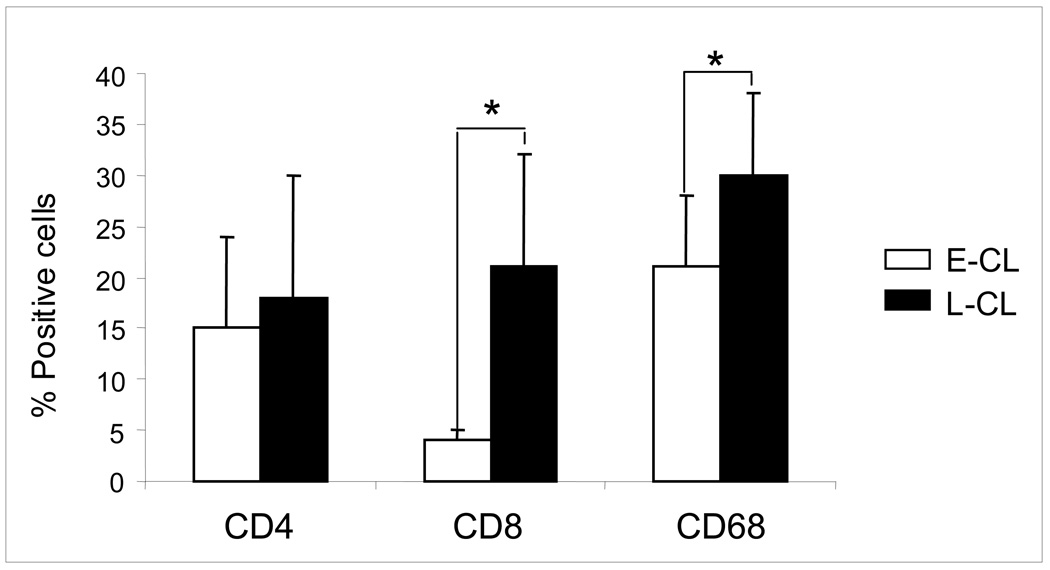
Analysis of the frequencies of CD68+, CD4+ and CD8+ cells was performed using confocal microscopy. The absolute numbers of these cell populations increased in L-CL, as compared to E-CL, compatible with the more exuberant inflammatory infiltrate observed in the former group. Thus, we determined the percentages of these populations within the infiltrates of both forms, to access the contribution of each population to the overall inflammatory infiltrate, comparing between E-CL and L-CL. Our analysis showed that the percentages of CD4+ cells and CD68+ cells were higher in L-CL as compared to E-CL (Figure 1). Strikingly, a five-fold increase in the percentage of CD8+ cells was observed in L-CL when compared to E-CL group (Figure 1).
Figure 1.
Percentages of T CD4+, T CD8+ and CD68+ cells in the inflammatory infiltrate from early cutaneous leishmaniasis (E-CL) (n=6) and late cutaneous leishmaniasis (L-CL) (n=9) lesions. Frozen tissue sections were stained with FITC-labeled anti-CD4, anti-CD8 or anti-CD68 monoclonal antibodies and were counterstained with DAPI as described in Materials and Methods. Results are expressed as bars of the mean percentages for each group. Standard deviation indicated by above line bars. Asterisks indicate statistically significant differences between groups at a p value of <0.05.
