Abstract
Aminopeptidase B (AP-B) is a metallopeptidase that removes basic residues from the N-termini of neuropeptide substrates in secretory vesicles. This study assessed zinc regulation of AP-B activity, since secretory vesicles contain endogenous zinc. AP-B was inhibited by zinc at concentrations typically present in secretory vesicles. Zinc effects were dependent on concentration, incubation time, and the molar ratio of zinc to enzyme. AP-B activity was recovered upon removal of zinc. AP-B with zinc became susceptible to degradation by trypsin, suggesting that zinc alters enzyme conformation. Zinc regulation demonstrates the metallopeptidase property of AP-B.
Keywords: Arg/Lys aminopeptidase, zinc regulation, inhibition, neuropeptides, secretory vesicles
1. Introduction
Proteolytic processing of prohormone and proneuropeptide precursors is required for the biosynthesis of active peptide hormones and neurotransmitters, known as neuropeptides. We recently identified secretory vesicle cathepsin L as a proneuropeptide processing enzyme for proenkephalin and others [1,2]. Cathepsin L represents a novel cysteine protease pathway for prohormone processing, in addition to the subtilisin-like prohormone converteases 1 and 2 (PC1/3 and PC2) [3–5]. Cathepsin L processing of proenkephalin within secretory vesicles results in peptide intermediates containing basic residue extensions at the NH2-termini. Subsequently, Arg/Lys aminopeptidase is required to generate the mature enkephalin and related neuropeptides.
The Arg/Lys aminopeptidase activity in neuropeptide-containing chromaffin secretory vesicles was identified as aminopeptidase B (AP-B) by our recent molecular cloning studies [6]. AP-B generates mature (Met)enkephalin (ME) from Arg-ME and Lys-ME peptide intermediates [6]. Sequence analyses of bovine and rat AP-B cDNAs [6–8] illustrate the presence of the metal-binding HEXXH motif which is typical of members of metalloprotease families [9,10]. Most metalloproteases are regulated by zinc metal ion, but a few metalloproteases, such as methionyl aminopeptidase in E. coli (M24 family of metalloprotease) and aminopeptidase T from thermus aquaticus (M29 family) utilize cobalt for activity [10]. Since AP-B is present in secretory vesicles that are known to contain zinc [11–13], it was of interest in this study to explore the regulation of AP-B by zinc.
This study evaluated the zinc metal ion dependence of AP-B. Results indicated that at zinc concentrations representative of in vivo levels in secretory granules, zinc inhibited AP-B at micromolar concentrations (5–50 μM), but low levels of zinc transiently activated AP-B. Kinetics for zinc inhibition of AP-B was assessed. Zinc inhibition was reversible upon emoval of zinc. Furthermore, when incubated with inhibitory levels of zinc, AP-B became sensitive to degradation by trypsin, suggesting that zinc may alter the conformation of AP-B in a manner that increased its susceptibility to proteolytic degradation. The presence of micromolar concentrations of zinc within secretory granules [11] implicates in vivo inhibition of AP-B within secretory vesicles of endocrine and neuronal cells. These findings for zinc regulation of aminopeptidase B demonstrate its metalloprotease properties.
2. Materials and Methods
Expression and purification of recombinant aminopeptidase B (AP-B)
The rat AP-B cDNA [6] was expressed in E. coli, and purified by a Ni2-column by affinity binding to the N-His-tag of recombinant AP-B, as we have described previously [6]. The resultant AP-B is enzymatically active with Arg-MCA substrate used for monitoring AP-B activity. Purified AP-B was stored in 50% glycerol at −20°C.
AP-B activity and regulation by zinc
AP-B was preincubated with 0–80 μM ZnCl2 for 5 minutes at room temperature; Arg-MCA substrate was then added to a final concentration of 200 μM in the AP-B assay buffer (100 μl assay volume, containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl). Enzyme samples were incubated at 37°C for 5–40 minutes, and AP-B activity was measured by the release of fluorescent AMC, as described previously [6]. Relative AP-B activity in the presence of zinc was compared to control AP-B activity without zinc. AP-B activity was also assessed at different molar ratios of zinc to AP-B enzyme. Assays were conducted at pH 7.5 to allow comparison to other studies of the effects of zinc on metalloproteases; AP-B activity at pH 7.5 represents approximately 80% of its activity measured at pH 5.5–6.5 [6].
