Abstract
The human multidrug resistance transporter P-glycoprotein (P-gp) prevents the entry of compounds into the brain by an active efflux mechanism at the blood brain barrier (BBB). Treatment of neurodegenerative diseases, therefore, has become a challenge and the development of new reversible inhibitors of P-gp is pertinent to overcome this problem. We report the design and synthesis of a crosslinked agent based on the Alzheimer’s disease treatment galantamine (Gal-2) that inhibits P-gp-mediated efflux from cultured cells. Gal-2 was found to inhibit the efflux of the fluorescent P-gp substrate rhodamine 123 in cancer cells that over express P-gp with an IC50 value of approximately 0.8 µM. In addition, Gal-2 was found to inhibit the efflux of therapeutic substrates of P-gp, such as doxorubicin, daunomycin and verapamil with IC50 values ranging from 0.5 µM – 2 µM. Through competition experiments, it was determined that Gal-2 modulates P-gp mediated efflux by competing for the substrate binding sites. These findings support a potential role of agents, such as Gal-2, as inhibitors of P-gp at the BBB to augment treatment of neurodegenerative diseases.
Keywords: P-glycoprotein, inhibition, galantamine, dimer, ATP-Binding-Cassette transporter, blood brain barrier
INTRODUCTION
The treatment of neurodegenerative diseases is a challenge due to the presence of transporters such as P-glycoprotein (P-gp) in the endothelial cells lining the capillaries at the blood brain barrier (BBB). The presence of P-gp at the BBB prevents accumulation of therapeutic drugs in the brain by actively effluxing these drugs back into the blood stream [1]. P-gp belongs to the superfamily of ATP Binding Cassette (ABC) membrane transporters and is encoded by the MDR-1 (ABCB1) gene in humans [1–4]. This transporter consists of two homologous transmembrane spanning domains each containing an ATP binding site in the cytosol and substrate transport out of the cell is powered by the hydrolysis of ATP [1–4]. P-gp extrudes a broad range of therapeutics that differ in their structure and function. These drugs include, but are not limited to, antiepileptic drugs [5] such as phenytoin and phenobarbital, drugs used for treatment of schizophrenia/psychosis [6] such as quetiapine and aripiprazole and antidepressants such as venlafaxine [7] and escitalopram [8].
The important role of P-gp in excluding therapeutics from the brain has sparked interest in developing reversible inhibitors against the transporter [9]. P-gp has been shown to contain at least two distinct substrate binding sites, which are located in the transmembrane regions of the protein [10–12,20–22,27]. One of the promising strategies of modulating P-gp is to develop agents that target these drug binding sites simultaneously with designed dimeric compounds [13,14]. We now describe a novel bivalent agent based on the Alzheimer’s disease treatment galantamine, Gal-2, that is an effective inhibitor of the binding and transport activity of P-gp.
MATERIALS AND METHODS
Materials
Galantamine hydrochloride was purchased from TCI America (Portland, OR). Doxorubicin hydrochloride was purchased from Oakwood Products (West Columbia, Sc). Rhodamine 123 and BODIPY-FL-verapamil were obtained from Molecular Probes, Inc (Eugene, OR). [3H]daunomycin and [125I]Iodoarylazidoprazosin ([125I]IAAP) were purchased from Perkin-Elmer Life and Analytical Sciences (Boston, MA). Sebacic acid and diisopropylamine (DIEA) were purchased from Sigma-Aldrich (St. Louis, MO), N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC) was obtained from AKscientific (Mountain View, CA) and 4-dimethylaminopyridine (DMAP) was obtained from GenScript (Piscataway, NJ).
Synthesis of Gal-2
To a solution of commercially available sebacic acid (10.5 mg, 0.07 mmol) in dry dimethylformamide (DMF) (2 mL) at 0°C was added EDC (39.7 mg, 0.28 mmol), DMAP (2.5 mg, 0.03 mmol) and DIEA (36 µL, 0.28 mmol). After 20 min at 0°C, galantamine hydrochloride (50 mg, 0.21 mmol) was added. The mixture was allowed to warm to room temperature and was stirred for 12 hours. The solvent was removed in vacuo and the residue was dissolved in DMSO. The resulting material was purified by reverse phase HPLC using a C5 column (Phenomenex, USA) with an eluent consisting of solvent A (water and 0.1 % trifluoroacetic acid (TFA)) and solvent B (acetonitrile (CH3CN) and 0.1 % TFA) with a 60 min gradient of 10–90% solvent A, a flow rate of 8.0 mL/min and UV detection at 214 nm and 287 nm. The pure compound (purity 98% by analytical HPLC, retention time 15.25 minutes using a C5 analytical column, with a 30 minute gradient of 10–90% solvent A at a flow rate of 1.20 mL/min) was characterized by MALDI-TOF mass spectrometry. Gal-2 [M+H]+ : 740.92 calculated, 739.92 observed.