Recruitment of CD8+ T cells expressing a cytolytic molecule, granzyme A, is associated with lesion ulceration in CL
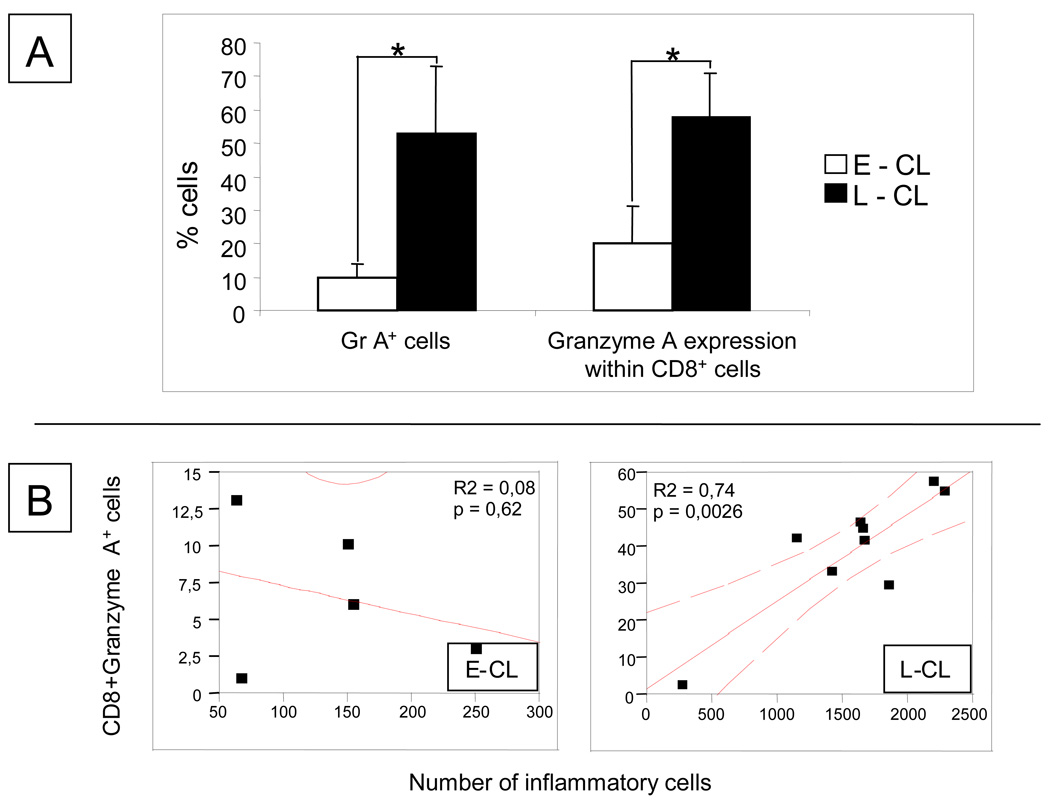
In order to evaluate whether the establishment of CL lesions was associated to cytotoxic responses, we determined the frequency of cells expressing granzyme A, a cytolytic molecule, in E-CL and L-CL lesions. Our results showed that the expression of granzyme A by cells within the inflammatory infiltrate was over 5 times higher in L-CL that E-CL (Figure 2 and Figure 3B). Moreover, while approximately 20% of the CD8+ cells from E-CL expressed granzyme A, approximately 50% of the CD8+ cells from L-CL expressed this cytolytic molecule. Interestingly, a positive correlation was observed between the intensity of the inflammatory infiltrate and the number of CD8+granzymeA+ cells in lesions from L-CL but not E-CL (Figure 2). These results suggest that the expression of granzyme A by CD8+ T cells is involved in tissue destruction during the progression of CL to the ulcerated stage.
Figure 2.
(A) Percentages of cells expressing granzyme A (Gr A+) and % granzyme A expression within CD8+ T cells in early cutaneous leishmaniasis (E-CL) (n=6) and late cutaneous leishmaniasis (n= 9) lesions. (B) Correlation analysis between the frequency of CD8+granzyme A+ cells and intensity of inflammatory infiltrate in lesions from E-CL and L-CL
Figure 3.