Kinetics of zinc inhibition of AP-B was evaluated by Lineweaver-Burk plots [14] which showed mixed inhibition kinetics, which may represent inhibitor binding to free enzyme ([E]) or the enzyme-substrate complex ([ES]), respectively. Inhibitor binding to E and ES are represented by the kinetic constants Ki = ([E][I])/[EI] and Ki′ = ([ES][I])/[ESI]. Ki and Ki′ can be calculated by α = 1 + [I]/Ki and α′ = 1 + [I]/Ki′, with the Lineweaver-Burk plot equation represented by 1/vo = (αKm/Vmax)(1/[S]) + α′/Vmax [14]. Furthermore, the effect of zinc on the catalytic efficiency, kcat/Km, of AP-B was evaluated [14].
All assays of AP-B under different zinc conditions were performed in duplicate or triplicate, and each experiment was repeated at least two-three times. Based on the replicate assays for each condition tested for AP-B activity, the averages of the activity assays are plotted in figures 1–6. The average values for replicate assays varied by less than 5% of the average enzyme activity value. Furthermore, the mean values of aminopeptidase activities in the presence of zinc, compared to the absence of zinc, were statistically significant based on student’s t-tests with p < 0.05 (for figures 1–5). Also, the mean values of aminopeptidase activities with desalting were statistically significant compared to the enzyme activities without desalting (student’s t-test, p < 0.05) (fig. 6).
Figure 1. Zinc inhibition of aminopeptidase B (AP-B) at in vivo levels of zinc in secretory vesicles.
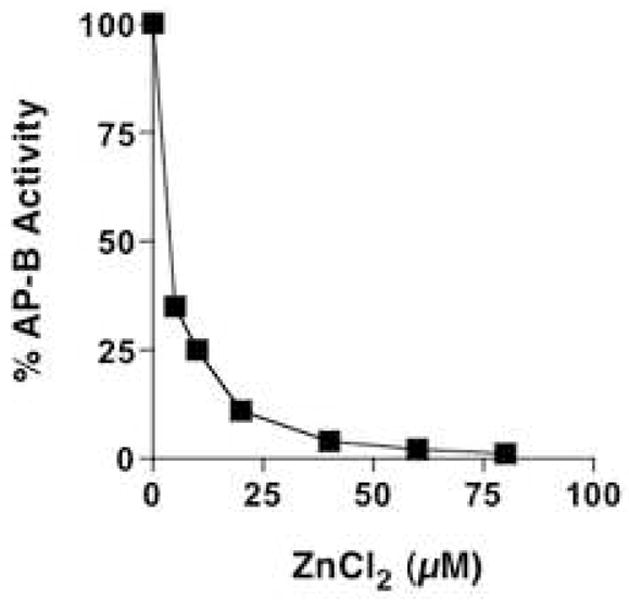
AP-B activity was assessed at different concentrations of zinc of 5–80 μM ZnCl2). AP-B (22 nM) was monitored with Arg-MCA substrate, with aminopeptidase activity detected by the formation of fluorescent AMC (aminomethylcoumarinamide). Results show inhibition of AP-B by zinc in a concentration-dependent manner.
Figure 6. Zinc increases susceptibility of AP-B to degradation by trypsin.
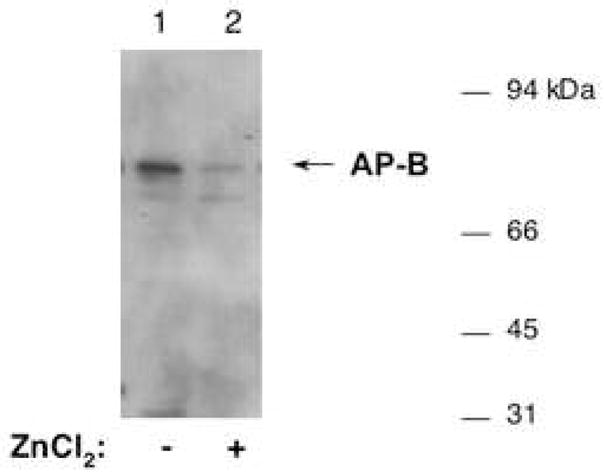
Purified AP-B (835 ng) was incubated without (lane 1) or with (lane 2) ZnCl2 (250 μM) (for 5 min.), and was then subjected to incubation with trypsin (50 ng) at 30°C for 40 minutes. The integrity AP-B was then assessed by western blots with anti-AP-B, with equal amounts of AP-B (58 ng) present in each of lanes 1 and 2. Results show that zinc induced the susceptibility of AP-B to degradation by trypsin.
