Abstract
Objective
The frequency of a second child with cardiac manifestations of Neonatal Lupus (cardiac NL) is critical to understanding the pathogenesis of anti-SSA/Ro mediated injury, counseling regarding future pregnancies, and powering preventative trials. Accordingly, this study addressed the recurrence rates of cardiac NL and associated risk factors in a large U.S.—based cohort.
Methods
Evaluation of cardiac NL in families enrolled in the Research Registry for Neonatal Lupus with a focus on pregnancies immediately following an affected child.
Results
The overall recurrence rate of cardiac NL in mothers with anti-SSA/Ro antibodies was 17.4% (95% CI: 11.1%-23.6%). For evaluation of potential risk factors, data from 129 mothers with a pregnancy immediately following the birth of a cardiac NL child were analyzed. Maternal diagnosis was not associated with outcome: 23% of asymptomatic/UAS mothers had a second child with cardiac NL compared to 14% of mothers with SLE or SS (p=0.25). The recurrence rate was not statistically significant in mothers who used steroids compared to no steroids, (16% v/s 21%, respectively; p=0.78). Antibody status of the mother did not predict outcome. Death of the first child with cardiac NL did not predict recurrence in the subsequent pregnancy (p=0.31). Risk of cardiac NL was similar in male and female children (17.2% vs. 18.3%, respectively; p=1.0).
Conclusions
The overall recurrence rate for cardiac NL is 17% and appears to be unaffected by maternal health, steroids, antibody status, severity of cardiac disease in the first affected child or gender in the subsequent child.
Keywords: congenital heart block, anti-SSA/Ro antibodies, anti-SSB/La antibodies, neonatal lupus
Introduction
Neonatal lupus (NL) is a pathologic readout of maternal anti-SSA/Ro and or SSB/La autoantibodies (Abs) affecting only a minority of exposed offspring. Cardiac manifestations (cardiac NL) are the most serious and can range from clinically benign first degree heart block to complete heart block (most characteristic) and fatal cardiomyopathy [1, 2]. An intriguing aspect of cardiac NL is that it is an injury unique to some phase(s) of development, since it has only once been reported in the maternal heart despite the presence of identical Abs in the maternal circulation [3]. Cardiac NL carries a substantial mortality (~30%) and morbidity, with >60% of children requiring lifelong pacemakers [4, 5]. Despite the attempt of large multicenter studies to forestall disease by careful monitoring, irreversible complete block and extensive myocardial injury have been documented within 7 days of a normal rhythm and PR interval [6, 7]. Moreover, postnatal incomplete block [EKG identification of 1st or 2nd degree] can progress despite clearance of maternal Abs from the neonatal circulation [8]. In contrast to cardiac NL, the cutaneous manifestations of NL, despite being associated with the same maternal autoantibodies, are most common several weeks after birth and are transient [9].
Identification of isolated fetal conduction disease may be the first indication of previously unrecognized autoimmunity in the mother. It is this link that so powerfully associates anti-SSA/Ro and SSB/La Abs with cardiac disease. Since mothers with established Systemic Lupus Erythematosus (SLE) and Sjögren’s Syndrome (SS) often have these reactivities, they are the clinical groups most frequently counseled regarding NL. Two prospective studies to date, one from Italy and the other U.S. based, have identified the risk of cardiac NL for a mother previously known to have SSA/Ro antibodies at 2% [3, 6]. Anti-SSB/La antibodies may increase the risk to 5% [10]. The specificity of maternal Abs for aa 200-239 of the 52 component of SSA/Ro has received considerable attention with some differences (dependent on assays used and cohorts studied) as to the extent of added risk [11-13]. While these aggregate risks are still relatively low at <10%, the burden of disease is high and mothers contemplating a subsequent pregnancy entreat their managing physicians for advice regarding recurrence rates.
