Abstract
Fulminant inflammation in the liver is often accompanied by the accumulation of IFN-γ-producing T cells. The BALB/c-Tgfb1−/− mouse exhibits extensive, spontaneously developing necroinflammation in the liver, accompanied by the accumulation of IFN-γ-producing CD4+ and CD8+ T cells. Liver damage depends on the presence of an intact Ifng gene. We determined the relevant cellular source(s) of IFN-γ. In Tgfb1−/− liver, CD4+ T cells were more numerous than CD8+ T cells and NK cells, and produced more IFN-γ. Depletion of CD4+ T cells eliminated both the elevation in plasma IFN-γ and aspartate aminotransferase, whereas depletion of CD8+ T cells did not. Rag1−/−Tgfb1−/− mice exhibited neither IFN-γ elevation nor tissue damage, indicating that NK cells are not sufficient. IFN-γ was required for strong overexpression of class II genes but not for CD4+ T cell activation, oligoclonal expansion, or accumulation in the liver. The T cell inhibitory molecule PD-L1 was strongly expressed in Tgfb1−/− livers, ruling out a lack of PD-L1 expression as an explanation for aberrant liver T cell activation. Finally, whereas Tgfb1−/− CD4+ T cells overexpressed Fas ligand, hepatocellular damage was observed in Faslpr/lprTgfb1−/− mice, indicating that liver pathology is Fas independent. We conclude that liver damage in this model of fulminant autoimmune hepatitis is driven by CD4+ T cell production of IFN-γ, is independent of both CD8+ T cells and the Fas ligand/Fas pathway, and is not explained by a lack of PD-L1 expression.
Hepatocellular damage mediated by CD4+ T cell is an important pathological component of several inflammatory liver diseases, including autoimmune hepatitis (AIH),3 chronic hepatitis due to hepatotropic viruses, and posttransplantation ischemia-reperfusion injury (1–5). CD4+ T cells carry out their effector functions principally by elaborating cytokines such as IFN-γ and IL-17. Expression of the Th1 cytokine IFN-γ is characteristic of acute liver injury. In AIH patients, IFN-γ expression correlates strongly with disease activity (6). In vivo neutralization of IFN-γ in mice is protective in acute liver injury due to either Con A (7) or acetaminophen (8). Pretreatment with IFN-γ exacerbates liver damage in murine experimental hepatic posttransplantation ischemia-reperfusion injury (9).
AIH is considered to be mediated by T cells that recognize one or more liver-specific Ags (10–12). Analysis of TCR usage in liver T cells from AIH patients demonstrates oligoclonality (13, 14), a hallmark of Ag-specific responses. Prominent features of AIH include a strong genetic influence (15, 16) and the accumulation in liver of CD4+ T cells and CD8+ T cells (17). The prominence of CD4+ T cells distinguishes AIH from viral hepatitis, dominated by CD8+ T cells (1). In liver biopsies from AIH patients, CD4+ T cells are found located in portal tracts and, upon isolation and stimulation ex vivo, produce IFN-γ (2, 18). IFN-γ can injure the liver through a variety of mechanisms, including direct cellular injury (19, 20), modulation of Ag presentation, and the recruitment and activation of other immune cells that participate in liver damage. It is likely that IFN-γ produced by the CD4+ T cell compartment is important for the development of hepatocellular damage. However, this hypothesis has not been directly tested in patients or in an appropriate animal model.
The BALB/c-Tgfb1−/− mouse model recapitulates certain features of human AIH, including a strong genetic influence, extensive necroinflammation in the liver, the spontaneous development of pathology, and the accumulation of CD4+ and CD8+ T cells in the liver (21, 22). Disease is CD4+ T cell dependent (23) and liver CD4+ T cell populations are oligoclonal (24). Further supporting an Ag-specific basis for disease, experimental restriction of the CD4+ TCR repertoire is protective (25).
IFN-γ is critical to the pathogenesis of liver disease in this model system. Tgfb1−/− mice rendered doubly deficient in IFN-γ (Ifng−/−Tgfb1−/− mice) exhibit portal inflammation but not the widespread parenchymal loss associated with Ifng+/+Tgfb1−/− mice. The measured concentration of IFN-γ in circulation correlates with the extent of tissue damage in Tgfb1−/− mice, underscoring the importance of determining which cell types are its relevant source(s). In this study, we show that CD4+ T cells are the sole source of IFN-γ relevant to pathology, with no significant contribution from either CD8+ T cells or NK cells. We also show that tissue damage is independent of CD8+ T cells and of Fas.
Materials and Methods
Mice
Mice were bred at Dartmouth Medical School (Lebanon, NH) and treated humanely according to National Institutes of Health guidelines. All experiments were done according to protocols approved by Dartmouth’s Institutional Animal Care and Use Committee. BALB/c background Tgfb1−/− mice, Ifng−/−Tgfb1−/− mice, and Rag1−/−Tgfb1−/− mice were generated as published (23, 26). Faslpr/lprTgfb1−/− mice were generated by intercrossing BALB/c-Tgfb1+/− mice with BALB/c-Faslpr/lpr mice (27). Litters were screened by PCR of tail DNA for Tgfb1, Ifng, Rag1, and Fas genotypes as described (22, 27). Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), and IFN-γ were measured as published (26) from day 11 and 12 mice. Mice were depleted of CD4+ T cells via i.p. injections of the mAb GK1.5 according to the previously published dose and schedule (23). Tgfb1−/− mice were similarly depleted of CD8+ T cells using the mAb T1B-210. Cell subset depletions were confirmed by flow cytometry.