Figure 5. Reversibility of zinc inhibition of AP-B.
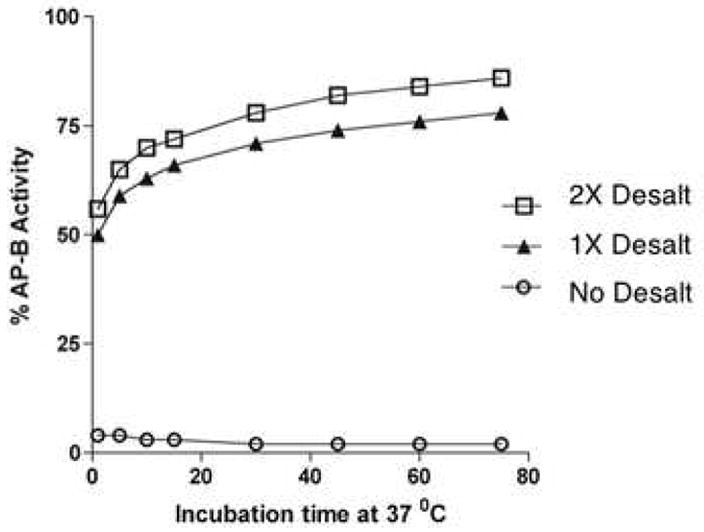
AP-B (22 nM) was incubated with ZnCl2 (50 μM) for 5 min., and was then assayed for AP-B activity with different incubation times (2–75 min.). AP-B activity without removal of zinc (○), and after removal of ZnCl2 by one (▲) or two (□) desalting column steps, was assessed. Recovery of AP-B activity is evident upon removal of ZnCl2.
Reversibility of zinc inhibition of AP-B
AP-B (22 nM) was incubated with 50 μM ZnCl2 for 5 minutes at room temperature, and was then desalted on a Zebra desalting column (Pierce Biotechnology, Rockford, IL). A second desalting step was included to insure maximal removal of ZnCl2. AP-B activity before and after desalting was monitored to assess the reversibility of zinc inhibition.
Susceptibility of AP-B to degradation by trypsin during incubation with zinc
AP-B (120 nM) was incubated in AP-B assay buffer wth zinc (250 μM ZnCl2) for 5 minutes. The uffer was exchanged to trypsin digestion buffer (100 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 0.005% Triton X-100 by a desalting column. The AP-B sample was incubated with or without trypsin (50 ng bovine trypsin, Worthington, Piscataway, NJ) at 30° (for 40 min.). The integrity of AP-B was then assessed by western blots with anti-AP-B, as described previously [1,2,6].
3. Results
Zinc regulation of AP-B
AP-B activity, measured with Arg-MCA as substrate, was inhibited by increasing concentrations of ZnCl2 with nearly complete inhibition occuring at 50–80 μM (fig. 1). These assays measured AP-B activity with Arg-MCA substrate for 30 minutes (37°C). Based on the estimated in vivo levels of zinc in secretory vesicles of 10–50 μM [11], it is likely that AP-B in secretory vesicles may be present in an inhibited condition by zinc.
At lower levels of zinc and with shorter incubation times (10 and 15 minutes) low levels of zinc at 0.25 to 2 μM resulted in some activation of AP-B activity up to 25–40% above controls (without zinc) (fig. 2). However, the longer incubation times (30 and 45 minutes) result in inhibition of AP-B at ZnCl2 of greater than 3 μM. In addition, inhibition of AP-B by zinc was shown to depend on the molar ratio of zinc to enzyme (fig. 3). Since the in vivo levels of zinc in secretory vesicles is estimated at 10–50 μM [11], it is predicted AP-B in these vesicles is largely inhibited at in vivo levels of zinc.
