Abstract
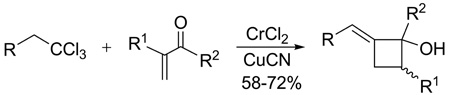
A facile, one-pot reaction cascade condenses 1,1,1-trichloroalkanes with α,β-unsaturated ketones to unexpectedly furnish moderate to good yields of (E)-2-alkylidenecyclobutanols
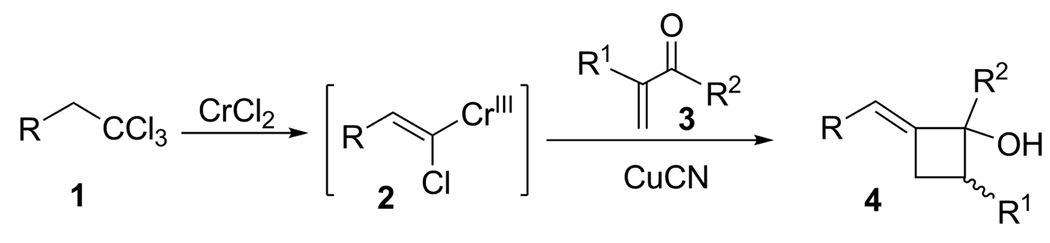
In recent years, our laboratories1 and others2 have introduced an assortment of organochromium reagents and exploited their unique physical/chemical properties for access to a wide range of natural products and high value targets.3 In continuation of these studies, we sought to extend the utility of select chromium reagents via in situ transmetalation and subsequent reaction with electrophiles. In one such example, chromium carbenoid 2 was generated from 1,1,1-trichloroalkane 1 using excess anhydrous CrCl2, except both copper cyanide and an α,β-unsaturated ketone 3 were present. We anticipated the (E)-vinylchromium(III)-intermediate 2 would undergo transmetalation and subsequent 1,4-conjugate addition with 3. Unexpectedly, however, (E)-2-alkylidenecyclobutanol 4 was isolated as the major product in moderate to good yields (Scheme 1).
Scheme 1.
Synthesis of (E)-2-Alkylidenecyclobutanols
Alkylidenecyclobutanols, and the cyclobutanols which are readily derived from them, appear as substructures4 in many architecturally interesting and/or bioactive natural products.5 They also display unique reaction manifolds that make them useful as synthetic intermediates.6 Access to these strained ring systems is generally restricted to [2+2]-cycloadditions,7 ring expansions or contractions of the corresponding homologs,8 Wittig 9 and, to a lesser extent, via intramolecular alkylations.10
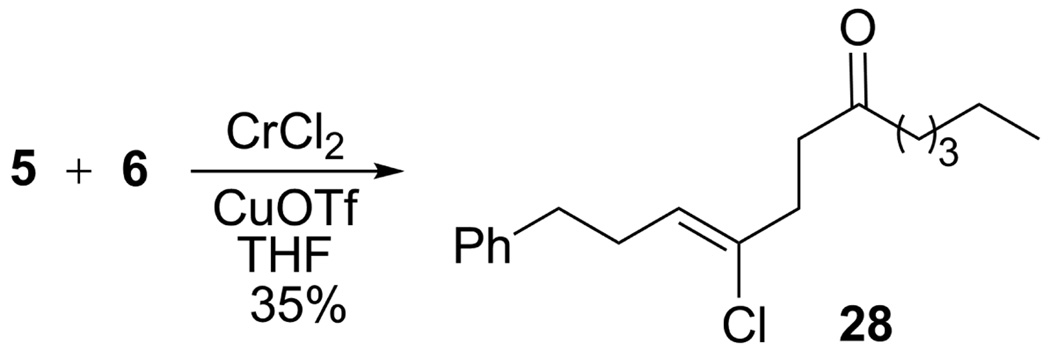
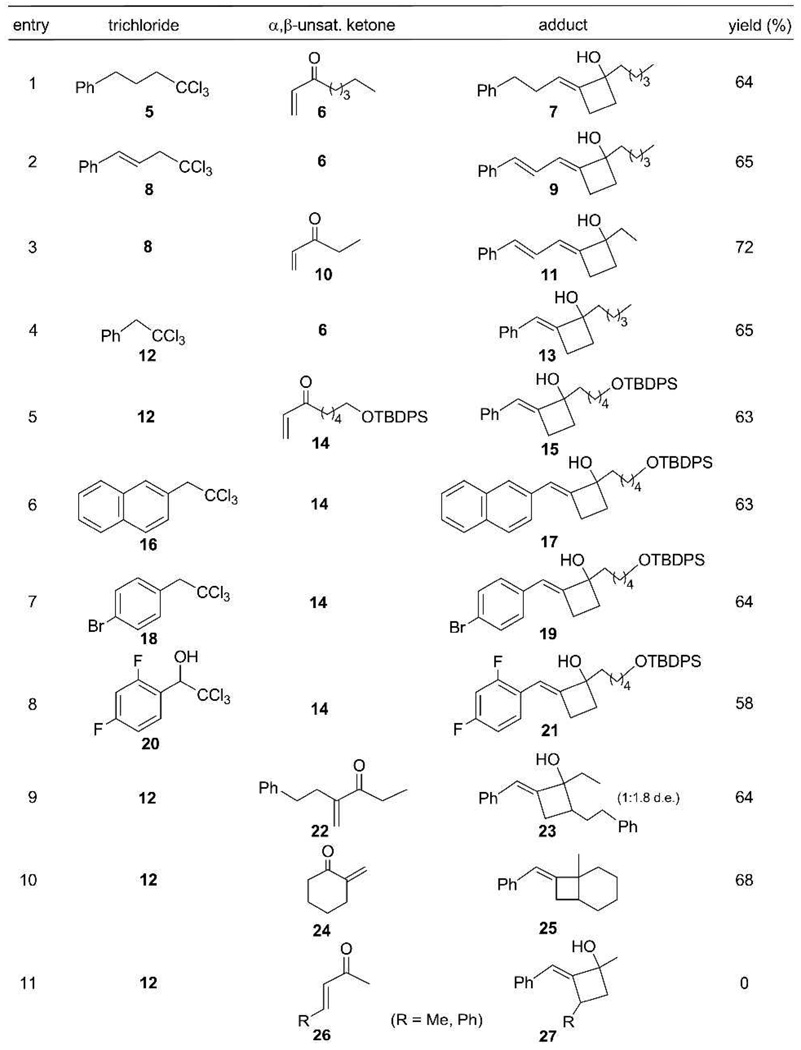