Isolation of liver lymphocytes and thymocytes
Liver nonparenchymal cells (NPC) were prepared from 11 to 12-day-old mice as described (24). NPC were counted and either stained for flow cytometric analysis or used for positive selection of CD4+ cells (for liver) or CD8+ negative selection (for thymus) to obtain CD4+ single positive (CD4SP) thymocytes using the Miltenyi system.
Flow cytometry
Analyses were performed as described by Robinson et al. (25). Ficoll-purified NPC were washed with FACS buffer (2% FCS in PBS) and incubated with Abs (all from BD Pharmingen) to label CD3ε, CD4, CD8, DX5, CD11b, CD11c, class II MHC, CD69, CD62L, CD44, and/or PD-L1, washed, fixed in 1% methanol-free formaldehyde in FACS buffer, and analyzed using a FACSCanto cytometer (BD Biosciences). For each analyte, positive staining was established using appropriate isotype controls. PMA/ionomycin stimulations and intracellular IFN-γ analyses were performed as described (28).
mRNA and expression analyses
Expression levels of mRNAs encoding class II MHC genes, CIITA, PD-L1, and β-actin were determined by mining a database of liver mRNA expression. In brief, total liver RNA was prepared from 11 day old mice of the following genotypes (three mice per genotype): Ifng+/+Tgfb1+/+, Ifng+/+Tgfb1−/−, Ifng−/−Tgfb1+/+, and Ifng−/−Tgfb1−/−. Gene expression analysis used a total of twelve GeneChip Mouse Genome 430 2.0 microarrays (Affymetrix), which were processed with standard labeling, 1-cycle amplification, hybridization, washes, and scanning, completed at the Dartmouth Genomics and Microarray Laboratory. Resulting data were analyzed using GeneTraffic version 3.2 (Stratagene). The accession number for the microarrays is GSE9892 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=jjmtrseosyqownu&acc=GSE9892).
A more complete description of these analyses has been submitted for publication (M. W. Milks, J. G. Cripps, H. Lin, J. Wang, R. T. Robinson, J. L. Sargent, M. L. Whitfield, and J. D. Gorham, submitted). Expression of Fas ligand (FasL) from purified liver CD4+ T cells was done by RT-PCR. Total RNA was isolated using the RNeasy method (Qiagen). cDNA was synthesized using the Omniscript RT-PCR kit (Qiagen) with oligo(dT) primers. FasL was detected by RT-PCR as described (29), and spectratype analyses were performed as described (24).
Graphing and statistics
Figures were prepared using Graph Pad Prism version 4.0. Statistical analyses used the bundled software. Bars in the figures show mean + SD. Numbers shown above horizontal lines or next to vertical lines represent p values for the comparisons indicated. Statistical comparisons involving AST levels used the nonparametric Mann-Whitney U test. Other statistical comparisons used Student’s t test. Fig. 1B used linear regression.
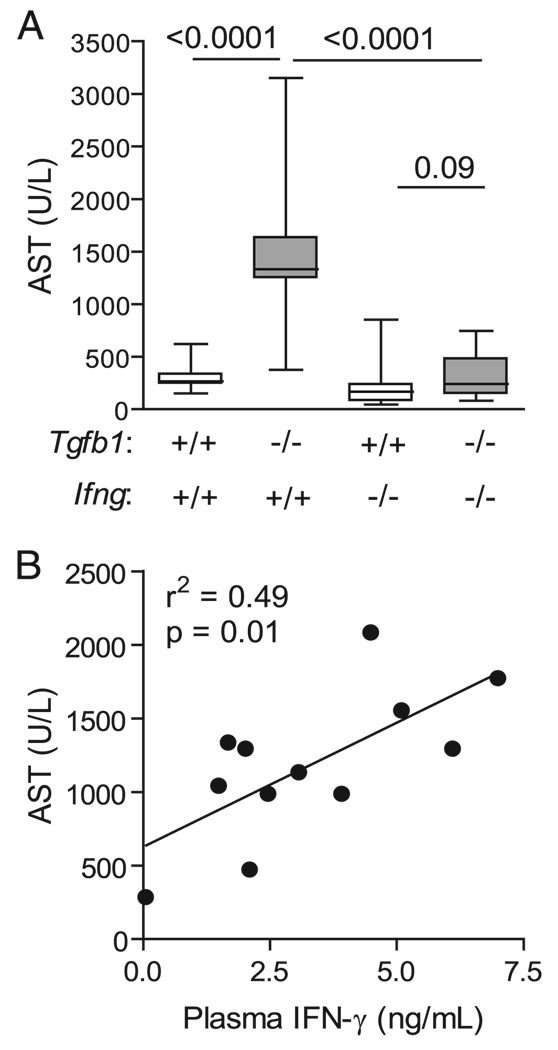
FIGURE 1.
IFN-γ determines organ damage in Tgfb1−/− mice. A, Damage is Ifng dependent. Mice of the indicated genotypes were euthanized on day 11–12 after birth. AST was measured from plasma. Data are shown as box and whiskers plots indicating means, 75% confidence intervals, and 95% confidence intervals. Numbers above lines show p values for group comparisons by Mann-Whitney U test (n ≥ 12 mice per group). B, Plasma concentrations of IFN-γ correlate with end-organ damage (AST). Data points show individual Tgfb1−/− mice (n = 12; r2 = 0.49; p = 0.01).