Cell Culture
MCF-7/DX1 cells (breast adenocarcinoma overexpressing P-gp [15]) were cultured at 37°C in 5% CO2 and maintained in RPMI 1640 supplemented with 2 mM L-glutamine, 50 µg/mL streptomycin (Cellgro Mediatech), 50 units/mL of penicillin, 10 % fetal bovine serum (Cambrex bioscience, WalkersVille, Inc) and 1 µM doxorubicin. High Five cells expressing P-gp were cultured at 27°C in Express Five SFM medium (Invitrogen) supplemented with 18 mM L-glutamine (Mediatech) and 0.5X antibiotic-antimycotic (Invitrogen). Sf9 cells were cultured at 27°C in SF-900 II SFM medium (Invitrogen) supplemented with 0.5X antibiotic-antimycotic (Invitrogen).
Expression of P-gp in Insect Cells
Sf9 or High Five cells (9.3 × 106 cells/flask) cultured in 75-cm2 flasks were infected with BV-MDR1 that expresses human P-gp with a 6x His tag at the N-terminus at a multiplicity of infection of 10 for 2 h at 27°C in 2.5 ml of culture medium as described previously [16]. After incubation for 2 h, 7.5 ml of media were added to the cells and the flasks incubated at 27°C for 72 h.
Preparation of Crude Insect Cell Membranes
BV-MDR1-infected High Five cells were harvested and crude membranes were prepared as described previously. P-gp expression was confirmed by immunoblot with C219 primary antibody (1:4000) and an HRP-linked secondary antibody probe bands were visualized using enhanced chemiluminescence (ECL) (Pierce).
Flow Cytometry Assays
Substrate accumulation assays were performed as previously described [17] with slight modifications. The studies were performed using 125,000 MCF-7/DX1 cells in suspension (1 mL) that were incubated with either 0.5 µg/mL (1.3 µM) rhodamine 123, 3 µM doxorubicin, or 0.5 µM BODIPY-FL-verapamil alone or with increasing concentrations of Gal-2 for 30 to 40 min at 37°C. One µM GF120918 was used as a positive control. For rhodamine 123 and doxorubicin assays, cells were first harvested by centrifugation at 300g, resuspended in fresh media with 1 µM GF120918 or different concentrations of Gal-2 and incubated for an additional 30 to 40 min at 37°C. These cells, and those treated with BODIPY-FL-verapamil were harvested by centrifugation at 300g, the media was removed and the cells were resuspended in 500 µL of ice cold PBS, pH 7.4. The cells were analyzed using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA) equipped with a 488 nm argon laser and a 530 band pass filter (FL1) for rhodamine 123 and BODIPY-FL-verapamil, and a 585/42 band pass filter (FL2) for doxorubicin. Ten thousand cells were counted for each data point and histogram plots were obtained. The IC50 value for Gal-2 was acquired from the concentration-dependent data that were fitted using Sigma Plot.
Radioactive Substrate Accumulation Assays
Radioactive assays were performed as previously described [17] with minor modifications. Six-well plates were seeded with 500,000 cells per well in RPMI 1640 media a day before the assay. The next day, the media in the wells was removed and the cells were washed twice with cold PBS. The cells were incubated with Basal Medium Eagle media supplemented with 5% calf serum and 0.25 µCi/mL [3H]daunomycin alone or with varying concentrations of Gal-2 or 1 µM GF120918 for 40 min at 37°C. The media was removed and the cells were washed twice with 1 mL ice cold PBS. Pre-warmed trypsin-EDTA (1 mL) was added and the cells were incubated for 1 hour at 37°C. The contents of the wells were then transferred into scintillation vials containing 18-mL of EcoLite (+) scintillation fluid (MR Research products, Irvin, CA). The wells were each washed twice with 500 µL cold PBS and the contents added to the scintillation vials. Each vial was counted using a 1600 CA, tri-CARB liquid scintillation analyzer (Packard instrument company, Downers Grove, IL). The data obtained was normalized to counts per 10,000 cells. The IC50 value for Gal-2 was acquired from the concentration dependent data that was fitted using Sigma Plot.