Figure 2. Zinc regulation of AP-B.
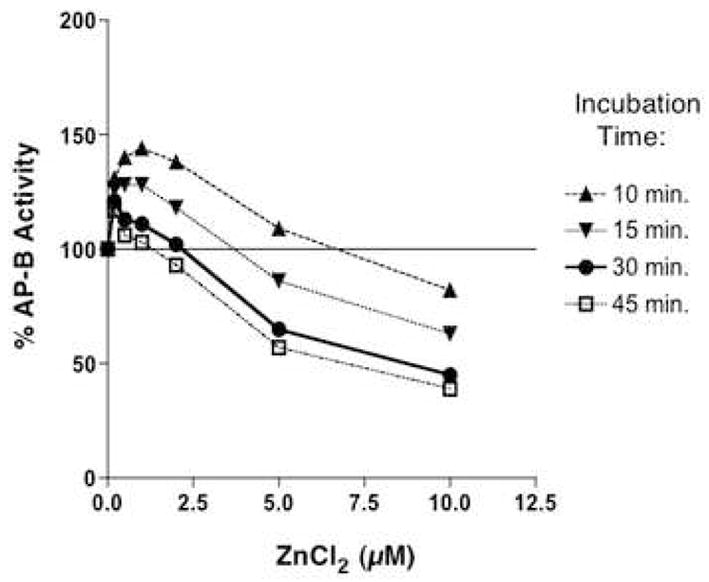
Aminopeptidase B (AP-B, 22 nM) was assessed at different concentrations of ZnCl2 in time course assays that monitored activity at 10, 15, 30, and 45 minutes of incubation. AP-B activity was expressed as percent of control AP-B activity (100%) assayed in the absence of zinc.
Figure 3. Effects of different molar ratios of zinc to AP-B for aminopeptidase activity.
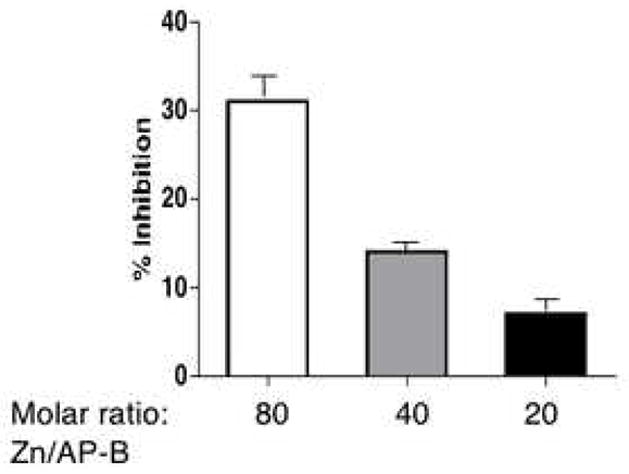
AP-B was assayed in the presence of ZnCl2 (0.5 μM) at different molar ratios of 80, 40, and 20 of zinc to AP-B at 37°C for 60 min. The percent inhibition of AP-B relative to AP-B control without zinc (0% inhibition) is shown. The mean of replicate assays (triplicate) with s.e.m. (standard error of the mean) is shown.
Kinetic evaluation of zinc inhibition of AP-B by Lineweaver-Burk analysis (fig. 4) showed zinc inhibition to display properties of a mixed inhibitor [14]. A mixed inhibitor is hypothesized to bind to the free enzyme (E) and the enzyme-substrate complex (ES). Thus, the Ki value for inhibition of E was calculated as 6 μM, and the Ki′ value for inhibition of ES was calculated as 12 μM zinc. In addition, the presence of zinc reduced the catalytic efficiency of AP-B assessed by its kcat/Km value. AP-B in the absence of zinc showed a kcat/Km value of 3.3 × 104 M−1s−1, which was reduced to 1.0 × 104 M−1s−1 in the presence of 10 μM ZnCl2 (Table 1).