Results
CD4+ T cells are the principal IFN-γ-producing lymphocyte subset
Compared with Ifng+/+Tgfb1−/− mice, Ifng−/−Tgfb1−/− mice developed less tissue damage as measured by either AST (Fig. 1A), or ALT (data not shown) confirming our previous study (26). A significant correlation was observed between the extent of tissue damage, measured by plasma AST, and concentration of circulating IFN-γ (Fig. 1B; n = 12; r2 = 0.49; p = 0.01). IFN-γ production is typically associated with T cells and with NK cells. We therefore measured cell number and IFN-γ production for three liver lymphoid cell types: CD4+ T cells, CD8+ T cells, and NK (CD3−DX5+) cells. NKT cells could potentially be a source but are nearly absent in Tgfb1−/− livers (23) and so were not examined for IFN-γ. The numbers of CD4+ T cells, CD8+ T cells, and NK cells were expanded in Tgfb1−/− livers compared with Tgfb1+/+ livers (27-, 18-, and 5-fold, respectively; Fig. 2A). To identify the source of IFN-γ, liver lymphocytes were stained for intracellular IFN-γ production upon fresh isolation, without additional ex vivo stimulation. In a representative experiment, 15.0% of CD4+ T cells stained for IFN-γ production, compared with 4.2% of CD8+ T cells and 1.4% of NKT cells (Fig. 2B). In littermate control Tgfb1+/+ liver lymphocytes, IFN-γ production was low for all three cell types. After pharmacologic stimulation with PMA/ionomycin, for all three subsets, a significant proportion (30–60%) produced IFN-γ (data not shown), ruling out the possibility that Tgfb1−/− liver CD8+ T cells and NK cells are incapable of producing IFN-γ. Thus, among the three populations, liver CD4+ T cells are both most numerous and produce the most IFN-γ.
FIGURE 2.
Tgfb1−/− liver CD4+ T cells produce copious IFN-γ. A, Liver NPC isolated from 11-day-old Tgfb1−/− mice, and littermate control Tgfb1+/− mice were counted and stained with Abs to identify CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), and NK cells (CD3−DX5+). Data show mean + SD of calculated cell numbers (n = 3 mice per genotype); p values are shown. B, Freshly isolated liver NPC were surface stained to classify cell subset, and stained intracellularly to determine IFN-γ production. Unshaded profiles, anti-IFN-γ mAb; shaded profiles, isotype mAb. Similar data were acquired from three independent experiments.
To definitively test which lymphocyte subset contributes to IFN-γ in vivo, we depleted specific lymphocyte subsets. Tgfb1−/− mice rendered deficient in Rag1, and therefore lacking in adaptive lymphocytes but retaining innate immunity, had levels of plasma IFN-γ equivalent to control mice (Fig. 3A). This background level reflects IFN-γ produced by innate immune cells, including NK cells, and independently confirms that Tgfb1−/− NK cells and other innate immune cells do not appreciably contribute to IFN-γ production in Tgfb1−/− mice. To eliminate individual T cell subsets specifically, Tgfb1−/− mice were treated with subset-specific mAbs before the onset of tissue damage. Depletion of CD4+ T cells completely prevented the elevation in plasma IFN-γ, whereas depletion of CD8+ T cells did not (Fig. 3A). In addition, CD8+ T cell depletion did not prevent tissue damage in Tgfb1−/− mice, whereas elimination either of all adaptive lymphocytes (Rag1−/−Tgfb1−/− mice) or specifically of CD4+ T cells (anti-CD4) did prevent tissue damage (Fig. 3B). Together with the intracellular IFN-γ analysis, these results directly implicate CD4+ cells as the important source of pathologic IFN-γ in the Tgfb1−/− mouse. Because plasma levels of IFN-γ and AST correlate closely (Fig. 1B), we propose that CD4+ T cell production of IFN-γ is the key determinant of tissue damage in this mouse model of fulminant hepatitis.
FIGURE 3.
CD4+ T cells but not CD8+ T cells are required for both high plasma IFN-γ and end-organ damage. Plasma from mice of the indicated genotypes and/or treatments was obtained at day 11–12, and IFN-γ (A) and AST (B) were measured. Data show mean + SD (n ≥ 3 mice per group); p values are shown. Black bars, data from Tgfb1−/− mice; white bars, data from Tgfb1+/+ mice and Tgfb1+/− mice combined.
Class II overexpression in Tgfb1−/− mice is Ifng dependent
We explored potential mechanisms by which IFN-γ might contribute to tissue damage in the liver. We examined the involvement of IFN-γ in CD4+ T cell responses. We have previously shown that Tgfb1−/− liver CD4+ T cells are activated (26) and have a skewed TCR repertoire (23). It has been shown that Tgfb1−/− mice strongly overexpress MHC class II genes in multiple organs (30). Because IFN-γ potently activates class II MHC expression in APC, we hypothesized that IFN-γ-mediated class II up-regulation in turn drives CD4+ T cell activation and TCR repertoire deviation. In this way, IFN-γ might contribute to tissue damage through a positive feedback loop involving augmentation of Ag presentation and CD4+ T cell responses. In cells propagated in vitro, class II expression is positively regulated by IFN-γ and negatively regulated by TGF-β1, through antagonistic effects on the gene encoding CIITA (31). Indeed, both I-Eα and I-Eβ were strongly overexpressed in Tgfb1−/− livers, and overexpression of each was nearly completely dependent upon Ifng (Fig. 4A). Similar patterns were observed for the expressions of I-Aα and I-Aβ (data not shown). Expression patterns for class II genes correlated well with that for CIITA (Fig. 4A), consistent with a model in which TGF-β1 and IFN-γ counter-regulate class II expression in vivo through this key class II gene coactivator. Expression of the housekeeping gene β-actin was equivalent between samples. At the protein level, analyses of isolated Tgfb1−/− liver NPC indicated that class II protein overexpression was observed on CD11b+CD11c− and CD11b+CD11c+ cell populations (Fig. 4B), and that overexpression was Ifng dependent.