Accordingly, the present study was undertaken to address the recurrence rates of cardiac NL and associated risk factors in a large extensively characterized U.S.-based cohort. Such information would be expected to improve pregnancy counseling of anti-SSA/Ro-SSB/La positive mothers, provide power for the design of prevention trials, and to gain insight into the pathogenesis of cardiac injury particularly with regard to a fetal genetic contribution. This study leveraged the Research Registry for Neonatal Lupus (RRNL), established in 1994 [5], to accomplish these goals. One hundred and twenty-nine families in whom a pregnancy followed the birth of a child with cardiac NL were evaluated for the overall recurrence rate of cardiac NL. In addition, factors which might amplify or decrease effects of the “necessary” antibody (assuming overall profile of antibodies remained similar between pregnancies) were explored. These included maternal factors such as health status, medications and antibody specificities and titer. Fetal risk factors included severity of cardiac disease in the first affected child and gender in the subsequent child.
PATIENTS AND METHODS
Subjects
As previously described, mothers enrolled in the RRNL must satisfy two requirements 1) Abs to SSA/Ro or SSB/La and 2) a child with any manifestation of NL [5], verified by review of medical records. The RRNL and its informed consent are approved by the NYU School of Medicine IRB. The enrollment period for this study extended from September 1994 to January 2009. However, a mother could enter the RRNL in 1994 having had a child with NL years prior. Inclusion criteria for the present study were 1) enrollment in the RRNL, 2) documented maternal anti-SSA/Ro and/or SSB/La Abs, 3) a previous child with cardiac NL defined herein as the presence of heart block (first-, second-, or third-degree) documented by electrocardiogram, echocardiogram, history of pacemaker, or statement in the medical record; and/or presence of cardiac injury which specifically included autopsy evidence of a mononuclear infiltrate in the endocardium, myocardium and pericardium and/or endocardial fibroelastosis (EFE) on echocardiogram always associated with cardiac dysfunction. A prolonged PR interval ≥150 msec identified in utero was only counted if confirmed by the birth EKG 4) a pregnancy immediately subsequent to the child with cardiac disease.
Maternal health status, ethnicity and medications were based on phone interviews and information obtained from medical records as well as from enrollment and follow up questionnaires available in the RRNL. For the purposes of this study, the maternal health status at the time of the subsequent pregnancy following the child with cardiac NL was chosen.
Subsequent Pregnancies
An overview of the birth order data on families enrolled in the RRNL in which at least one child has cardiac NL is presented in Figure 1. In families with more than one child following the birth of a baby with cardiac NL, another affected child could have been the immediate next child or follow a healthy child (three families) or be the third affected child (two families). For the purposes of calculating the overall recurrence rate, all pregnancies from 129 families in which there were subsequent pregnancies following an initial child with cardiac NL were included (N=161 pregnancies). For the evaluation of potential risk factors of recurrence, data from only the pregnancy occurring immediately following the initial child with cardiac NL was analyzed (N=129 pregnancies). Fetuses that died secondary to heart block, i.e. hydrops, were also included.
Figure 1.
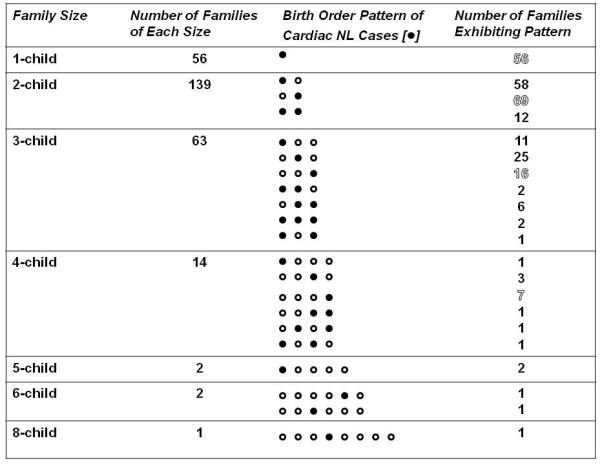
Forty-five families entered the RRNL with a subsequent non cardiac NL pregnancy having already occurred, 10 families entered the RRNL with a subsequent cardiac NL having already occurred and were prospectively evaluated for subsequent pregnancies, 72 families entered the RRNL at the time of birth of the initial child with cardiac NL and all subsequent pregnancies were evaluated prospectively.
Detection of antibodies to SSA/Ro and SSB/La protein
Determination of antibodies to SSA/Ro and SSB/La was done by the clinical immunology laboratory at the Hospital for Joint Diseases using a commercial ELISA kit (Diamedix, Miami, FL). Reactivity to the 52-kDa SSA/Ro, 60-kDa SSA/Ro, or 48-kDa SSB/La ribonucleoproteins was done by ELISA using recombinant proteins and/or SDS immunoblot (MOLT4) as previously described [14, 15].