ATPase Assay
Crude membranes obtained from High Five cells expressing P-gp were analyzed for ATP consumption in the presence or absence of Gal-2 as previously described [17]. Crude membranes (10 µg) in the absence or presence of increasing concentrations of Gal-2 were incubated in a total volume of 100 µL assay buffer (45 mM Tris-HCl pH 7.5, 5 mM sodium azide, 2 mM ethylene glycol tetraacetic acid (EGTA), 1 mM ouabain, 2 mM dithiothreitol (DTT), 10 mM MgCl2), 300 µM sodium orthovanadate and 5 mM ATP at 37 °C for 20 minutes. The reaction was stopped with 100 µL of 5% (w:v) sodium dodecyl sulfate (SDS). Vanadate-sensitive P-gp activity was quantitated by colorimetric detection of inorganic phosphate at 880 nm as previously described [17].
IAAP Photoaffinity Labeling
[125I]Iodoarylazidoprazosin ([125I]IAAP) (specific activity of 2,200 Ci/mmol) was used to label P-gp as described previously [17]. Crude Sf9 membranes (25 µg) expressing P-gp were treated with either 2.5 % DMSO or increasing concentrations of Gal-2 and were incubated at room temperature in 50 mM Tris-HCl, pH 7.5, 1% aprotinin, 1 mM DTT and 2 mM of 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) with ([125I]IAAP (3 nM) for 10 min in the dark. The samples were illuminated with a UV lamp (wavelength 365 nm) for 20 min on ice. The samples were separated by SDS-PAGE on a 7.5 % Tris gel, and the gel was fixed and dried overnight. The gel was exposed to X-ray film (RPI) at −80°C for 5 h. Each band on the gel was quantified using image J (NIH, Bethseda, MD) to determine the amount of [125I] IAAP photocrosslinked to P-gp. Values are presented as a percentage of the DMSO control. The IC50 value for Gal-2 was obtained by fitting using GraphPad.
RESULTS AND DISCUSSION
Studies have shown that a wide range of structurally dissimilar compounds that interact with P-gp are recognized by binding sites that are localized to the transmembrane regions of the transporter [18,19,21,22]. At least two of these substrate binding sites have been identified on P-gp that can recognize and efflux multiple substrates [10,12,20–22]. We previously developed bivalent inhibitors based on the crosslinked P-gp substrates emetine and quinine that resulted in potent inhibition of P-gp efflux, with IC50 values in the µM range. To expand on this strategy, we have designed homodimeric inhibitors based on agents that are used to treat brain disorders. Given the important role that P-gp plays in protecting the brain from entry of these drugs, the design of such dimeric agents would be useful for blocking the efflux of neurodegenerative disease drugs at the BBB. We chose galantamine, an approved symptomatic therapy for Alzheimer’s disease [23], as a viable compound for dimerization as it has a secondary alcohol that may act as a point of attachment to a crosslinking agent. Although galantamine has not been reported to be a substrate of P-gp, it has a structure that is very similar to the known P-gp substrate morphine [24]. As such, we sought to dimerize galantamine in an effort to obtain a novel P-gp inhibitor.