FIGURE 4.
IFN-γ is required for class II overexpression. A, Expression levels of mRNAs indicated were mined from microarray data from liver RNA isolated from mice of the indicated genotypes (n = 3 per group); p values are shown. B, Liver NPC were isolated from mice of the indicated genotypes and stained for CD11c, CD11b, and class II, gated as shown. Similar data were acquired from three independent experiments.
CD4+ T cell activation, TCR repertoire deviation, and T cell accumulation in liver are independent of IFN-γ
Despite effects of Ifng genotype on class II expression, Ifng genotype had no discernible impact on expression of markers indicating CD4+ T cell activation, that is liver Tgfb1−/− CD4+ T cells were CD69highCD62LlowCD44high, whether on an Ifng+/+ or Ifng−/− background (Fig. 5A). TCR repertoire skewing, as assessed by TCR spectratype, was also independent of Ifng. Spectratype analyses of control liver Ifng−/−Tgfb1+/+ CD4+ T cells yielded largely Gaussian distributions, indicating that the population is polyclonal, whereas spectratype analyses of liver Ifng−/−Tgfb1−/− CD4+ T cells yielded non-Gaussian spectratype distributions, indicative of strong oligoclonal T cell responses (Fig. 5B, top). Ifng−/−Tgfb1−/− CD4SP thymocytes yielded polyclonal profiles, similar to littermate controls (Fig. 5B, bottom). Thus, skewing of the CD4+ TCR repertoire in Ifng−/−Tgfb1−/− mice occurs in the periphery, not in the thymus, similar to what we have observed for Ifng+/+Tgfb1−/− mice (24). Finally, CD4+ T cells accumulated in Ifng−/− Tgfb1−/− liver at d 11 in numbers similar to those found in Ifng+/+ Tgfb1−/− liver (Fig. 5C). Thus, although liver disease in Tgfb1−/− mice requires Ifng, we find no evidence to a support a role for Ifng in the activation, oligoclonal expansion, or accumulation in liver of CD4+ T cells.
FIGURE 5.
IFN-γ is not required for liver Tgfb1−/− CD4+ T cell activation, TCR repertoire deviation, or accumulation in liver. A, Liver CD4+ T cells were isolated from 11-day-old mice and stained with labeled Abs to CD69, CD62L, and CD44. Data are representative of at least three mice per genotype. B, Liver CD4+ T cells and CD4SP thymocytes were purified from 11-day-old mice. mRNA was isolated, converted to cDNA and CDR3 lengths assessed by the PCR-based spectratype technique for the Vβ segments depicted. Similar data were acquired from three independent experiments. C, Liver NPC isolated from 11-day-old mice were counted and stained for CD3+CD4+ T cells. Data show mean + SD of calculated cell numbers (n ≥ 3 mice per genotype); p values are indicated.
Liver T cell activation is not due to an absence of PD-L1 expression
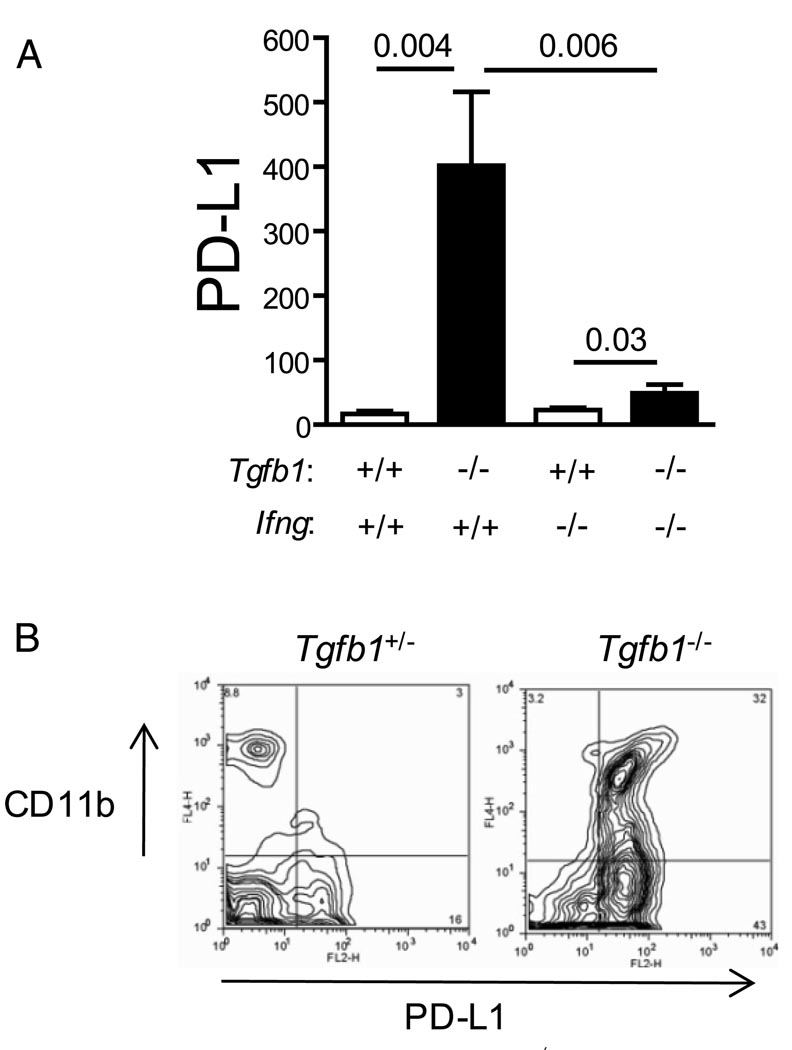
The B7-like molecule PD-L1 inhibits T cell activation and function through interaction with PD-1, expressed on T cells. PD-L1 is expressed by a variety of liver parenchymal and nonparenchymal cells and is important for the down-regulation of intrahepatic T cell responses (32–34). We therefore considered the possibility that the high degree of T cell activation observed in Tgfb1−/− liver T cells is due to a deficiency of PD-L1 expression. PD-L1 mRNA expression was not suppressed in Tgfb1−/− liver, but, conversely, was strongly up-regulated (Fig. 6A). In addition, PD-L1 protein was overexpressed on some populations of Tgfb1−/− liver mononuclear cells, CD11b+ cells in particular (Fig. 6B). Consistent with previous studies (35–37), PD-L1 expression was largely dependent on Ifng.