Statistical Analysis
The overall recurrence rate of cardiac NL was computed as the proportion of cardiac NL cases among all pregnancies following an initial child with cardiac NL. Because data from multiple pregnancies from the same subject were included in the estimate, the 95% confidence interval for the recurrence rate was computed using a standard error based on the approach of [16] for clustered binary data. For the analysis of the potential risk factors for recurrent cardiac NL, only data from the pregnancy immediately following the initial child with cardiac NL were used. The effects of maternal health status, steroid use, titer of maternal antibodies, death in the first child with cardiac NL, and gender of the second child on risk of a recurrence were evaluated with the Fisher’s exact test. The titers of anti-SSA/Ro antibodies in the pregnancies complicated by cardiac NL were compared to those whose fetuses had no disease with the Mann-Whitney Test. Two-sided p values less than 0.05 were considered statistically significant.
RESULTS
Outcome of Pregnancies Subsequent to a Child with CHB
Over the 15 year study period, 129 of the 277 families (verified to comprise a mother with anti-SSA/Ro antibodies and a child with cardiac NL) currently enrolled in the Research Registry for Neonatal Lupus (RRNL) included a pregnancy immediately subsequent to a child with cardiac NL. Seventy-nine percent of the mothers were Caucasian, 9% were African-American, 6% were Asian, 6% were Hispanic and one mother (<1%) was American Indian.
The overall recurrence rate including all pregnancies following an initial one with cardiac NL is 17.4% (95% CI: 11.1% - 23.6%). As summarized in Table 1, of the 28 children with cardiac NL, 22 had either 2nd or 3rd degree block. Two children had 1st degree block, one detected in utero, one at birth, both confirmed by EKG which has persisted through one year of follow up. One child had EFE on echocardiogram in utero and died postpartum with severe cardiac dysfunction. Two children have had persistent EFE on echocardiogram, one of them remains alive at seven month and the other child remains alive at two years. The majority of cardiac NL cases (75%) were detected between 18 and 25 weeks of gestation. The mortality rate was 21%. (Two at birth, three within three months after birth, and one at age five). Sixty-eight percent of the children required pacemakers.
Table 1.
| Dead N (%) |
Detected between 18 - 25 wks of gestation N (%) |
Paced N (%) |
Total | |
|---|---|---|---|---|
| 1st Degree Heart Block |
0 | 0 | 0 | 2 |
| 2nd Degree Heart Block |
1 (4) | 1 (3.5) | 0 | 1 |
| 3rd Degree Heart Block |
5 (17.8) | 5 (17.8) | 19 (68) | 22 |
| EFE | 1 (4) | 2 (7) | 0 | 3 |
For the evaluation of potential risk factors for recurrent cardiac NL only one pregnancy immediately following the birth of a cardiac NL child was included in each family. Of 129 pregnancies studied, 23 (18%) resulted in a child with cardiac NL. Twelve (9%) subsequent children had a rash and 94 (73%) were healthy.
Maternal Risk Factors and Association with Pregnancy Outcome
Table 2 summarizes the results of the maternal parameters evaluated. Maternal health status was determined at the time of birth of the child immediately following the birth of the child with cardiac NL. Pregnancy outcome was not associated with maternal diagnosis. Specifically, 14 (22.6%) of 62 mothers characterized as asymptomatic or having an undifferentiated autoimmune syndrome had a recurrent cardiac NL child compared to nine (13.9%) of 65 mothers who were diagnosed with SLE or SS (p = 0.25). In two mothers giving birth to non-cardiac NL, information regarding health status was unavailable.
