Nitric oxide (·NO or EDRF, endothelial-derived relaxing factor) plays several important biological roles, including smooth-muscle relaxation, regulation of neurotransmission, and inhibition of platelet aggregation.[1] The NO derivative of vitamin B12, known as nitrosylcobalamin (NOCbl), has recently attracted a lot of attention in the literature. Mammalian vitamin B12-dependent enzyme reactions, methylcobalamin-dependent methionine synthase, and adenosyl-cobalamin-dependent methylmalonyl-CoA mutase are inhibited by nitric oxide or nitric oxide donors[2] in vitro and in vivo.[3, 4] Importantly, it is postulated that the inhibition of these enzymes is a consequence of NOCbl formation.[5] Evidence supporting formation of an NO derivative of vitamin B12 includes observations that hydroxocobalamin suppresses NO-induced relaxation of smooth muscle in rodents,[6] NO-induced vasodilation,[7] and NO-mediated inhibition of cell proliferation.[5] Vitamin B12 can also reverse NO-induced neural tube defects.[8] Furthermore, it has been proposed that NOCbl has applications in the treatment of cancer and in wound healing.[9] NOCbl itself has been associated with considerable controversy in the literature, notwithstanding its existence,[10] and, more recently, whether or not aquacobalamin reacts directly with NO to form NOCbl (it is now generally accepted that this is not the case).[11, 12] Indeed, NOCbl was referred to as “elusive” in a recent comprehensive review of vitamin B12 structures,[13] in which the absence of structural information on NOCbl was noted. This situation has led to speculation on the geometry, bond lengths, and bond angles of NOCbl.[13, 14] The oxidation state of the Co center of NOCbl is also of considerable interest.[11, 12, 15–17]
Herein, we report the synthesis and X-ray structural characterization of NOCbl, which was prepared by reacting hydroxycobalamin hydrochloride with the NO donor 2-(N,N-diethylamino)diazenolate-2-oxide (DEA-NONOate) under strictly anaerobic, alkaline conditions (pH 8.9). Isolation of the bulk material by precipitation with acetone gave a bright orange product in 85% yield that was approximately 97% pure (see the Supporting Information). The UV/Vis and 1H NMR spectra of the product redissolved in anaerobic buffer (pH 7.4; Figures S1 and S2 in the Supporting Information) are identical to reported spectra for NOCbl. To our knowledge, this is the first time that pure, well-characterized NOCbl has been isolated. Solid NOCbl is stable in air in the absence of moisture. A crystal of NOCbl (ca. 0.1 × 0.1 × 0.3 mm, obtained from aqueous acetone) was mounted under paraffin oil in a nylon loop in a glove box and flash frozen in liquid nitrogen. Diffraction experiments were carried out at the Stanford Synchrotron Radiation Laboratory.
NOCbl·15H2O crystallizes in the orthorhombic space group P212121 with one molecule per asymmetric unit.[18] A thermal ellipsoid plot is given in Figure 1. The atom numbering is given in Scheme 1. The solvent structure in NOCbl·15H2O has been modeled as 15 water molecules, all of which are involved in hydrogen-bonding interactions with either oxygen or nitrogen atoms on the cobalamin moiety. The cobalamin is in the “base-on” conformation, and the NO ligand is found in three different orientations, such that three positions for the oxygen atom are detected (O71A, O71B, and O71C). Rotational disorder is common for NO ligands.[19] The Co-N-O angle provides important information on the oxidation state of the Co center, since the Co-N-O group of low-spin nitroxyl CoIII complexes is bent (ca. 12°) as a consequence of the lone pair on the N atom of nitroxyl (NO−),[20] while Co-N-O is essentially linear for nitrosyl (·NO) complexes.[20] The Co-N-O angle in NOCbl·15H2O ranges from 117.4–121.4° (Table 1); hence, NOCbl is best described as nitroxylcob-(III)alamin in both the solid and solution states.[12, 16]
Figure 1.
Thermal ellipsoid plot (ellipsoids set at 30% probability) of NOCbl·15 H2O; view of the entire cobalamin complex. The NO ligand is bound to the Co center through the N atom (Co–N 1.927(6) Å); O71A–O71C are the three positions of the disordered oxygen atom. For clarity, H atoms and water solvent molecules are not shown; Co green, N blue, O red, P pink, C white.