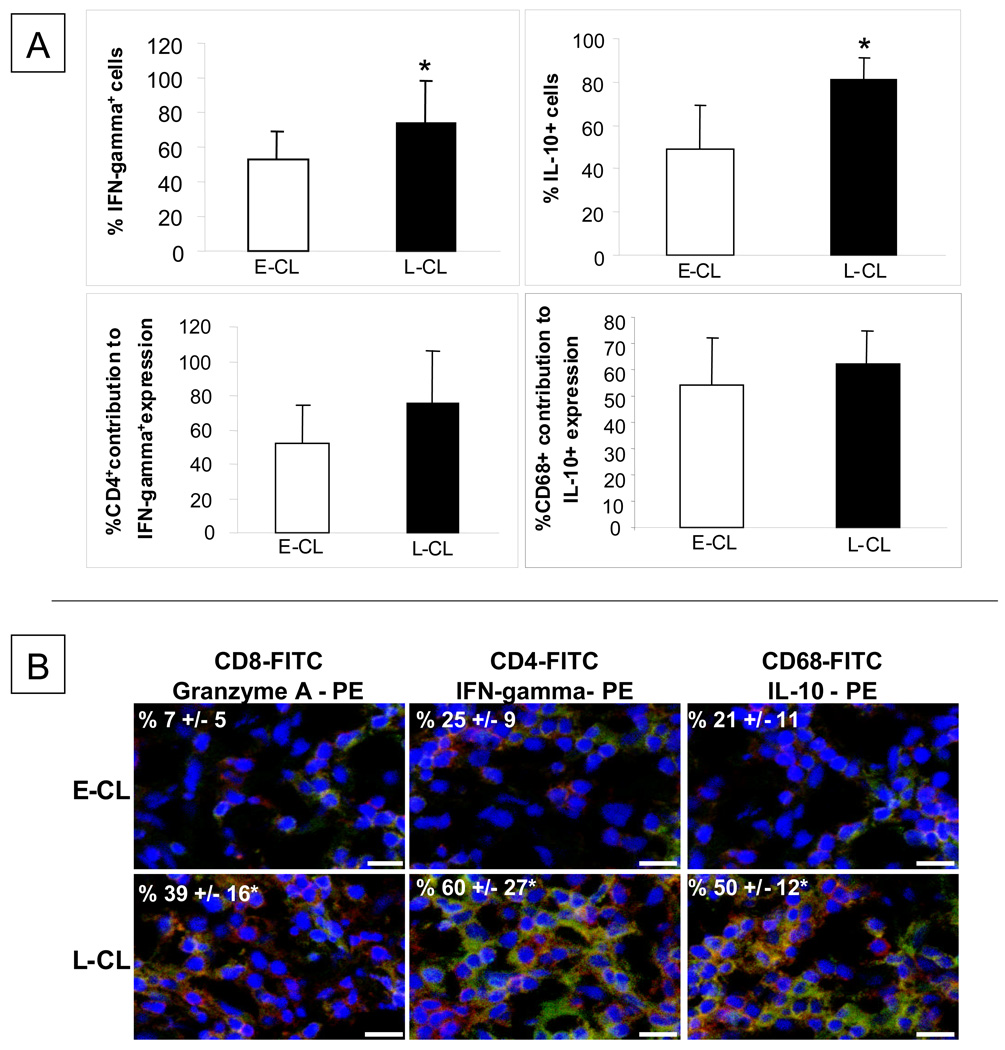
(A) Percentages, in early cutaneous leishmaniasis (E-CL) (n=6) and late cutaneous leishmaniasis (n= 9) lesions, of cells expressing IFN-gamma and IL-10 and the percentage contribution of CD4+ and CD68+ cells for the expression of IFN-gamma and IL-10, respectively. Frozen tissue sections were stained with FITC-labeled anti-CD4 or anti-CD68 monoclonal antibodies and with PE-labeled anti-IFN-gamma or anti-IL-10 antibody, and were counterstained with DAPI as described in Materials and Methods. Standard deviation indicated by above line bars. Asterisks indicate statistically significant differences between groups at a p value of <0.05 (t test). (B) Representative images from confocal microscopy analyses for determination of the frequencies of CD8+granzyme A, CD4+IFN-gamma+ and CD68+IL-10+ cells in E-CL and L-CL lesions. Frozen tissue sections were co-stained with FITC-labeled anti-CD8 or anti-CD4 or anti-CD68 monoclonal antibody and PE-labeled anti-granzyme A or anti-IFN-gamma or anti-IL-10, and were counterstained with DAPI as described in Materials and Methods. The three optical sections for each patient were obtained simultaneously with 363, 488 and 568 line of the argon/krypton laser and the proper set of filters. The overlay for CD4 or CD8 or CD68 (green), granzyme A or IFN-gamma or IL-10 (red) and DAPI (blue) in E-CL and L-CL lesions is shown. The cells that are double positive for each pair of staining (CD8+GranzymeA+ or CD4+IFN-gamma+ or CD8+IL-10+) appear in yellow. These images are representative of each group. Values represent the average +/− standard deviation of each group following numeric determination of the percentage of positive cells for the indicated molecules. * indicates statistically significant differences at a p value of <0.05. The bar = 10 µm.