Table 2.
| Subsequent cardiac NL N (%) |
Subsequent non-cardiac NL N (%) |
Total N |
P value (Fisher’s exact) |
|
|---|---|---|---|---|
| Maternal Diagnosis | ||||
| Asym/UAS | 14 (22.6) | 48 (77.4) | 62 | 0.25 |
| SLE/SS | 9 (13.9) | 56 (86.1) | 65 | |
| Maternal Medications | ||||
| Steroids | 4 (16) | 21 (84) | 25 | 0.78 |
| No Steroids | 19 (20.9) | 72 (79.1) | 91 | |
| Maternal Antibody Status | ||||
| anti-Ro/La | 15 (17.9) | 69 (82.1) | 84 | 1.0 |
| Anti-Ro | 8 (17.8) | 37 (82.2) | 45 | |
| Anti-Ro52 (+) | 14 (16.5) | 85 (83.5) | 99 | 1.0 |
| Anti-Ro52(-) | 0 (0) | 91 (100) | 91 |
Since steroids, both fluorinated and non-flourinated, have been considered in the prevention of cardiac NL [17-19], the use of prednisone and dexamethasone was assessed. Only mothers who were taking steroids from the time of conception with continuation through at least 34 weeks of gestation were considered in the analysis. Although not always stated, the use of prednisone was mostly for maternal disease. The indication of dexamethasone was prophylaxis against recurrence of cardiac-NL. Four (16%) of the 25 mothers taking steroids had a recurrent cardiac NL child compared to 19 (20.9%) of 91 mothers who were not taking steroids (p = 0.78). Three of four mothers with a recurrent cardiac NL child that were taking steroids received prednisone and one of them dexamethasone (dose not stated). Seventeen of the mothers who received steroids and had non-cardiac NL children were taking prednisone (mean dose 23 mg/day) and four took dexamethasone (mean dose 4mg/day) In 13 mothers of the non-cardiac NL group, information about medications was not available.
The maternal antibody status did not influence the recurrence rate. Specifically, 15 (17.9%) of the 84 mothers with antibodies to both SSA/Ro and SSB/La had a cardiac NL child, compared to 8 of 45 (17.8%) with antibodies to SSA/Ro only (P= 1.0). Only one mother had antibodies against SSB/La only and had one child with cardiac NL that died and a subsequent healthy child. Antibodies reactive with Ro52 were almost universally present but did not associate with outcome (P=1.0). Furthermore, the titer of anti-SSA/Ro antibodies within 6 months of the subsequent pregnancy did not predict outcome (p= 0.287).
Fetal factors associated with pregnancy outcome
As summarized in Table 3, the contribution of fetal factors to the risk of recurrence was considered. Death was used as a proxy to address whether severity of cardiac involvement in a first child with cardiac NL influences the recurrence rate. Eight (23.5%) of the 34 cardiac NL index children who died in the index pregnancy, were followed by a recurrence of cardiac NL compared to 15 (15.8%) of the 95 index cardiac NL children who remained alive (p=0.31).
Table 3.
| Subsequent cardiac NL N (%) |
Subsequent non- cardiac NL N (%) |
Total N |
P value (Fisher’s exact) |
|
|---|---|---|---|---|
| Index Cardiac NL Death | ||||
| Yes | 8 (23.5) | 26 (76.5) | 34 | 0.31 |
| No | 15 (15.8) | 80 (84.2) | 95 | |
| Fetal Gender | ||||
| Female | 13 (18.3) | 58 (81.7) | 71 | 1.0 |
| Male | 10 (17.2) | 48 (82.8) | 58 |
Fetal gender was evaluated with regard to recurrence and overall association with cardiac NL. Evaluation of the entire RRNL revealed that female gender was observed in 57% of the children with cardiac NL, 55% of the children with rash, and 49% of the completely unaffected children (P=0.062 comparing cardiac NL to completely healthy). Comparison with national statistics (F:M=1000:1048, National Vital Statistics Report 2003) revealed significant female skewing for cardiac NL likely due to the larger sample size. (P=0.013) Thirteen (18.3%) of the 71 girls born subsequent to the index cardiac NL child, had cardiac NL compared to 10 (17.2%) of 58 subsequent boys (P=1.0).
Outcome of four pregnancies immediately subsequent to the birth of two consecutive children with cardiac NL
Four pregnancies of the mothers included in this study immediately followed two consecutive children with cardiac NL. In two, the third child was again affected. In one, the third child had a rash and in the other, the third child was healthy. Although not considered in the analyses of this study, in another mother with two consecutive cardiac NL children, the use of a surrogate carrier for a subsequent pregnancy resulted in an unaffected child.