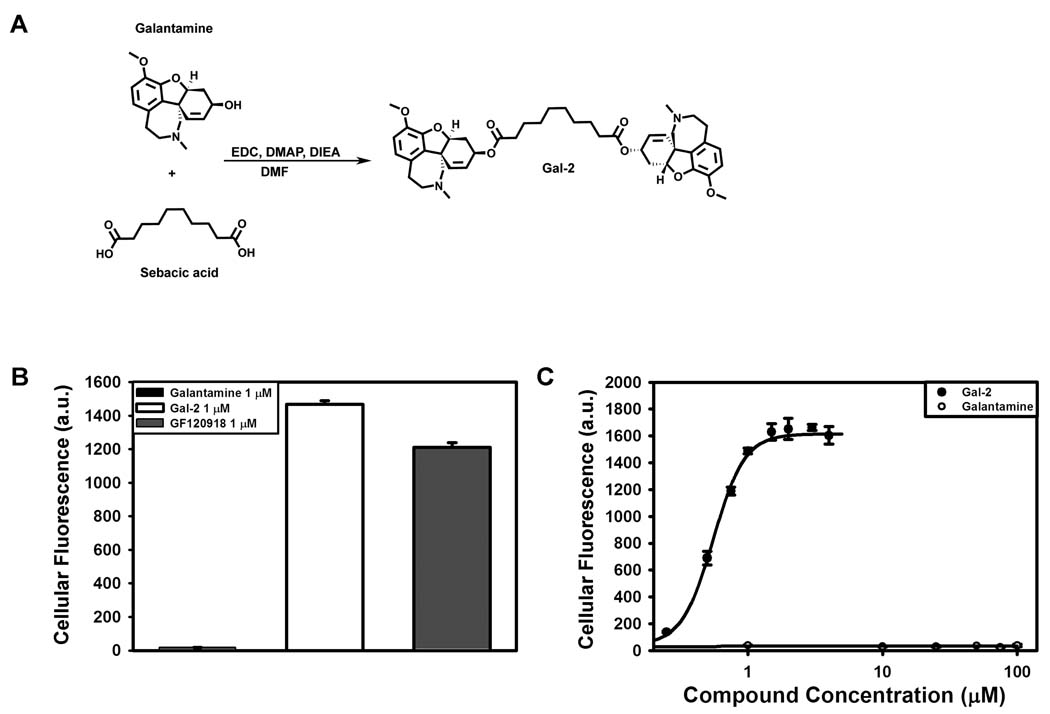
The secondary hydroxyl group of galantamine was used as the site of dimerization with an appropriate di-carboxylic acid containing tether. We previously demonstrated that quinine could be effectively dimerized via its secondary alcohol with sebacic acid to yield a potent P-gp efflux inhibitor [13]. Therefore, we evaluated the ability of sebacic acid to crosslink galantamine. To synthesize the homodimeric compound, galantamine hydrochloride was treated with sebacic acid and EDC with the addition of base (Fig. 1A). The desired di-ester compound was purified to homogeneity by reverse phase HPLC and characterized by MALDI-TOF mass spectroscopy.
Fig. 1.
Synthesis of Gal-2 and inihibitory effects of galantamine versus Gal-2 on the efflux of rhodamine 123 using MCF-7/DX1 cells. Cells were incubated with 0.5 µg/mL rhodamine 123 and in the presence and absence of galantamine agents at varying concentrations and analyzed by flow cytometry. (A) Synthesis of Gal-2 starting with monomeric galantamine. (B) Inhibitory effect of galantamine (1 µM), Gal-2 (1 µM) and GF120918 (1 µM, positive control) on rhodamine 123 accumulation. (C) Inhibition of rhodamine 123 accumulation over a concentration range of Gal-2 (●) and galantamine (○)
The inhibitory activity of the synthesized Gal-2 on P-gp transport of substrate was evaluated in cells that over-express P-gp (MCF-7/DX1). First, accumulation assays with the fluorescent P-gp substrate rhodamine 123 were performed to assess the effects of Gal-2 and monomeric galantamine on P-gp efflux. In this assay, using flow cytometry, the inhibition of P-gp efflux is quantified by an increase in cellular fluorescence due to the accumulation of rhodamine 123 in MCF-7/DX1 cells. Initially, cells were treated with galantamine (monomer), Gal-2, or the positive control GF120918 at 1 µM and rhodamine 123 accumulation was quantified (Fig. 1B). Dimeric Gal-2 treatment was found to greatly increase the cellular fluorescence as compared to the monomeric galantamine (85-fold). At this concentration, Gal-2 was also approximately 20% more active than the known P-gp inhibitor GF120918 [25]. A concentration dependent assay was performed to assess the effectiveness of Gal-2 versus monomeric galantamine (Fig. 1C). Gal-2 inhibited rhodamine 123 efflux with an IC50 of 0.8 ± 0.1 µM, while the galantamine monomer demonstrated no inhibition of P-gp efflux up to 100 µM. These data confirm that dimerization of galantamine is essential for inhibition of P-gp transport, and that a highly potent inhibitor can be obtained.