Figure 4. Analysis of zinc inhibition of AP-B by Lineweaver-Burk plot.
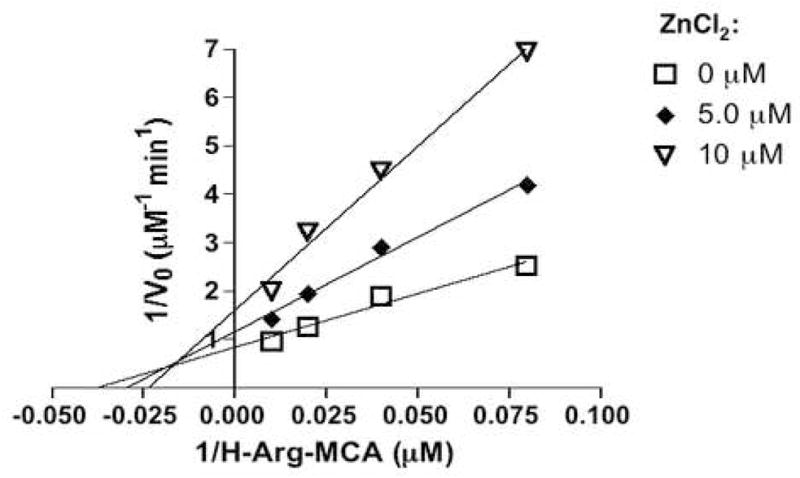
Inhibition of AP-B (22 nM) by zinc (at 1, 2, 5, and 10 μM) was assessed by inverse Lineweaver-Burk plots [15], which indicated zinc as a mixed inhibitor. The Lineweaver-Burk plot also showed that AP-B (without zinc) has Km value of 27 μM Arg-MCA and Vmax of 1.2 μM/min. The Ki and Ki′ values for zinc inhibition of enzyme and enzyme/substrate complexes were calculated as 6 μM and 12 μM zinc, respectively.
Table 1.
Catalytic efficiency of aminopeptidase B in the absence and presence of zinc
| Zinc Concentration (μM) | kcat/Km of Aminopeptidase B (M−1s−1) |
|---|---|
| 0 | 3.3 × 104 |
| 5 μM | 1.6 × 104 |
| 10 μM | 1.0 × 104 |
AP-B was evaluaed for its catalytic efficiency by determining the kcat/Km value in the absence or presence of ZnCl2 (5 μM and 10 μM).
Reversibility of zinc inhibition of AP-B
After inhibition of AP-B by zinc, AP-B activity was recovered after removal of zinc by a desalting column (fig. 5). Recovery of AP-B activity was facilitiated by subjecting AP-B to one or two desalting steps for removal of zinc. These results demonstrate the reversible nature of zinc inhibition of AP-B.
Alteration of AP-B conformation demonstrated by zinc-induced susceptibility to degradation by trypsin
After incubation of AP-B with ZnCl2 (250 μM), AP-B was more susceptible to degradation by trypsin. In the absence of ZnCl2, intact AP-B of 74 kDa was observed by western blots after incubation with trypsin (fig. 6, lane 1). However, the presence of ZnCl2 (250 μM) resulted in AP-B degradation by trypsin (fig. 6, lane 2). These results suggest that ZnCl2 alters the conformation of AP-B in a manner that faciliates its degradation by trypsin. Thus, zinc inhibition of AP-B may involve conformational changes in the AP-B enzyme.
4. Discussion
Aminopeptidase B (AP-B) is a metallopeptidase that removes NH2-terminal basic residues (Arg, Lys) from peptide substrates. AP-B was recently found to be localized within neuropeptide-containing secretory vesicles for the production of the (Met)enkephalin neuropeptide. AP-B is predicted to be regulated by zinc metal ion for its activity, as predicted from its HEXXH metallopeptidase motif. The presence of endogenous zinc in neuropeptide-containing secretory granules [11–13] that contain AP-B [6,15] raised the question of how AP-B activity may be regulated by zinc.