Discussion
Based on data from this large U.S.-based cohort, the overall recurrence rate of cardiac NL is 17% and 18% for the immediately subsequent pregnancy. This is nearly tenfold the risk for an anti-SSA/Ro positive mother who has not had a previous child with cardiac NL. The estimated recurrence rate for a pregnancy subsequent to two consecutive cardiac NL-children is 50%. Maternal health status, use of steroids during pregnancy, antibody status, severity of disease in a first affected child and gender in second child do not predict the outcome of subsequent pregnancies.
Over the last decade, several groups have published rates for recurrence of cardiac NL. One study from Finland comprised 33 pregnancies immediately subsequent to a child with CHB and reported a recurrence rate of 18% (6/33). In addition to being a smaller study, the presence of maternal anti SSA/Ro-SSB/La antibodies was not an inclusion criterion, consequently women without documented anti-SSA/Ro antibodies were pooled with those having anti-SSA/Ro antibodies [20]. This latter shortcoming may be critical since the etiology of congenital conduction disease includes such entities as complex structural congenital heart diseases (Heterotaxia and Endocardial Cushion Defect) as well as long QT syndrome [21] which would not be applicable to mothers with autoantibodies seeking counsel. Gladman et al. reported the results of a prospective follow up of 118 pregnancies from 105 anti-SSA/Ro positive women followed in a Toronto cohort. Only 15 of these mothers had a previous child with NL (rash or cardiac NL were not specified). In 16 subsequent pregnancies, one (6%) resulted in a second child with cardiac NL [22]. A previous report based on data from anti-SSA/Ro positive mothers enrolled in the RRNL evaluated the outcome of 87 pregnancies immediately following the birth of a child with CHB. The recurrence rate was 19.5% [23].
In this cohort the majority (75%) of the cardiac NL cases were detected in the fetal period, specifically between 18 and 25 weeks of gestation. This is consistent with previous literature supporting that cardiac NL is usually diagnosed before 30 weeks of gestation with a peak incidence between 20 and 24 weeks of gestation [24, 25]. Two of the five children with AV conduction abnormalities first identified after birth were noted to have 1st degree block, one despite normal measurements of the PR interval throughout pregnancy and the other in the early 1990’s prior to the more widespread use of this technique [6]. The other three children with 3rd degree block were born before 1990 when considerably less information about the disease and fewer diagnostic tools were available. The overall mortality rate found in this group of recurrent CHB was 21% which is consistent with the literature [5, 24, 25]. The requirement of pacemaker implantation in the surviving newborns was likewise consistent with previous reports [4, 5, 24, 25].
Only a minority (2%) of anti-SSA/Ro positive mothers give birth to a child with cardiac NL, suggesting that maternal autoantibodies, while necessary, are not sufficient to cause disease. Recurrence rates for siblings of affected infants are 3000 times higher than the population prevalence estimated at 1:15,000 [26, 27, 28]. However, if only Ro+ mothers are considered in the prevalence, this risk is still tenfold higher suggesting a genetic influence on susceptibility to cardiac NL in an anti-SSA/Ro environment. It is widely held that genes predispose to but do not solely account for disease expression. Other factors such as events in utero can modulate the penetrance and severity of genetic predisposition. In the case of cardiac NL, the phenotype is well characterized and covers a limited range of presentation, an advantage for studying a fetal genetic component. A candidate gene approach based on the histologic hallmark of disease, fibrosis of the conduction tissue, has focused on TGF-β. The TGF-β polymorphism Leu10 (associated with increased fibrosis) was found to be significantly enriched in cardiac-NL children vs. unaffected offspring and controls [29].
Several limitations of this study should be noted. Although the present data have been obtained from a large cohort, the predominance of Caucasians restricts an extrapolation to other ethnicities. The mothers included in this study are all enrolled in the RRNL which specifically seeks families with NL. There may be a bias in attracting families with more than one affected child. However, if only prospectively evaluated pregnancies are included, the recurrence rate remains substantial at 16%.
The substantial morbidity and mortality of cardiac NL drive the search for preventive therapies. Given the low event rate of disease in anti-SSA/Ro positive mothers overall, such therapy cannot be easily evaluated in mothers without previously affected children. Thus, the target for preventive studies are the mothers with a prior affected child. Overall it is expected that these data will serve as an important reference point for family counseling, power calculations for evaluation of preventative studies, estimation of genetic burden, and basic science studies on pathogenesis of cardiac NL.