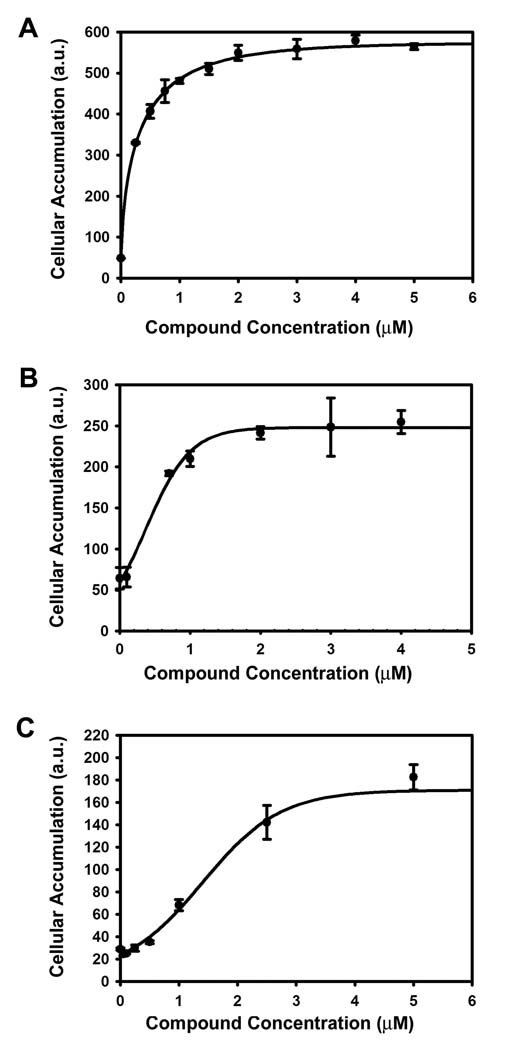
P-gp is known to efflux a wide range of therapeutic substrates [26]. To demonstrate that the inhibitory effect exhibited by Gal-2 was not limited to a single substrate, three additional P-gp substrates were evaluated: BODIPY-FL-verapamil, doxorubicin and the radioactive agent [3H]daunomycin. Cellular fluorescence was monitored in the MCF-7/DX1 cell line with BODIPY-FL-verapamil and doxorubicin while cellular radioactivity was monitored with [3H]daunomycin as a function of increasing amounts of Gal-2 (Fig. 2). In each case, Gal-2 was found to increase the cellular accumulation of the P-gp substrate in a concentration dependent fashion, with IC50 values of 0.5 ± 0.1 µM, 0.5 ± 0.1 and 2.0 ± 0.4 µM for BODIPY-FL-verapamil, doxorubicin and [3H]daunomycin, respectively. These data demonstrated that Gal-2 reverses the effluxes of a wide range of P-gp substrates including therapeutic drugs, such as doxorubicin and verapamil, that are effluxed by the transporter.
Fig. 2.
Inhibitory effects of galantamine versus Gal-2 on the efflux of therapeutic substrates. Cells were treated with varying concentrations of Gal-2 with (A) BODIPY-FL-verapamil 0.5 µM (B) doxorubicin 3 µM and (C) [3H]daunomycin 0.25 µCi/mL. (A) and (B) were analyzed by flow cytometry while (C) was analyzed using a scintillation counter.
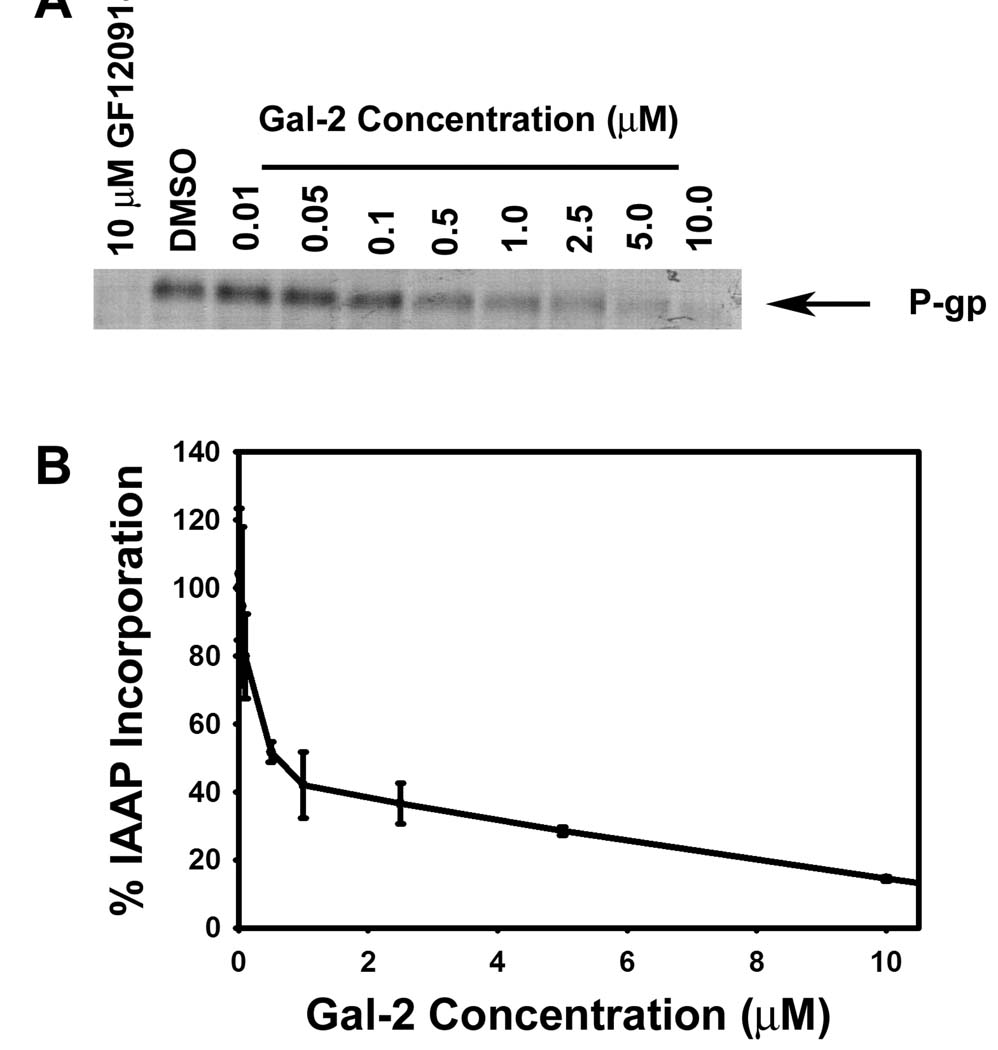
The inhibition observed with Gal-2 could be a result of binding to numerous sites on P-gp. To support that Gal-2 interacts with the transport binding sites of P-gp, photoaffinity crosslinking competition experiments were performed with [125I]IAAP, a photoactive analog of the P-gp substrate prazosin, an agent that is known to bind to