To better understand the implications of this unusual cascade reaction, we investigated its scope and possible mechanism and report our findings herein. The reaction parameters were systematically optimized using 1,1,1-trichloroalkane 5, α,β-unsaturated ketone 6, CrCl2 (6 equiv) and CuCN (1.2 equiv) as the benchmark system. Yields of 7 were best in THF (Table 1, entry 1), somewhat lower in DME, CH3CN and dioxane, and poor in DMF, HMPA, DMSO and EtOAc. The reaction was also highly dependant upon the copper salt. CuCN was superior to all others for producing alkylidenecyclobutanols; little, if any, 7 or conjugate addition was observed with CuI, CuBr, CuCl, PhSCu, or CuTc whereas CuOTf gave a 35% yield of the 1,4-adduct 28, but no alkylidenecyclobutanol (Scheme 2). Adjuvants, e.g., NiCl2, BF3·Et2O, and KCN were likewise unhelpful as were higher (70 °C) or lower (4 °C) reaction temperatures. The amount of CrCl2 could be reduced from 6 equiv to 1 equiv using Mn(0) powder as a regeneration agent,11 although the yield of 7 declined to 24%. Substoichiometric amounts of CuCN also led to significantly lower yields.
Table 1.
Synthesis of (E)-2-Alkylidenecyclobutanolsa
 |
see footnote 12 for general procedure
Scheme 2.
1,4-Conjugate Adduct
Both allylic 8 (entries 2 and 3) and benzylic 12 (entry 4) trichloroalkanes behaved analogously to 5 and afforded adducts 9, 11, and 13, respectively, from ketones 6 and 10.12 Importantly, the cascade was compatible with silyl ether 14 (entries 5–8), electron-rich napthalene 16 (entry 6), and even the aryl bromide 18 (entry 7). X-ray analysis (see Supporting Information) of adduct 17, following desilylation, confirmed its identity and the E-olefinic geometry. The latter was a key insight that must be accommodated by any proposed annulation process (vide infra).