FIGURE 6.
PD-L1 is overexpressed in Tgfb1−/− liver. A, Expression levels of PD-L1 mRNA were mined from microarray data from liver RNA isolated from mice of the indicated genotypes (n = 3 per group). B, Liver NPC were isolated from mice of the indicated genotypes and stained for CD11b and PD-L1. Similar data were acquired from three independent experiments.
Tissue damage is Fas independent
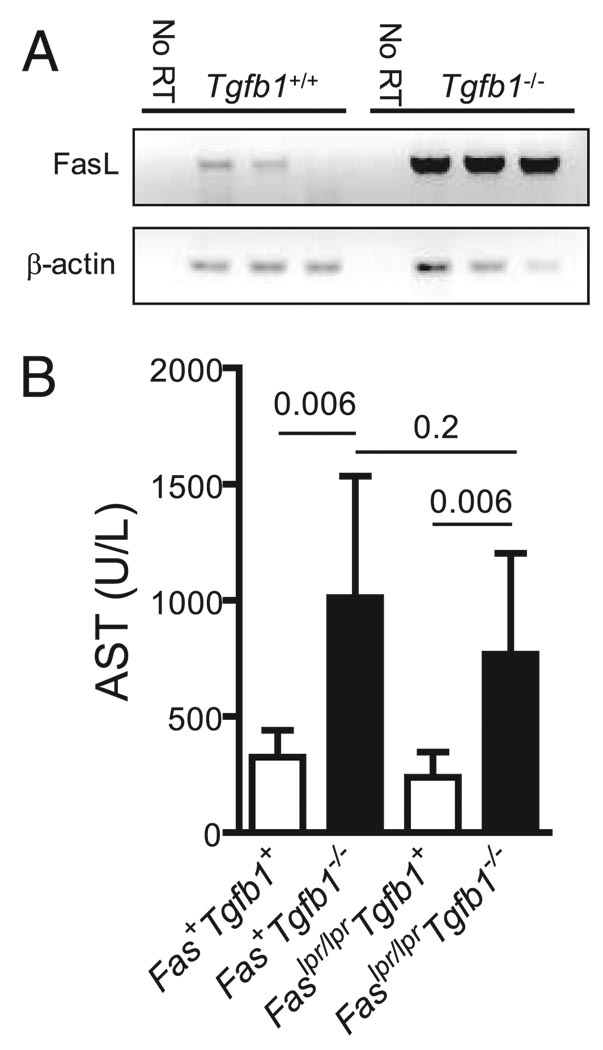
We examined whether liver injury requires the FasL/Fas death receptor pathway, as we have previously shown that FasL mRNA is overexpressed in Tgfb1−/− livers in an Ifng-dependent fashion (26). Indeed, highly purified Tgfb1−/− liver CD4+ T cells overexpressed FasL mRNA (Fig. 7A). Fas is constitutively expressed on hepatocytes and its engagement rapidly results in hepatocyte apoptosis in vivo (38). Moreover, the direct apoptotic effects of IFN-γ on murine hepatocytes may require FasL/Fas interactions (39). With these considerations in mind, we hypothesized that liver injury in Tgfb1−/− mice is Fas dependent. To test this, we rendered Tgfb1−/− mice Fas-deficient by crossing them with Faslpr/lpr mice (27). Faslpr/lprTgfb1−/− mice and Fas+Tgfb1−/− mice developed equivalent elevations in AST (Fig. 7B) and ALT (not shown) as well as the characteristic necroinflammatory hepatic lesions at histologic examination (not shown), indicating that hepatocellular damage is Fas independent.
FIGURE 7.
Liver damage is Fas independent. A, Liver CD4+ T cells were isolated from 11-day-old littermate Tgfb1+/− mice (n = 3) and Tgfb1−/− mice (n = 3). mRNA was purified, converted to cDNA, and FasL and β-actin were measured by RT-PCR. “No RT” controls indicate mRNA samples to which reverse transcriptase was not added, showing that the PCR amplicons were not templated by contaminating genomic DNA. B, Plasma AST was measured from 11- to 12-day-old mice. Bars indicate mean + SD. Values of p are shown (n = 4–7 mice per group).