Ongoing immunoregulatory mechanism is observed during the evolution of CL lesions
The frequencies of cells expressing the pro-inflammatory cytokine IFN-gamma or the anti-inflammatory cytokine IL-10 were determined using confocal analysis. The results showed that the percentages of cells expressing IFN-gamma and IL-10 were higher in L-CL as compared to E-CL (Figure 3A and B). However, no differences were observed as for the sources of these cytokines between the groups, with CD4+ and CD68+ cells as the main sources of IFN-gamma and IL-10, respectively.
Discussion
The most common clinical form of the disease caused by L. braziliensis is localized cutaneous leishmaniasis (CL), which is characterized by single or multiple ulcerated dermal lesions that usually heal spontaneously. Patients with a short period of illness (<2 weeks) have acneiform lesions or small bleeding ulcers that clearly differ from the classical cutaneous leishmaniasis ulcer observed in patients with late cutaneous leishmaniasis. We have previously shown that early treatment of CL fails to prevent ulcer development4. In the present study, we compared the cellular composition, cytokine and granzyme A expression in lesions between individuals with E-CL and L-CL to better understand the progression of this disease towards lesion formation.
The composition of the inflammatory infiltrate in lesions from E-CL and L-CL patients was determined using conventional histological analysis, and it was verified that both clinical forms showed diffuse chronic inflammation, with the presence of dense fibrous connective tissue classified as productive and exudative type with a predominantly mononuclear cellular infiltrate. Moreover, lesions from patients with E-CL presented higher frequencies of polymorphonuclear (PMN) neutrophils and eosinophils when compared to lesions from L-CL. Previous studies had documented that ulcerated lesions from CL patients are mainly composed of mononuclear cells11,13. Although the frequency of PMN cells is low in relation to mononuclear cells (less than 2%), the presence of PMN cells in E-CL suggests a role for these cells during early infection. A recent paper using the murine model of Leishmania infection has demonstrated a role for PMN cells, specifically neutrophils, in hosting the parasite during early infection, favoring parasite survival14. Although we did not evaluate the frequency of Leishmania-infected PMN cells in E-CL lesions due to material limitation and preservation, it is possible that these cells also host the parasite in early stages of human infection. Further analysis to evaluate parasite burden in E-CL and L-CL will be performed. Rocha et al.12 showed that a down regulation of the Th1-type response occurs during the early phases of L. braziliensis infection. Moreover, they suggested that this phenomenon would allow the parasite survival and growth, leading to the development of disease.
We observed that the intensity of inflammation was higher in lesions from patients with L-CL than E-CL, compatible with lesion progression. Furthermore, this increased inflammatory infiltrate was associated with a striking recruitment of CD8+ T cells to L-CL lesions. We hypothesized that this increased inflammatory infiltrate was associated with the expression of the inflammatory cytokine IFN-gamma. IFN-gamma has been considered a critical cytokine involved in the pathogenesis of CL, due to its parasiticidal ability and also due to its inflammatory activity15,11. Our data showed an increase in IFN-gamma expression in L-CL as compared to E-CL lesions, which is consistent with our hypothesis. We have previously shown that CD4+ Th1 cells are responsible for the majority of IFN-gamma production in PBMC from CL patients5. Recently, our group demonstrated that CD4+ cells represented the majority of IFN-gamma-producing cells, followed by CD8+ cells and CD4− CD8− cells in PBMC and lesions from CL patients11,16. The analyses performed here showed that CD4+ T cells are the main source of IFN-gamma in L-CL and also E-CL, accounting for over 50% of the IFN-gamma expression in E-CL and 75% in L-CL. Thus, with progression of the disease, a higher frequency of CD4+ T cells become engaged with expression of this cytokine, although statistical analysis of these data did not show significance (p=0.06). Our suggestion that IFN-gamma is associated with disease progression is in accordance with previous studies showing that the higher the frequencies of IFN-gamma or TNF-alpha producing circulating T lymphocytes, the larger the ulcerated lesion of CL patients17.