both the R and H binding pockets of P-gp [27]. Crude Sf9 membranes that contain P-gp were incubated with Gal-2 and [125I] IAAP followed by photocrosslinking, separation by SDS-PAGE and autoradiography (Fig. 3). In the absence of Gal-2, a band is evident on the gel that corresponds to [125I]IAAP crosslinked to P-gp. With increasing levels of Gal-2 added prior to photolysis, the band corresponding to the prazosin – P-gp adduct decreased. These data show that Gal-2 inhibited the crosslinking of [125I]IAAP to P-gp with an IC50 value of approximately 0.5 µM. These findings indicate that dimerized galantamine competed for [125I]IAAP binding to P-gp.
Fig. 3.
Evaluation of the ability of Gal-2 to compete with radiolabeled prazosin ([125I]IAAP) for P-gp drug binding sites in a photoaffinity labeling assay. Crude Sf9 insect cell membranes expressing P-gp (25 µg) were incubated with [125I]IAAP and increasing concentrations of Gal-2 followed by UV crosslinking. (A) Autoradiogram of SDS-PAGE gel after photoaffinity labeling of P-gp in the presence of Gal-2. (B) Levels of [125I]IAAP incorporation into P-gp in the presence of Gal-2. Values are given as the percentage of the DMSO control.
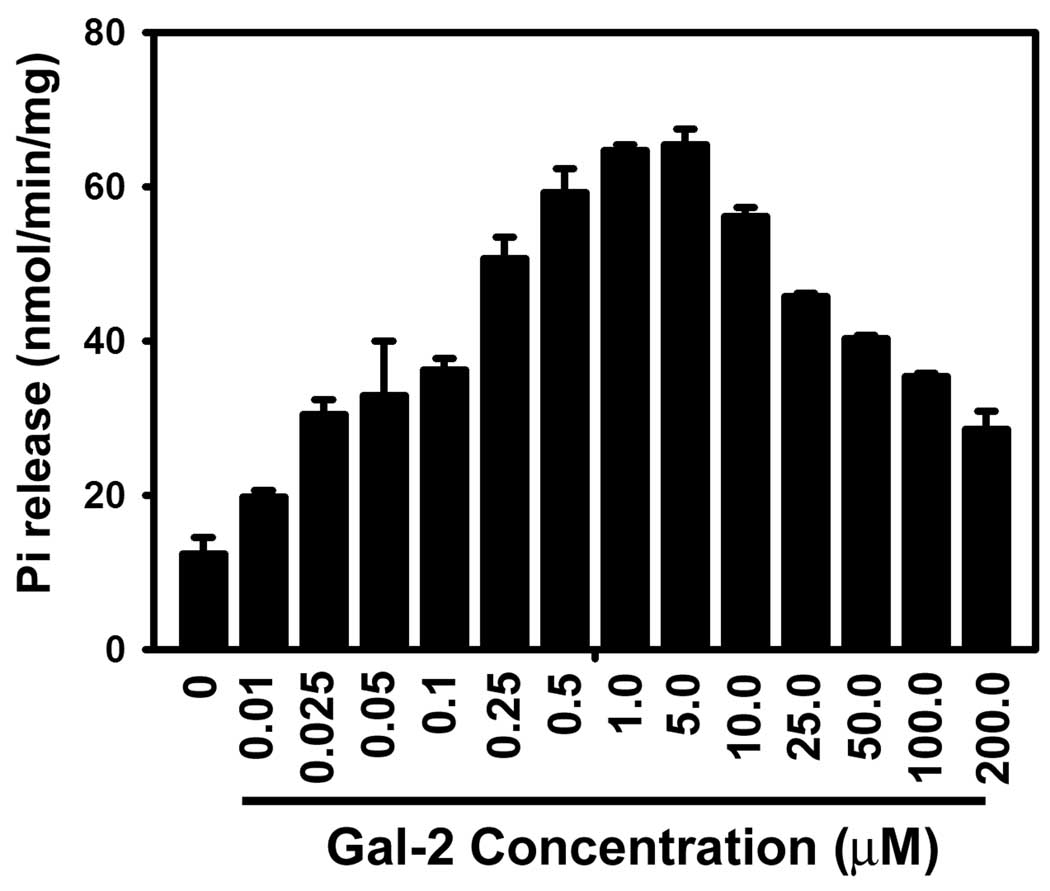
The transport function of P-gp, is tightly coupled to its ATPase activity, as ATP hydrolysis drives the transport process [28]. Substrate transport by P-gp, therefore, can be detected by the hydrolysis of ATP and the production of the inorganic phosphate. To determine if Gal-2 is itself transported by P-gp we evaluated the effect of Gal-2 on P-gp ATPase activity in crude membrane vesicles derived from insect cells containing P-gp. These vesicles were incubated with increasing amounts of