Discussion
We have previously shown that IFN-γ is necessary for the development of hepatocellular damage in the Tgfb1−/− mouse. In the current work, we make several observations that advance the understanding of pathogenesis in this mouse model of inflammatory liver disease. First, the levels of circulating IFN-γ correlate with AST, indicating that IFN-γ production predicts the extent of tissue damage. Second, the most important, and perhaps only relevant, source of IFN-γ is the CD4+ T cell, with no significant contributions from either CD8+ T cells or NK cells. Third, CD8+ T cells are dispensable for liver disease in Tgfb1−/− mice. Fourth, while IFN-γ is important for overexpression of class II molecules in Tgfb1−/− liver, it has no detectable role in the activation, expansion, or accumulation of hepatic CD4+ T cells. Fifth, the relative expression level of the T cell inhibitor molecule PD-L1, while strongly dependent on IFN-γ, is not a determining factor in T cell activation in the Tgfb1−/− liver. Finally, although IFN-γ is required for the overexpression of FasL in Tgfb1−/− liver (26), tissue damage in this model system is Fas independent.
AIH is believed to be caused by the failure of normal immune tolerance mechanisms in the liver, resulting in an immune attack upon hepatocytes (40). Studies of biopsy material show that AIH is characterized by the expansion of predominantly CD4+ Th cells and chronic viral hepatitis by the expansion of CD8+ T cells (1). To classify AIH as a CD4+ T cell disease and chronic viral hepatitis as a CD8+ T cell disease is, however, undoubtedly an over-simplification of the pathologies, which are more complex. CD4+ T cells are important for the immune response to hepatitis C virus, as shown in studies in primates (41). In addition, recent in vitro studies of T cell clones derived from AIH patients strongly suggest that CD8+ T cells participate in disease pathogenesis (6). These findings notwithstanding, the studies presented here demonstrate that, at least in this model system, CD4+ T cells, through the production of IFN-γ, can elicit tissue damage without the apparent participation of other lymphocytes of the cellular immune system. Like CD4+ T cells, CD8+ T cells are expanded in Tgfb1−/− livers, but mAb-mediated depletion of the CD8+ T cell subset had no discernible effect on the development of hepatocellular damage. Although we did not directly test the requirement for NK cells, they are not sufficient for the pathology. NKT cells are abundant in normal murine liver (42), but evidence suggests that they are significantly underrepresented in Tgfb1−/− livers (23, 24). As such, their contribution to pathology is probably minimal; however, we cannot formally rule out the possibility that part of the effectiveness of anti-CD4 treatment is via depletion of NKT cells.
How might CD4+ T cells elicit tissue damage here? IFN-γ is required for the development of hepatocellular damage, and the concentration of plasma IFN-γ quantitatively reflects the degree of tissue damage, as measured by AST. Moreover, the CD4+ T cell appears to be the only relevant source of IFN-γ here. Together, the data strongly support a model in which CD4+ T cells cause tissue damage through the production of IFN-γ. These results mirror those reported by Ma et al. (43). PBMCs isolated from type 2 AIH patients were stimulated in vitro with peptides derived from the microsomal liver enzyme cytochrome P450IID6, the principal target of autoimmunity in type 2 AIH. The level of IFN-γ produced in response strongly correlated with the degree of tissue damage, as assessed by serum AST. In the second part of this work, we considered hypotheses to explain how CD4+ T cells producing IFN-γ might lead to hepatocellular damage.
One hypothesis is that the critical role of IFN-γ is in the activation and expansion of CD4+ T cells themselves. IFN-γ can modulate Ag presentation to CD4+ T cells by inducing APC expression of class II proteins. Indeed, we found that class II genes are strongly overexpressed in Tgfb1−/− livers, and that overexpression is almost entirely IFN-γ dependent. In vitro, IFN-γ and Tgfb1 modulate class II levels through regulated expression of the critical coactivator protein CIITA (31). Consistent with this model, CIITA mRNA is overexpressed in Tgfb1−/− livers, but not in Ifng−/−Tgfb1−/− livers. Following this line of reasoning, IFN-γ could, through enhanced CIITA expression, induce the overexpression of class II proteins, which would then be responsible for the aberrant activation, oligoclonal expansion, and accumulation of CD4+ T cells in the Tgfb1−/− liver. However, we find no role for IFN-γ in the early stages of the CD4+ T cell response.
Another possibility is that the lack of TGF-β1 leads to a failure to express the T cell inhibitor PD-L1, a cell surface molecule that inhibits T cell mediated liver inflammation in other model systems. However, there was no failure in PD-L1 expression; indeed, in the absence of TGF-β1, PD-L1 was strongly overexpressed. As PD-L1’s effects in the liver may be more selective for CD8+ T cells than for CD4+ T cells (34), we cannot rule out the possibility that high PD-L1 expression may be responsible for the (relatively) lower expression of IFN-γ from Tgfb1−/− CD8+ T cells than from Tgfb1−/− CD4+ T cells. That notwithstanding, the relative expression level of the T cell inhibitor molecule PD-L1 is not a determining factor in T cell activation in the Tgfb1−/− liver.
A third possibility is that the critical role of IFN-γ is to regulate the effector function of hepatotoxic CD4+ T cells. The ligand FasL is expressed on activated Th1 cells (44, 45). The Fas receptor is constitutively expressed on hepatocytes (46), and its triggering rapidly leads to apoptosis (47). We hypothesized that IFN-γ’s pathologic role is to drive FasL expression on CD4+ T cells, and that CD4+ T cell engagement of hepatocellular Fas is the final effector pathway in the Tgfb1−/− liver. The predominant use of FasL by CD4+ CTLs in the killing of target tissue has precedence in a mouse model of graft-vs-host disease (48). Consistent with this hypothesis, FasL mRNA expression is up-regulated in Tgfb1−/− liver in an IFN-γ-dependent fashion (26). Moreover, as we have shown, FasL is indeed overexpressed in purified liver Tgfb1−/− CD4+ T cells. However, experimental elimination of functional Fas from the system through breeding in the lpr Fas allele had no effect on the development of liver damage. Liver disease is Fas independent, so IFN-γ must drive pathology in this mouse model system through a distinct pathway.