Scheme 1.
Numbering scheme for NOCbl. The three orientations of the oxygen atom of the β-axial NO ligand are shown as O71A, O71B, and O71C.
Table 1.
N–O bond lengths and angles for NOCbl, NO2Cbl, and selected cobalt porphyrin complexes.
| Complexes | Reference | Co–NOn (n=1,2) |
N–O1[a] | N–O2 | Co-N-O1 Co-N-O2 |
|---|---|---|---|---|---|
| Co–NO complexes | |||||
| NOCbl | this work | 1.927(6) | 1.18(1) | 117.4 | |
| 1.20(2) | – | 121.3 | |||
| 1.14(1) | 121.4 | ||||
| [Co(NO)(tpp)] | [21] | 1.978(4) | 1.01(2) | – | 135.2(8) |
| [Co(NO)(tpp)(p-OCH3)] | [22] | 1.854(5) | 1.195(8) | – | 119.6(4) |
| [Co(NO)(oep)] | [23] | 1.8444(9) | 1.164(1) | – | 122.70(8) |
| [Co(NO)(tc-3,3)] | [24] | 1.785(6) | 1.137(7) | – | 127.3(6) |
| [Co(NO)(tc-4,4)] | [24] | 1.779(6) | 1.151(9) | – | 128.9(6) |
| 1.18(2)[b] | 134.9(9)[b] | ||||
| Co–NO2 complexes | |||||
| NO2Cbl·LiCl | [25] | 1.942(6) | 1.217(9) | 1.220(9) | 121.7(6) |
| 115.4(5) | |||||
| 1.324(15)[c] | 1.230(13)[c] | 121.3(8)[c] | |||
| 125.6(9)[c] | |||||
| NO2Cbl·NaCl | [25] | 1.912(5) | 1.221(8) | 1.292(8) | 124.4(5) |
| 120.1(5) | |||||
| NO2Cbl | [26] | 1.941(5) | 1.217 | 1.236 | 121.2 |
| 117.1 | |||||
| 129.1[c] | |||||
| 119.5[c] | |||||
| [Co(NO2)(TpivPP)(H2O)][d] | [27] | 1.891(5) | 1.217(8) | 121.7(9) | |
| 1.17(2) | 118.7(5) | ||||
| [Co(NO2)(TpivPP)(CH3OH)] | [27] | 1.881(4) | 1.241(5) | 1.217(5) | 119.6(3) |
| 119.5(3) |
For NOCbl, three N–O1 bond lengths are reported, corresponding to the three orientations of oxygen atom for the ligand: N–O71A, N–O71B, and N–O71C.
Two orientations of the NO ligand are observed.
Two orientations of the NO2 group are observed.
This structure is centrosymmetric; therefore the N–O1 and N–O2 distances are identical. This molecule shows two orientations for the NO2 group. TpivPP=5,10,15,20-tetrakis(α,α,α-2-pivalamidophenyl)porphyrin.