It should be noted that benzylic trichloromethylcarbinols, e.g., 20 (entry 8), which are readily prepared from aldehydes, were also suitable precursors for the casacde, albeit with slightly diminished yields of adduct.13 Addition to α-substituted α,β-unsaturated ketone 22 proceeded smoothly to furnish 23 as a 1:1.8 diastereomeric mixture (entry 9) and, notably, the polymerization-prone exocyclic ketone 24 was transformed into fused bicyclic 25 (entry 10). In contrast, analogous efforts using the β-substituted analogue 26 (R = Me, Ph) failed to give any 27 (entry 11).
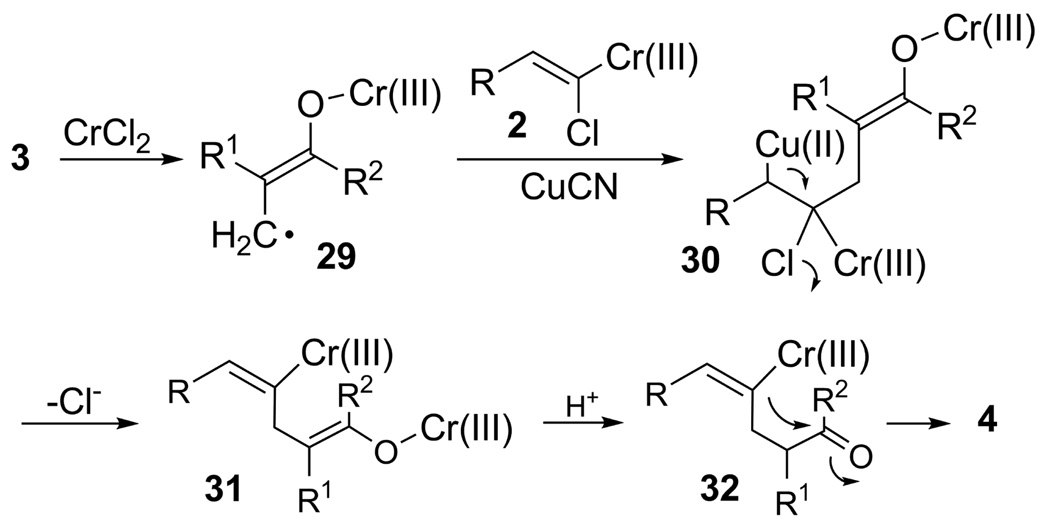
While the mechanistic details remain undefined at present, we speculate that one-electron reduction of enone 314 to enol radical 29 occurs concurrently with the production of α-halovinylidene chromium carbenoid 2 (Scheme 3).1j Subsequent copper mediated Kharasch-type addition15 and loss of copper chloride from the resultant adduct 30 deliver (E)-vinylchromium 31. Enol quench, perhaps by the previously identified internal proton return process1j,16 or adventitious water, gives 32 from which 4 is obtained by intramolecular ketone vinylation.17
Scheme 3.
Proposed Mechanism
In summary, we have demonstrated a convergent, (E)-selective synthesis of 2-alkylidenecyclobutanols based upon mechanistically unique, synergistic chemistry not achievable using either CrCl2 or CuCN alone.
Supplementary Material
Acknowledgment
Financial support provided by the Robert A. Welch Foundation and NIH (GM31278). The ANR (Agence National pour la Recherche) is acknowledge for a grant to DK.