Acknowledgments
We thank Dr. Jan Erikson (The Wistar Institute) for the generous gift of BALB/c-Faslpr/lpr mice. We are indebted to Christine Kretowicz and Beverly Gorham for expert breeding and screening of mice.
Footnotes
This work was supported by National Institutes of Health Grants AI053056 (J.D.G.), DK073904 (J.D.G.) and P20RR16437 from the Centers of Biomedical Research Excellence Program of the National Center for Research Resources, as well as by a grant from the Hitchcock Foundation (J.D.G.). J.W. was supported by a Samuel A. Hamacher Autoimmune Hepatitis Postdoctoral Research Fellowship from the American Liver Foundation. R.T.R. was supported by National Institutes of Health Training Grants T32AI07363 and T32AR07576.
Abbreviations used in this paper: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CD4SP, CD4+ single positive; FasL, Fas ligand; NPC, nonparenchymal cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lohr HF, Schlaak JF, Gerken G, Fleischer B, Dienes HP, Meyer Zum Buschenfelde KH. Phenotypical analysis and cytokine release of liver-infiltrating and peripheral blood T lymphocytes from patients with chronic hepatitis of different etiology. Liver. 1994;14:161–166. doi: 10.1111/j.1600-0676.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 2.Lohr HF, Schlaak JF, Lohse AW, Bocher WO, Arenz M, Gerken G, Meyer Zum Buschenfelde KH. Autoreactive CD4+ LKM-specific and anticlonotypic T-cell responses in LKM-1 antibody-positive autoimmune hepatitis. Hepatology. 1996;24:1416–1421. doi: 10.1002/hep.510240619. [DOI] [PubMed] [Google Scholar]
- 3.Rosen HR. Hepatitis C pathogenesis: mechanisms of viral clearance and liver injury. Liver Transpl. 2003;9:S35–S43. doi: 10.1053/jlts.2003.50253. [DOI] [PubMed] [Google Scholar]
- 4.Chang KM. Immunopathogenesis of hepatitis C virus infection. Clin. Liver Dis. 2003;7:89–105. doi: 10.1016/s1089-3261(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 5.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4+ T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J. Clin. Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, Vergani D. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 7.Kusters S, Gantner F, Kunstle G, Tiegs G. IFN-γ plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 8.Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-γ in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- 9.Langdale LA, Wilson L, Jurkovich GJ, Liggitt HD. Effects of immunomodulation with IFN-γ on hepatic ischemia-reperfusion injury. Shock. 1999;11:356–361. doi: 10.1097/00024382-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Zachou K, Rigopoulou E, Dalekos GN. Autoantibodies and autoantigens in autoimmune hepatitis: important tools in clinical practice and to study pathogenesis of the disease. J. Autoimmune Dis. 2004;1:2. doi: 10.1186/1740-2557-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergani D, Mieli-Vergani G. Mechanisms of autoimmune hepatitis. Pediatr. Transplant. 2004;8:589–593. doi: 10.1111/j.1399-3046.2004.00288.x. [DOI] [PubMed] [Google Scholar]
- 12.Lapierre P, Hajoui O, Homberg JC, Alvarez F. Formiminotransferase cyclodeaminase is an organ-specific autoantigen recognized by sera of patients with autoimmune hepatitis. Gastroenterology. 1999;116:643–649. doi: 10.1016/s0016-5085(99)70186-1. [DOI] [PubMed] [Google Scholar]
- 13.Arenz M, Meyer zum Buschenfelde KH, Lohr HF. Limited T cell receptor Vβ-chain repertoire of liver-infiltrating T cells in autoimmune hepatitis. J. Hepatol. 1998;28:70–77. doi: 10.1016/s0168-8278(98)80204-3. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizawa K, Ota M, Katsuyama Y, Ichijo T, Inada H, Umemura T, Tanaka E, Kiyosawa K. T cell repertoire in the liver of patients with autoimmune hepatitis. Hum. Immunol. 1999;60:806–815. doi: 10.1016/s0198-8859(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson PT. Genetics in autoimmune hepatitis. Semin. Liver Dis. 2002;22:353–364. doi: 10.1055/s-2002-35705. [DOI] [PubMed] [Google Scholar]
- 16.Czaja AJ, Donaldson PT. Genetic susceptibilities for immune expression and liver cell injury in autoimmune hepatitis. Immunol. Rev. 2000;174:250–259. doi: 10.1034/j.1600-0528.2002.017401.x. [DOI] [PubMed] [Google Scholar]
- 17.Senaldi G, Portmann B, Mowat AP, Mieli-Vergani G, Vergani D. Immunohistochemical features of the portal tract mononuclear cell infiltrate in chronic aggressive hepatitis. Arch. Dis. Child. 1992;67:1447–1453. doi: 10.1136/adc.67.12.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain MJ, Mustafa A, Gallati H, Mowat AP, Mieli-Vergani G, Vergani D. Cellular expression of TNF-α and IFN-γ in the liver biopsies of children with chronic liver disease. J. Hepatol. 1994;21:816–821. doi: 10.1016/s0168-8278(94)80244-0. [DOI] [PubMed] [Google Scholar]
- 19.Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyonaga T, Hino O, Sugai S, Wakasugi S, Abe K, Shichiri M, Yamamura K. Chronic active hepatitis in transgenic mice expressing IFN-γ in the liver. Proc. Natl. Acad. Sci. USA. 1994;91:614–618. doi: 10.1073/pnas.91.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorham JD. TGF-β1, Th1 responses, and autoimmune liver disease. Transfusion. 2005;45:51S–59S. doi: 10.1111/j.1537-2995.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin JT, Kitzmiller TJ, Cates JMM, Gorham JD. MHC-independent genetic regulation of liver damage in a mouse model of autoimmune hepatocellular injury. Lab. Invest. 2005;85:550–561. doi: 10.1038/labinvest.3700246. [DOI] [PubMed] [Google Scholar]