Therefore, this study examined and demonstrated zinc regulation of recombinant AP-B activity. AP-B was inhibited by zinc at 5–50 μM and higher levels that represent in vivo levels of zinc in secretory granules [11]. However, low levels of zinc activated AP-B activity with short incubation times, but inhibition was observed at longer incubation times. The regulatory effects of zinc were dependent on incubation time with AP-B, and on the molar ratio of zinc to AP-B enzyme. Zinc inhibition of AP-B was reversed by removal of zinc. In the presence of inhibitory levels of zinc, AP-B became susceptible to degradation by trypsin, suggesting that zinc alters the relative conformation of AP-B in a manner that facilitates trypsin degradation. Zinc modulation of AP-B activity demonstrates its metallopeptidase property, and implicates in vivo regulation of AP-B activity by zinc.
Kinetic studies demonstrated zinc as a mixed inhibitor of AP-B, demonstrated by the Lineweaver-Burk plot. Mixed inhibition is hypothesized to represent inhibition of the enzyme, AP-B, with calculation of Ki of 6 μM zinc, and inhibition of the enzyme/substrate complex with a Ki′ of 12 μM. Furthermore, evaluation of the catalytic efficiency of AP-B showed that zinc reduced its catalytic efficiency from 3.3 × 104 M−1s−1 in the absence of zinc, to 1.6 × 104 and 1.0 × 104 M−1s−1 in the presence of 5 μM and 10 μM zinc, respectively. These effects of zinc on AP-B, combined with the presence of zinc in secretory vesicles that contain AP-B, suggest that zinc may be involved in the in vivo regulation of AP-B in secretory vesicles.
The role of zinc for regulation of AP-B in neuropeptide-containing secretory vesicles is consistent with knowledge that zinc is stored and released from presynatic vesicles of neurons [11] which release peptide neurotransmitters [3]. Studies of endogenous release of zinc from synaptic vesicles estimates the in vivo level of zinc to be 10–50 μM and greater. At this range of zinc concentration, AP-B in secretory vesicles would be partially inhibited by zinc, as shown in this study. Zinc is also present in secretory granules of pituitary and pancreas that store and secrete peptide hormones [12,13]. AP-B has been demonstrated in such endocrine secretory vesicles [15]. Thus, the presence of zinc in secretory vesicles of neuroendocrine cells may allow zinc regulation of AP-B activity that is involved in the production of peptide neurotransmitters and peptide hormones.
AP-B has been proposed as an exopeptidase step in the cathepsin L protease pathway for proteolytic processing of proneuropeptides in secretory vesicles [3,6]. Following the prefered cleavage at the NH2-terminal side of dibasic processing sites within proneuropeptides by cathepsin L, peptide intermediates contain basic residues at their NH2-termini. AP-B is then required to remove NH2-terminal basic residues to generate the active neuropeptide. The presence of zinc within neuropeptide-containing secretory vesicles suggests a metal ion mechanism for the regulation of AP-B activity in this organelle.
In addition to AP-B, the carboxypeptidase E (CPE, also known as carboxypeptidase H) is another metallopeptidase utilized for neuropeptide production in secretory vesicles [3,16]. CPE removes COOH-terminal basic residues of peptide intermediates generated by proteolytic processing of proneuropeptides by the subtilisin-like prohormone convertases 1 and 2 (PC1/3 and PC2) [3–5]. Thus, both aminopeptidase and carboxypeptidase metallopeptidases are involved in secretory vesicle production of neuropeptides.
Studies of the metalloprotease carboxypeptidase A have also demonstrated that high levels of zinc inhibit the carboxypeptidase activity [17–18], with zinc shown as competitive inhibitor of CPA [19]. In addition, x-ray cyrstallography studies show that a second zinc binds to the enzyme active site that may perturb substrate catalysis [20]. These interesting studies, however, differ from zinc inhibition of AP-B shown in this report, since zinc shows mixed inhibition kinetics for AP-B (instead of zinc competitive inhibition of CPA). Furthermore, the rat AP-B shows low primary sequence homology to bovine CPA [21] of only 10.8%, although both contain the metal binding HEXXH motif; these comparisons show that AP-B differs substantially from the primary structure of bovine CPA. It will be of interest in future studies to gain structural knowledge of the mechanism for zinc inhibition of AP-B.