Footnotes
Supporting Information Available Experimental procedures, spectral data of all new compounds, and crystal structure data of 17 (after desilylation) in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Review: Baati R, Falck JR, Mioskowski C. Actualite Chim. 2009;326:25. Baati R, Mioskowski C, Kashinath D, Kodepelly S, Lu B, Falck JR. Tetrahedron Lett. 2009;50:402. Bejot R, He A, Falck JR, Mioskowski C. Angew. Chem, Internat. Ed. 2007;46:1719. doi: 10.1002/anie.200604015. Falck JR, Bejot R, Barma DK, Bandyopadhyay A, Joseph S, Mioskowski C. J. Org. Chem. 2006;71:8178. doi: 10.1021/jo061445u. Falck JR, He A, Reddy LM, Kundu A, Barma DK, Bandyopadhyay A, Kamila S, Akella R, Bejot R, Mioskowski C. Org. Lett. 2006;8:4645. doi: 10.1021/ol061953q. Baati R, Mioskowski C, Barma D, Kache R, Falck JR. Org. Lett. 2006;8:2949. doi: 10.1021/ol0607140. Bejot R, Tisserand S, Reddy LM, Barma DK, Baati R, Falck JR, Mioskowski C. Angew. Chem., Internat. Ed. 2005;44:2008. doi: 10.1002/anie.200461884. Barma DK, Kundu A, Zhang H, Mioskowski C, Falck JR. J. Am. Chem. Soc. 2003;125:3218. doi: 10.1021/ja029938d. Barma DK, Kundu A, Baati R, Mioskowski C, Falck JR. Org. Lett. 2002;4:1387. doi: 10.1021/ol025708s. Baati R, Barma DK, Falck JR, Mioskowski C. J. Am. Chem. Soc. 2001;123:9196. doi: 10.1021/ja016515n.
- 2.Recent examples: Takai K, Toshikawa S, Inoue A, Kokumai R, Hirano M. J. Organometal. Chem. 2007;692:520. Concellon JM, Mejica C. Eur. J. Org. Chem. 2007:5250. doi: 10.1021/jo050655o. Nakagawa M, Saito A, Soga A, Yamamoto N, Taguchi T. Tetrahedron Lett. 2005;46:5257. Takenaka N, Xia G, Yamamoto H. J. Am. Chem. Soc. 2004;126:13198. doi: 10.1021/ja045430u. Berkessel A, Menche D, Sklorz CA, Schroder M, Paterson I. Angew. Chem., Int. Ed. 2003;42:1032. doi: 10.1002/anie.200390265.
- 3.Pospisil J, Mueller C, Fürstner A. Chem. Eur. J. 2009;15:5956. doi: 10.1002/chem.200802681. [DOI] [PubMed] [Google Scholar]
- 4.(a) Zheng Y-B, Lu C-H, Zheng Z-H, Lin X-J, Su W-J, Shen Y-M. Helv. Chim. Acta. 2008;91:2174. [Google Scholar]; (b) Vasas A, Hohmann J, Forgo P, Szabo P. Tetrahedron. 2004;60:5025. [Google Scholar]; (c) Fujiwara Y, Naithou K, Miyazaki T, Hashimoto K, Mori K, Yamamoto Y. Tetrahedron Lett. 2001;42:2497. [Google Scholar]
- 5.Hansen TV, Stenstrom Y. Naturally Occurring Cyclobutanes. In: Hudlicky T, editor. Organic Synthesis: Theory and Applications. Vol. 5. New York, NY: Elsevier Science Ltd.; 2001. pp. 1–38. [Google Scholar]
- 6.(a) Kabalka GW, Yao M-L. Tetrahedron Lett. 2003;44:7885. [Google Scholar]; (b) Fujiwara T, Iwasaki N, Takeda T. Chem. Lett. 1998:741. [Google Scholar]; (b) Anderson EA, Alexanian EJ, Sorensen EJ. Angew. Chem., Internat. Ed. 2004;43:1998. doi: 10.1002/anie.200353129. [DOI] [PubMed] [Google Scholar]; (c) Liang Y, Jiao L, Wang Y, Chen Y, Ma L, Xu J, Zhang S, Yu Z-X. Org. Lett. 2006;8:5877. doi: 10.1021/ol062504t. [DOI] [PubMed] [Google Scholar]; (d) Jung ME, Nishimura N, Novack AR. J. Am. Chem. Soc. 2005;127:11206. doi: 10.1021/ja051663p. [DOI] [PubMed] [Google Scholar]; (e) Fu N-Y, Chan S-H, Wong HNC. The Application of Cyclobutane Derivatives in Organic Synthesis. In: Rappoport Z, Liebman JF, editors. Chemistry of Cyclobutanes. Vol. 1. Chichester, UK: John Wiley & Sons Ltd.; 2005. pp. 357–440. [Google Scholar]