- 23.Rudner LA, Lin JT, Park IK, Cates JM, Dyer DA, Franz DM, French MA, Duncan EM, White HD, Gorham JD. Necroinflammatory liver disease in BALB/c background, TGF-β1-deficient mice requires CD4+ T cells. J. Immunol. 2003;170:4785–4792. doi: 10.4049/jimmunol.170.9.4785. [DOI] [PubMed] [Google Scholar]
- 24.Robinson RT, Gorham JD. TGF-β1 regulates antigen-specific CD4+ T cell responses in the periphery. J. Immunol. 2007;179:71–79. doi: 10.4049/jimmunol.179.1.71. [DOI] [PubMed] [Google Scholar]
- 25.Robinson RT, French MA, Kitzmiller TJ, Gorham JD. Restriction of the CD4+ T-cell receptor repertoire prevents immune pathology in TGF-β1 knockout mice. Lab. Invest. 2006;86:815–828. doi: 10.1038/labinvest.3700439. [DOI] [PubMed] [Google Scholar]
- 26.Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF-β1-deficient mice develop necroinflammatory IFN-γ-dependent hepatitis. J. Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- 27.Fields ML, Sokol CL, Eaton-Bassiri A, Seo S, Madaio MP, Erikson J. Fas/Fas ligand deficiency results in altered localization of anti-double-stranded DNA B cells and dendritic cells. J. Immunol. 2001;167:2370–2378. doi: 10.4049/jimmunol.167.4.2370. [DOI] [PubMed] [Google Scholar]
- 28.Lin JT, Martin SL, Xia L, Gorham JD. TGF-β1 uses distinct mechanisms to inhibit IFN-γ expression in CD4+ T cells at priming and at recall: differential involvement of Stat4 and T-bet. J. Immunol. 2005;174:5950–5958. doi: 10.4049/jimmunol.174.10.5950. [DOI] [PubMed] [Google Scholar]
- 29.Ksontini R, Colagiovanni DB, Josephs MD, Edwards CK, 3rd, Tannahill CL, Solorzano CC, Norman J, Denham W, Clare-Salzler N, MacKay SL, Moldawer LL. Disparate roles for TNF-α and Fas ligand in concanavalin A-induced hepatitis. J. Immunol. 1998;160:4082–4089. [PubMed] [Google Scholar]
- 30.Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB. TGF-β1 controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-β1 null mouse phenotype. Proc. Natl. Acad. Sci. USA. 1993;90:9944–9948. doi: 10.1073/pnas.90.21.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YJ, Han Y, Lu HT, Nguyen V, Qin H, Howe PH, Hocevar BA, Boss JM, Ransohoff RM, Benveniste EN. TGF-β suppresses IFN-γ induction of class II MHC gene expression by inhibiting class II transactivator messenger RNA expression. J. Immunol. 1997;158:2065–2075. [PubMed] [Google Scholar]
- 32.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7–H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 33.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7–H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 35.Schoop R, Wahl P, Le Hir M, Heemann U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-γ-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol. Dial. Transplant. 2004;19:2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 36.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by IFN-α and -γ and mediates T cell apoptosis. J. Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 39.McCullough CT, Tura BJ, Harrison DJ. c-Myc partially mediates IFN-γ-induced apoptosis in the primary hepatocyte. Int. J. Exp. Pathol. 2007;88:129–136. doi: 10.1111/j.1365-2613.2006.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krawitt EL. Autoimmune hepatitis. N. Engl. J. Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 41.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 42.Exley MA, Koziel MJ. To be or not to be NKT: natural killer T cells in the liver. Hepatology. 2004;40:1033–1040. doi: 10.1002/hep.20433. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Bogdanos DP, Hussain MJ, Underhill J, Bansal S, Longhi MS, Cheeseman P, Mieli-Vergani G, Vergani D. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130:868–892. doi: 10.1053/j.gastro.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 44.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int. Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 45.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, Nakao K, Nagata S. Expression of the Fas ligand in cells of T cell lineage. J. Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 46.Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, Schmidt A, Debatin KM, Krammer PH, Moller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab. Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 47.Schlosser SF, Azzaroli F, Dao T, Hingorani R, C Nicholas I, Boyer JL. Induction of murine hepatocyte death by membrane-bound CD95 (Fas/APO-1)-ligand: characterization of an in vitro system. Hepatology. 2000;32:779–785. doi: 10.1053/jhep.2000.18422. [DOI] [PubMed] [Google Scholar]
- 48.Graubert TA, DiPersio JF, Russell JH, Ley TJ. Perforin/granzyme-dependent and independent mechanisms are both important for the development of graft-versus-host disease after murine bone marrow transplantation. J. Clin. Invest. 1997;100:904–911. doi: 10.1172/JCI119606. [DOI] [PMC free article] [PubMed] [Google Scholar]