Another important biological function of IFN-γ is the induction of cytotoxic activity, which is directly correlated with granzyme A and TIA-1 expression18,19,20. Previous studies using murine infection with L. major have shown that the frequency of T cells expressing granzyme A was significantly higher in susceptible BALB/c than in resistant C57BL/6 mice20. Also, we have previously demonstrated a higher frequency of granzyme A expression in lesions from ML patients, as compared to CL11 and that CL lesions display a high number of TIA-1+ cells21. In this work, we observed that the percentages of granzyme A+ cells and CD8+granzyme A+ cells were significantly higher in L-CL than in E-CL lesions. These data suggest that the higher frequency of cells expressing granzyme A is consistent with the extensive tissue destruction observed in L-CL and the main cell subpopulation involved in this process is the CD8+, which were preferentially recruited to L-CL. Thus, execution of cytolytic function by CD8+ T cells may be an important mechanism of tissue destruction in CL. Moreover, this cytolytic function could also be important for parasite killing. While eliminating the parasite, cell death would occur due to cytotoxicity and thus, tissue destruction and parasite elimination would be concomitant mechanisms. Our previous study showed that ML lesions have higher inflammation and tissue destruction that CL lesion11 and it has been shown that ML lesions have lower parasite burden2 that CL, strengthening the idea of a connection between parasite killing and tissue destruction.
In humans, Th1 and Th2 lymphocytes, as well as macrophages, can produce IL-10, a key macrophage deactivating cytokine22. IL-10 also promotes decreasing of IFN-gamma production and has been associated with parasite dissemination, as seen in human visceral leishmaniasis and diffuse cutaneous leishmaniasis23,24,25. Experimental models of cutaneous leishmaniasis have shown that IL-10 produced by naturally occurring T regulatory cells is crucial for persistent Leishmania major infection and generation of protective memory26. On the other hand, we have shown that a lack of response to IL-10 is associated to the hyperactive immune response seen in ML patients11. Our analysis of in situ IL-10 expression showed an increase in the expression of this cytokine in L-CL as compared to E-CL. Previous studies performed by us have shown a positive correlation between IFN-gamma and IL-10 expression by circulating leukocytes from CL27. Here we also observed an increase in IFN-gamma and IL-10 expression in L-CL. Moreover, we observed that CD68+ cells are the main source of IL-10 during both stages of the disease. The increase in IL-10 expression at later stages of CL may be important for the control of the inflammatory response and further healing of the lesion. This hypothesis is supported by the fact that ML patients do not respond to IL-10, have more intense inflammation than CL patients, and importantly, macrophages from ML patients do not display co-production of TNF-alpha and IL-10 as do CL patients11, 28.
Taken together, our data indicate that the progression of CL lesions from non-ulcerated to ulcerated states is associated with the presence of an intense inflammatory infiltrate, possibly amplified by the presence of IFN-gamma, and the recruitment of potentially cytolytic CD8+granzymeA+ cells. These analyses bring new insights towards the understanding of the progression of human CL lesions.
Acknowledgements
The authors are grateful to the financing agencies CNPq, FAPEMIG, CAPES and NIH/TMRC. We also would like to thank Drs. Alda Cruz, Cristina Guatimosim, Fátima Noronha e Annamaria Vago for critical review of the manuscript and valuable suggestions and Dr.José Barbosa Júnior for valuable help with the confocal figures.