Importantly, this study has demonstrated that estimated endogenous levels of zinc in secretory vesicles inhibits AP-B metalloprotease activity. Thus, zinc within secretory vesicles may represent an in vivo factor involved in the regulation of AP-B activity for the production of neuropeptides that function as peptide neurotransmitters and peptide hormones.
Acknowledgments
This research was supported by grants from the National Institutes of Health.
Abbreviations
- AP-B
aminopeptidase B
- ME
(Met)enkephalin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook V. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook V. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J Biol Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- 3.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Tox. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 5.Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol, and extracellular milieu. Essays Biochem. 2002;38:79–94. doi: 10.1042/bse0380079. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SR, O’Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J Neurochem. 2007;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 7.Cadel S, Foulon T, Viron A, Balogh A, Midol-Monnet S, Noël N, Cohen P. Aminopeptidase B from the rat testis is a bifunctional enzyme structurally related to leukotriene-A4 hydrolase. Proc Natl Acad Sci USA. 1997;94:2963–2968. doi: 10.1073/pnas.94.7.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukasawa KM, Fukasawa K, Kanai M, Fujii S, Harada M. Molecular cloning and expression of rat liver aminopeptidase B. J Biol Chem. 1996;271:30731–30735. doi: 10.1074/jbc.271.48.30731. [DOI] [PubMed] [Google Scholar]
- 9.Rawlings ND, Barrett AJ. Evolutionary families of peptidases. Biochem J. 1993;290:205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2008;36:D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nature Rev. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 12.Thorlacius-Ussing O. Zinc in the anterior pituitary of rat: a histochemical and analytical work. Neuroendocrinology. 1987;45:233–242. doi: 10.1159/000124731. [DOI] [PubMed] [Google Scholar]
- 13.Wolters GHJ, Pasma A, Wiegman JB, Konijnendijk W. Changes in histochemically detectable calcium and zinc during tolbutamide-induced degranulation and susequent regranulation of rat pancreatic islets. Histochemistry. 1983;78:325–338. doi: 10.1007/BF00496620. [DOI] [PubMed] [Google Scholar]
- 14.Voet D, Voet JG. Biochemistry. John Wiley & Sons, Inc; Hoboken, NJ: 2004. pp. 480–487. [Google Scholar]
- 15.Gainer H, Russell JT, Loh YP. An aminopeptidase activity in bovine pituitary secretory vesicles that cleaves the N-terminal arginine from beta-lipotropin60–65. FEBS Lett. 1984;175:135–139. doi: 10.1016/0014-5793(84)80586-4. [DOI] [PubMed] [Google Scholar]
- 16.Fricker LD. Carboxypeptidase E. Annu Rev Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- 17.Hirose J, Noji M, Kidani Y. Interaction of zinc ions with arsanilazotyrosine-248 carboxypeptidase A. Biochemistry. 1985;24:3495–3502. doi: 10.1021/bi00335a016. [DOI] [PubMed] [Google Scholar]
- 18.Hirose J, Ando S, Kidani Y. Excess zinc ions are a competitive inhibitor for carboxypeptidase A. Biochemistry. 1987;26:6561–6565. doi: 10.1021/bi00394a041. [DOI] [PubMed] [Google Scholar]
- 19.Larsen K, Auld DS. Carboxypeptidase A: mechanism of zinc inhibition. Biochemistry. 1989;28:9620–9625. doi: 10.1021/bi00451a012. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Ortiz M, Gomis-Turh FX, Huber R, Aviles FX. Inhibition of carboxypeptidase A by excess zinc: analysis of the structural determinants by X-ray crystallography. FEBS Lett. 1997;400:336–340. doi: 10.1016/s0014-5793(96)01412-3. [DOI] [PubMed] [Google Scholar]
- 21.Le Huerou I, Guilloteau P, Toullec R, Puigserver A, Wicker C. Cloning and nucleotide sequence of a bovine pancreatic preprocarboxypeptidase A cDNA. Biochem Biophys Res Commun. 1991;175:110–116. doi: 10.1016/s0006-291x(05)81207-0. [DOI] [PubMed] [Google Scholar]