- 7.(a) Carreira EM, Hastings CA, Shepard MS, Yerkey LA, Millward DB. J. Am. Chem. Soc. 1994;116:6622. [Google Scholar]; (b) Taylor DR, Warburton MR, Wright DB. J. Chem. Soc., Perkin Trans. 1;1972:1365. [Google Scholar]
- 8.(a) Chowdhury MA, Senboku H, Tokuda M. Tetrahedron Lett. 2003;44:3329. [Google Scholar]; (b) Bernard AM, Floris C, Frongia A, Piras PP. Synlett. 1998:668. [Google Scholar]
- 9.Wu Z, Nguyen ST, Grubbs RH, Ziller JW. J. Am. Chem. Soc. 1995;117:5503. [Google Scholar]
- 10. Krohn K, Boerner G. J. Org. Chem. 1994;59:6063. Avila-Zarraga JG, Maldonado LA. Chem. Lett. 2000:512. Other synthetic methodology: Okuma K, Kamahori Y, Tsubakihara K, Yoshihara K, Tanaka Y, Shioji K. J. Org. Chem. 2002;67:7355. doi: 10.1021/jo0202435. Mubarak MS, Jennermann TR, Ischay M, Peters DG. Eur. J. Org. Chem. 2007;32:5346. Barbero A, Cuadrado P, Garcia C, Rincon JA, Pulido FJ. J. Org. Chem. 1998;63:7531. doi: 10.1021/jo980874s. Bailey WF, Ovaska TV. J. Am. Chem. Soc. 1993;115:3080.
- 11.Fürstner A, Shi N. J. Am. Chem. Soc. 1996;118:12349. [Google Scholar]
- 12.A mixture of 1,1,1-trichloroalkane 1 (0.2 mmol) and α,β-unsaturated ketone 3 (0.24 mmol, 1.2 equiv) in dry tetrahydrofuran (5 mL) was added to a stirring, room temperature suspension of CrCl2 (1.2 mmol, 6 equiv; Aldrich Chem. Co.) and CuCN (0.24 mmol, 1.2 equiv) in dry tetrahydrofuran (5 mL) under an argon atmosphere. After 12 h, the reaction mixture was quenched with saturated aqueous ammonium oxalate (3 mL) and extracted with Et2O (3 × 30 mL). The combined ethereal extracts were washed with water (2 × 40 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by SiO2, column chromatography using a gradient of hexane to hexane/ethyl acetate (10:1) affording 2-alkylidenecyclobutanol 4 in the indicated yields (Table 1).
- 13.Baati R, Barma DK, Falck JR, Mioskowski C. Tetrahedron Lett. 2002;43:2183. [Google Scholar]
- 14.Comparable enone Cr(II)-reductions are well precedented: Takai K, Morita R, Toratsu C. Angew. Chem. Int. Ed. 2001;40:1116. doi: 10.1002/1521-3773(20010316)40:6<1116::aid-anie11160>3.0.co;2-t. Toratsu C, Fujii T, Suzuki T, Takai K. Angew. Chem., Internat. Ed. 2000;39:2725. Montgomery D, Reynolds K, Stevenson P. J. Chem. Soc., Chem. Commun. 1993:363.
- 15.Gossage RA, van de Kuil LA, van Koten G. Acc. Chem. Res. 1998;31:423. [Google Scholar]
- 16.As would be predicted for an internal proton return (IPR) process, there was no change in the yield of 4 if 1.2 equiv of water were intentionally added at the beginning of the reaction. Also, there was no deuterium incorporation into 4 using THF-d8 as solvent or when the final reaction mixture was quenched with D2O. Utilization of 2,2-dideuterated 1 led to 4 fully deuterated at the vinyl but nowhere else inthe molecule.
- 17.Vinyl chromium reagents add to ketones with retention of configuration: Trost BM, Pinkerton AB. J. Org. Chem. 2001;66:7714. doi: 10.1021/jo010593b.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.