Acknowledgments
This work was funded by NIH, Contract NO1-AR-4-2220 (Research Registry for Neonatal Lupus) and NIAMS, grant RO1 AR42455-01 (Maternal Autoantibodies: Pathogenesis of Neonatal Lupus) to Dr. Buyon, and S.L.E. Foundation NY Inc. grants to Dr. Llanos and Dr. Izmirly.
Footnotes
Disclosures: Dr. Friedman is a member of the speakers’ bureau of MedImmune. The authors report no conflict of interest.
References
- 1.Moak JP, Barron KS, Hougen TJ, Wiles HB, Balaji S, Sreeram N, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37:238–42. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 2.Nield LE, Silverman ED, Taylor GP, Smallhorn JF, Mullen JB, Silverman NH, et al. Maternal Anti-Ro and Anti-La Antibody—Associated Endocardial Fibroelastosis. Circulation. 2002;105:843–8. doi: 10.1161/hc0702.104182. [DOI] [PubMed] [Google Scholar]
- 3.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, et al. Risk of Congenital Complete Heart Block in Newborns of Mothers with Anti-Ro/SSA Antibodies Detected by Counterimmunoelectrophoresis. A prospective Study of 100 Women. Arthritis Rheum. 2001;44:1832–5. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Waltuck J, Buyon JP. Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Intern Med. 1994;120:544–51. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DM, Kim MY, Copel JA, Friedman DM, Kim MY, Copel JA, et al. PRIDE Investigators. Utility of Cardiac Monitoring in Fetuses at Risk for Congenital Heart Block. The PR interval and Dexamethasone evaluation (PRIDE) Prospective Study. Circulation. 2008;117:485–93. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 7.Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren-Herlenius M. Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52-kd antibodies. Arthritis Rheum. 2004;50:1253–61. doi: 10.1002/art.20126. [DOI] [PubMed] [Google Scholar]
- 8.Askanase AD, Friedman DM, Copel J, Dische MR, Dubin A, Starc TJ, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti SSA/Ro-SSB/La antibodies. Lupus. 2002;11:145–51. doi: 10.1191/0961203302lu173oa. [DOI] [PubMed] [Google Scholar]
- 9.Neiman AR, Lee LA, Weston WL, Buyon JP. Cutaneous Manifestations of Neonatal Lupus without Heart Block: characteristics of mothers and children enrolled in a national registry. J Pediatr. 2000;137:674–80. doi: 10.1067/mpd.2000.109108. [DOI] [PubMed] [Google Scholar]
- 10.Gordon P, Khamashta MA, Rosenthal E, Simpson JM, Sharland G, Brucato A, et al. Anti-52 kDa Ro, anti-60 kDa Ro, and anti-La antibody profiles in neonatal lupus. J Rheumatol. 2004;3:2480–7. [PubMed] [Google Scholar]
- 11.Salomonsson S, Dorner T, Theander E, Bremme K, Larsson P, Wahren-Herlenius M. A serologic marker for fetal risk of congenital heart block. Arthritis Rheum. 2002;46:1233–41. doi: 10.1002/art.10232. [DOI] [PubMed] [Google Scholar]
- 12.Clancy RM, Buyon JP, Ikeda K, Nozawa K, Argyle DA, Friedman DM, et al. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 13.Strandberg L, Winqvist O, Sonesson SE, Mohseni S, Salomonsson S, Bremme K, et al. Antibodies to amino acid 200-239 (p200) of Ro52 as serological markers for the risk of developing congenital heart block. Clin Exp Immunol. 2008;154:30–7. doi: 10.1111/j.1365-2249.2008.03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buyon JP, Winchester RJ, Slade SG, Arnett F, Copel J, Friedman D, et al. Identification of mothers at risk for congenital heart block and other neonatal lupus syndromes in their children. Comparison of enzyme-linked immunosorbent assay and immunoblot for measurement of anti-SS-A/Ro and anti-SS-B/La antibodies. Arthritis Rheum. 1993;36:1263–73. doi: 10.1002/art.1780360911. [DOI] [PubMed] [Google Scholar]