References
- 1.Schriefer A, Schriefer AL, Góes-Neto A, et al. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun. 2004;72:508–514. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bittencourt AL, Barral A. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 1991;86:51–56. doi: 10.1590/s0074-02761991000100009. [DOI] [PubMed] [Google Scholar]
- 3.Lee SA, Hasburn R. Therapy of cutaneous leishmaniasis. Int J Infect Dis. 2003;7:86–93. doi: 10.1016/s1201-9712(03)90002-6. [DOI] [PubMed] [Google Scholar]
- 4.Machado P, Araújo C, Da Silva AT, et al. Failure of early treatment of cutaneous leishmaniasis in preventing the development of an ulcer. Clin Infect Dis. 2002;34:69–73. doi: 10.1086/340526. [DOI] [PubMed] [Google Scholar]
- 5.Bottrel RL, Dutra WO, Martins FA, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69:3232–3239. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceição-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cáceres-Dittmar G, Tapia FJ, Sánchez MA, et al. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol. 1993;91:500–505. doi: 10.1111/j.1365-2249.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutinho SG, Oliveira MP, Da-Cruz AM, et al. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–155. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 9.Coutinho SG, Da-Cruz AM, Bertho AL, Santiago MA, De-Luca P. Immunologic patterns associated with cure in human American cutaneous leishmaniasis. Braz J Med Biol Res. 1998;31:139–142. doi: 10.1590/s0100-879x1998000100019. [DOI] [PubMed] [Google Scholar]
- 10.Bacellar O, Lessa H, Schriefer A, et al. Up-Regulation of Th1-Type responses in mucosal leishmaniasis patients. Infect. Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faria DR, Gollob KJ, Barbosa J, Jr, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha PN, Almeida RP, Bacellar O, et al. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J Infect Dis. 1999;180:1731–1734. doi: 10.1086/315071. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 14.Van Zandbergen G, Klinger M, Mueller A, et al. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 15.Liew FY, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN8 gamma in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 16.Antonelli LR, Dutra WO, Oliveira RR, et al. Disparate immunoregulatory potentials for double-negative (CD4- CD8-) alphabeta and gammadelta T cells from human patients with cutaneous leishmaniasis. Infect Immun. 2006;74:6317–6323. doi: 10.1128/IAI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101:226–230. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Anderson P, Nagler-Anderson C, O'Brien C, et al. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol. 1990;144:574–582. [PubMed] [Google Scholar]
- 19.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 20.Moll H, Müller C, Gillitzer R, Fuchs H, Röllinghoff M, Simon MM, Kramer MD. Expression of T-cell-associated serine proteinase 1 during murine Leishmania major infection correlates with susceptibility to disease. Infect Immun. 1991;59:4701–4705. doi: 10.1128/iai.59.12.4701-4705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado P, Kanitakis J, Almeida R, Chalon A, Araújo C, Carvalho EM. Evidence of in situ cytotoxicity in American cutaneous leishmaniasis. Eur J Dermatol. 2002;12:449–451. [PubMed] [Google Scholar]
- 22.Mosmann TR, Moore K. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today. 1991;12:49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho EM, Barral A, Pedral-Sampaio D, et al. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J Infect Dis. 1992;165:535–540. doi: 10.1093/infdis/165.3.535. [DOI] [PubMed] [Google Scholar]
- 24.Bomfim G, Nascimento C, Costa J, Carvalho EM, Barral-Netto M, Barral A. Variation of cytokine patterns related to therapeutic response in diffuse cutaneous leishmaniasis. Exp Parasitol. 1996;84:188–194. doi: 10.1006/expr.1996.0104. [DOI] [PubMed] [Google Scholar]
- 25.Bacellar O, D'Oliveira A, Jr, Jeronimo S, Carvalho EM. IL-10 and IL-12 are the main regulatory cytokines in visceral leishmaniasis. Cytokine. 2000;12:1228–1231. doi: 10.1006/cyto.2000.0694. [DOI] [PubMed] [Google Scholar]
- 26.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 27.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Gollob KJ. Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation. Clin Exp Immunol. 2004;136:341–348. doi: 10.1111/j.1365-2249.2004.02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaze ST, Dutra WO, Lessaz M, Lessaz H, Guimaraes LH, de Jesus AR, Carvalho LP, Machado P, Carvalho EM, Gollob KJ. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scan. J. Immunol. 2006;63:70–78. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]