As expected, the NO ligand is bound to the cobalt center through a nitrogen atom, with a Co–NO bond length of 1.93 Å (Table 1). This bond length is similar to the Co–NO2 bond length found in the three reported structures of NO2Cbl (1.91–1.94 Å, Table 1) and is also consistent with Co–NOn (n = 1, 2) bond lengths observed in nitroxyl and nitro complexes of cobalt porphyrin complexes (Table 1). Evidence for the presence of a NO− group as opposed to the oxidized form NO2− comes from comparison of the N–O bond lengths of NOCbl with those of other structures. The N–O bond lengths for the three orientations of NOCbl range from 1.14 to 1.20 Å and are entirely consistent with a N–O double bond, as reported for the nitroxyl complexes of tetraphenylporphyrin (tpp),[21, 22] octylethylporphyrin (oep),[23] and two tropocoronand ligands (tc-3,3 and tc-4,4; Table 1).[24] Nitro ligands, on the other hand, typically show two distinct N–O bond lengths (N–O1 and N–O2, Table 1), and the N–O/N=O bond lengths for the three reported NO2Cbl structures are in the range 1.22–1.32 Å. Further support for the presence of a nitroxyl ligand compared to NO2− comes from a comparison of the Co–Nα axial bond length (Co–NB3, Scheme 1) in NOCbl with those found in the three NO2Cbl structures (2.13 versus 1.99–2.01 Å, respectively; Table 2). The nitroxyl anion is a moderate π acceptor in addition to being a strong σ-donor ligand[19] and therefore exhibits a stronger trans weakening influence than NO2−, which results in a longer Co–NB3 bond. Indeed, the Co–NB3 bond length is similar to that reported for the strong donor ligands SO32− (Co–NB3 2.13 Å) and CH3 (Co–NB3 2.16 Å).[13] Importantly, the UV/V is spectrum of a sample of dried crystals redissolved in anaerobic buffer solution (pH 7.4) was identical to that of NOCbl. The final piece of evidence for NOCbl rather than NO2Cbl is more qualitative and relates to the observed color of the crystals. As noted above, the crystals and the solution from which they grew were bright orange. The crystal chosen for X-ray diffraction studies remained orange throughout data collection. Subsequently, the vial containing the remaining crystals was opened to the air, and the mother liquor surrounding the crystals immediately turned deep red—the color expected for NO2Cbl. The aerobic decomposition of NOCbl to NO2Cbl has been previously reported.[16, 17] The crystals in the vial slowly changed from orange to deep red over the course of several days.
Table 2.
Comparison of the Co coordination sphere in NOCbl and NO2Cbl structures.
| NOCbl[a] | NO2Cbl·2 LiCl[b] | NO2Cbl·NaCl[b] | NO2Cbl[c] | |
|---|---|---|---|---|
| Co–NOn[d] (n=1, 2) |
1.927(6) | 1.942(6) | 1.912(5) | 1.941(5) |
| Co–NB3 | 2.126(5) | 1.992(6) | 2.014(5) | 2.008(4) |
| Co–N21 | 1.858(4) | 1.873(5) | 1.868(5) | 1.888(4) |
| Co–N22 | 1.908(5) | 1.920(4) | 1.902(5) | 1.917(4) |
| Co–N23 | 1.915(4) | 1.919(5) | 1.900(5) | 1.922(4) |
| Co–N24 | 1.887(5) | 1.894(5) | 1.870(5) | 1.906(4) |
Numerous crystal structures of cobalamins have been described, with the majority of structures crystallizing in the P212121 space group;[13] NOCbl is no exception. NOCbl·15H2O belongs to cluster type I,[13] with c/a and b/a ratios of 1.511 and 1.343, respectively. The cobalamin molecules are oriented such that the plane of the corrin ring is roughly parallel to the ab plane of the unit cell, with the axial base and the NO ligand extending out into solvent pockets. All potential hydrogen-bonding donors and acceptors are involved in at least one hydrogen bond, with the exception of O2P, the oxygen atom bridging the ribose and phosphate groups. There are significant intermolecular contacts in the crystal lattice, including six direct hydrogen bonds linking neighboring cobalamin molecules (O28–N45′, N29–N52′, N34–O5P′, N34–O3P′, N45–N52′, and O51–N63′), with the other interactions involving water-mediated hydrogen bonds. Although the relatively high final R factor of 9.64% is typical of cobalamin structures, it might be explained by the presence of disorder in the solvent structure and the relative lack of direct hydrogen-bonding contacts versus water-mediated interactions.