- 15.Tseng CE, Caldwell K, Feit S, Chan EK, Buyon JP. Subclass distribution of maternal and neonatal anti-Ro (SSA) and La (SSB) antibodies in congenital heart block. J Rheumatol. 1996;23:925–32. [PubMed] [Google Scholar]
- 16.Donald A, Donner A. Adjustments to the Mantel-Haenszel chi-square statistic and odds ratio variance estimator when the data are clustered. Stat Med. 1987;6:491–9. doi: 10.1002/sim.4780060408. [DOI] [PubMed] [Google Scholar]
- 17.Costedoat-Chalumeau N, Amoura Z, Le Thi Hong D, Wechsler B, Vauthier D, Ghillani P, et al. Questions about dexamethasone use for the prevention of anti-SSA related congenital heart block. Annals of Rheumatic Diseases. 2003;62:1010–12. doi: 10.1136/ard.62.10.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinohara K, Miyagawa S, Fujita T, Aono T, Kidoguchi K. Neonatal lupus erythematosus: results of maternal corticosteroid therapy. Obstet Gynecol. 1999;93:952–7. doi: 10.1016/s0029-7844(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 19.Buyon JP, Slade SG, Reveille JD, Hamel JC, Chan EK. Autoantibody responses to the “native” 52-kDa SS-A/Ro protein in neonatal lupus syndromes, systemic lupus erythematosus, and Sjogren’s syndrome. J Immunol. 1994;152:3675–84. [PubMed] [Google Scholar]
- 20.Julkunen H, Eronen M. The Rate of Recurrence of Isolated Congenital Heart Block: A Population Based Study. Arthritis Rheum. 2001;44:487–8. doi: 10.1002/1529-0131(200102)44:2<487::AID-ANR70>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan S, Strasburger J. Overview of Fetal Arrhythmias. Curr Opin Pediatr. 2008;20:522–31. doi: 10.1097/MOP.0b013e32830f93ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gladman G, Silverman ED, Yuk-Law, Luy L, Boutin C, Laskin C, et al. Fetal echocardiographic screening of pregnancies of mothers with anti-Ro and/or anti-La antibodies. Am J Perinatol. 2002;19:73–80. doi: 10.1055/s-2002-23555. [DOI] [PubMed] [Google Scholar]
- 23.Solomon DG, Rupel A, Buyon JP. Birth order, gender and recurrence rate in autoantibody-associated congenital heart block: implications for pathogenesis and family counseling. Lupus. 2003;12:646–7. doi: 10.1191/0961203303lu425xx. [DOI] [PubMed] [Google Scholar]
- 24.Friedman DM, Rupel A, Buyon JP. Epidemiology, etiology, detection and treatment of autoantibody-associated congenital heart block in neonatal lupus. Curr Rheumatol Rep. 2007;9:101–8. doi: 10.1007/s11926-007-0003-4. [DOI] [PubMed] [Google Scholar]
- 25.Izmirly PM, Rivera TL, Buyon JP. Neonatal Lupus Syndromes. Rheum Dis Clin North Am. 2007;33:267–85. doi: 10.1016/j.rdc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Fritzler MJ, Pauls JD, Kinsella TD, Bowen TJ. Antinuclear, anticytoplasmic, and anti-Sjögren’s syndrome antigen A (SS-A/Ro) antibodies in female blood donors. Clin Immunol Immunopathol. 1985;36:120–8. doi: 10.1016/0090-1229(85)90045-5. [DOI] [PubMed] [Google Scholar]
- 27.Harmon CE, Lee LA, Huff JC, Norris DA, Weston WL. The frequency of autoantibodies to the SS-A/Ro antigen in pregnancy sera. Arthritis Rheum. 1984;27:S20. [Google Scholar]
- 28.Siren MK, Julkunen H, Kaaja R. The increasing incidence of isolated congenital heart block in Finland. J Rheumatol. 1998;25:1862–4. [PubMed] [Google Scholar]
- 29.Clancy RM, Backer CB, Yin X, Kapur RP, Molad Y, Buyon JP. Cytokine polymorphisms and histologic expression in autopsy studies: contribution of TNF-alpha and TGF-beta 1 to the pathogenesis of autoimmune-associated congenital heart block. J Immunol. 2003;171:3253–61. doi: 10.4049/jimmunol.171.6.3253. [DOI] [PubMed] [Google Scholar]