Gal-2, and the ATPase activity was measured by detecting the levels of inorganic phosphate (Pi) released (Fig. 4). These data show that Gal-2 stimulated ATPase activity at low concentrations (< 1 µM), but at higher concentrations (>5 µM) ATPase stimulation was found to decrease in a concentration-dependent manner (Fig. 4). These data suggest that Gal-2 may be a substrate of P-gp at lower concentration and an inhibitor at higher concentrations. This dual behavior has similarly been seen for other P-gp inhibitors, such as the FDA approved therapeutic, verapamil [26].
Fig. 4.
The effect of Gal-2 on basal P-gp ATPase activity. Crude High Five insect membranes expressing P-gp (10 µg) were treated with increasing concentrations of Gal-2 and activity was measured by colorimetric detection of inorganic phosphate at 880 nm.
In conclusion, we have designed and synthesized a novel dimeric inhibitor of P-gp based on the anti-Alzheimer’s treatment galantamine (Gal-2). Gal-2 was found to potently inhibit P-gp transport of the fluorescent substrate rhodamine 123, as well as the therapeutic substrates verapamil, doxorubicin and daunomycin. Furthermore, we have demonstrated that the dimeric inhibitor Gal-2 is a competitive inhibitor that binds to the substrate binding sites through competition studies with the photoaffinity substrate [125I] IAAP. Gal-2 was found to stimulate the ATPase activity of P-gp at low concentrations and inhibit this activity at higher concentrations, suggesting that Gal-2 may function both as an inhibitor as well as a substrate of P-gp. The results presented demonstrate that Gal-2 is a potent dimeric inhibitor of P-gp that may ultimately have potential as a modulator of P-gp function at the BBB.
ACKNOWLEDGEMENTS
The work was supported by NIH Grant (EY018481) and the Purdue Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schinkel AH, Borst P. Multidrug resistance mediated by P-glycoproteins. Semin. Cancer Biol. 1991;2:213–226. [PubMed] [Google Scholar]
- 2.Hrycyna CA. Molecular genetic analysis and biochemical characterization of mammalian P-glycoproteins involved in multidrug resistance. Semin. Cell Dev. Biol. 2001;12:247–256. doi: 10.1006/scdb.2000.0250. [DOI] [PubMed] [Google Scholar]
- 3.Higgins CF. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 5.Lazarowski A, Czornyj L, Lubienieki F, Girardi E, Vazquez S, D'Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48 Suppl. 5:140–149. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 6.Boulton DW, DeVane CL, Liston HL, Markowitz JS. In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci. 2002;71:163–169. doi: 10.1016/s0024-3205(02)01680-6. [DOI] [PubMed] [Google Scholar]
- 7.Uhr M, Grauer MT, Holsboer F. Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol. Psychiatry. 2003;54:840–846. doi: 10.1016/s0006-3223(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 8.Uhr M, Grauer MT, Yassouridis A, Ebinger M. Blood-brain barrier penetration and pharmacokinetics of amitriptyline and its metabolites in p-glycoprotein (abcb1ab) knock-out mice and controls. J. Psychiatr. Res. 2007;41:179–188. doi: 10.1016/j.jpsychires.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802–832. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]
- 10.Dey S, Ramachandra M, Pastan I, Gottesman MM, Ambudkar SV. Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc. Natl. Acad. Sci. U S A. 1997;94:10594–10599. doi: 10.1073/pnas.94.20.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro AB, Ling V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 1997;250:130–137. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 12.Loo TW, Bartlett MC, Clarke DM. Simultaneous binding of two different drugs in the binding pocket of the human multidrug resistance P-glycoprotein. J. Biol. Chem. 2003;278:39706–39710. doi: 10.1074/jbc.M308559200. [DOI] [PubMed] [Google Scholar]
- 13.Pires MM, Emmert D, Hrycyna CA, Chmielewski J. Inhibition of P-glycoprotein-mediated paclitaxel resistance by reversibly linked quinine homodimers. Mol. Pharmacol. 2009;75:92–100. doi: 10.1124/mol.108.050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pires MM, Hrycyna CA, Chmielewski J. Bivalent probes of the human multidrug transporter P-glycoprotein. Biochemistry. 2006;45:11695–11702. doi: 10.1021/bi0608109. [DOI] [PubMed] [Google Scholar]
- 15.Mealey KL, Barhoumi R, Burghardt RC, Safe S, Kochevar DT. Doxycycline induces expression of P glycoprotein in MCF-7 breast carcinoma cells. Antimicrob. Agents Chemother. 2002;46:755–761. doi: 10.1128/AAC.46.3.755-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey S, Ramachandra M, Pastan I, Gottesman MM, Ambudkar SV. Photoaffinity labeling of human P-glycoprotein: effect of modulator interaction and ATP hydrolysis on substrate binding. Methods Enzymol. 1998;292:318–328. doi: 10.1016/s0076-6879(98)92025-0. [DOI] [PubMed] [Google Scholar]
- 17.Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Functional expression of human P-glycoprotein from plasmids using vaccinia virus-bacteriophage T7 RNA polymerase system. Methods Enzymol. 1998;292:456–473. doi: 10.1016/s0076-6879(98)92035-3. [DOI] [PubMed] [Google Scholar]
- 18.Homolya L, Hollo Z, Germann UA, Pastan I, Gottesman MM, Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J. Biol. Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- 19.Tsuji A, Tamai I, Sakata A, Tenda Y, Terasaki T. Restricted transport of cyclosporin A across the blood-brain barrier by a multidrug transporter. P-glycoprotein, Biochem. Pharmacol. 1993;46:1096–1099. doi: 10.1016/0006-2952(93)90677-o. [DOI] [PubMed] [Google Scholar]
- 20.Martin C, Berridge G, Higgins CF, Mistry P, Charlton P, Callaghan R. Communication between multiple drug binding sites on P-glycoprotein. Mol. Pharmacol. 2000;58:624–632. doi: 10.1124/mol.58.3.624. [DOI] [PubMed] [Google Scholar]
- 21.Bruggemann EP, Germann UA, Gottesman MM, Pastan I. Two different regions of P-glycoprotein [corrected] are photoaffinity-labeled by azidopine. J. Biol. Chem. 1989;264:15483–15488. [PubMed] [Google Scholar]
- 22.Bruggemann EP, Currier SJ, Gottesman MM, Pastan I. Characterization of the azidopine and vinblastine binding site of P-glycoprotein. J. Biol. Chem. 1992;267:21020–21026. [PubMed] [Google Scholar]
- 23.Pearson VE. Galantamine: a new alzheimer drug with a past life. Ann. Pharmacother. 2001;35:1406–1413. doi: 10.1345/aph.1A092. [DOI] [PubMed] [Google Scholar]
- 24.Hamabe W, Maeda T, Fukazawa Y, Kumamoto K, Shang LQ, Yamamoto A, Yamamoto C, Tokuyama S, Kishioka S. P-glycoprotein ATPase activating effect of opioid analgesics and their P-glycoprotein-dependent antinociception in mice. Pharmacol. Biochem. Behav. 2006;85:629–636. doi: 10.1016/j.pbb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–4602. [PubMed] [Google Scholar]
- 26.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro AB, Fox K, Lam P, Ling V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur. J. Biochem. 1999;259:841–850. doi: 10.1046/j.1432-1327.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 28.Horio M, Gottesman MM, Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc. Natl. Acad. Sci. U S A. 1988;85:3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]