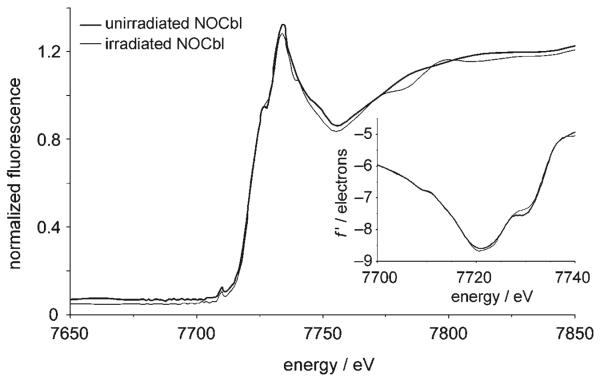
The cobalt absorption spectra of cobalamin crystals can provide information on the degree of irradiation damage incurred on the crystal during X-ray diffraction data collection. The cobalt absorption spectrum for the NOCbl crystal used for X-ray diffraction data collection (irradiated NOCbl) and the spectrum for a fresh crystal (unirradiated NOCbl) between 7650 and 7850 eV are shown in Figure 2. The overall shape of the spectra are similar to the spectra reported for other cobalamins.[28] The intensity and position of the absorption peak as calculated from the second derivative (not shown) is unchanged (7734.1 eV, f″ = 5.46 for the unirradiated sample and 7733.7 eV, f″ = 5.38 for the irradiated sample), and the overall shape of the XANES and low-energy EXAFS regions are very similar (XANES = X-ray absorption near-edge structure, EXAFS = extended X-ray absorption fine structure). Therefore, there appear to be only minor differences following prolonged irradiation, and hence practically no evidence of radiation damage. This result is in contrast to the recently reported study on N-acetylcysteinyl-cobalamin (sodium salt, NACCbl), for which the spectrum of irradiated NACCbl showed a significant drop in peak intensity relative to unirradiated NACCbl, in addition to differences in some of the XANES and near-EXAFS features.[29] It is not clear why susceptibility to radiation damage should differ between cobalamin complexes. The inflection point, or threshold energy of the absorption edge, was calculated from the first derivative of the edge spectrum (7720.8 and 7720.6 eV for the unirradiated and irradiated NOCbl samples, respectively; see inset, Figure 2). Upon comparing these values to values obtained for other cobalamin complexes, it was concluded that the threshold energy is not a reliable indicator of the cobalt oxidation state (see the Supporting Information). A similar conclusion was recently reached for N-acetylcysteinylcobalamin.[29]
Figure 2.
XAS spectrum of the NOCbl·15H2O crystal before and after irradiation during data collection for X-ray structure analysis. The inset shows the first derivatives f′ of the edge spectra.
To summarize, an efficient procedure to synthesize NOCbl is reported, and the X-ray structure of NOCbl has been determined. NOCbl is base-on, and the bent Co-N-O group suggests that NOCbl is best described as nitroxylcob-(III)alamin in the solid state. The Co–NB3 bond length is typical of that seen when there are strong β-axial ligands trans to the Co–DMB moiety (DMB = 5,6-dimethylbenzimidazole), and, as expected, is longer (by ca. 0.13 Å) than that observed for NO2Cbl, since NO2− is a weaker donor ligand. XAS measurements show that minimal damage to the NOCbl crystal occurs during X-ray data collection. Mechanistic studies are currently underway to elucidate the mechanism of formation of NOCbl from the reaction between hydroxy-cobalamin hydrochloride and DEA-NONOate. Our structural characterization of NOCbl, combined with the array of spectroscopic data obtained by others for NOCbl in solution, means that NOCbl can no longer be regarded as mysterious; the extreme air sensitivity of NOCbl in solution is undoubtedly responsible for some of the earlier confusion regarding the existence and the properties of this compound.
Supplementary Material
Footnotes
This research was funded by Kent State University (N.E.B. and L.H.) and the National Heart Lung and Blood Institute of the National Institutes of Health (HL71907) (D.W.J.).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Bian K, Murad F. Front. Biosci. 2003;8:d264–d278. doi: 10.2741/997. [DOI] [PubMed] [Google Scholar]
- 2.The terms “nitric oxide” and “nitric oxide donors” can refer to other nitrogen monoxide species (NO+ and NO−) in addition to ·NO.
- 3.Nicolaou A, Kenyon SH, Gibbons JM, Ast T, Gibbons WA. Eur. J. Clin. Invest. 1996;26:167–170. doi: 10.1046/j.1365-2362.1996.122254.x. [DOI] [PubMed] [Google Scholar]
- 4.Kambo A, Sharma VS, Casteel DE, Woods VL, Jr., Pilz RB, Boss GR. J. Biol. Chem. 2005;280:10073–10082. doi: 10.1074/jbc.M411842200. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer M, Chamulitrat W, Ferruzzi G, Sauls DL, Weinberg JB. Blood. 1996;88:1857–1864. [PubMed] [Google Scholar]
- 6.Schubert R, Krien U, Wulfsen I, Schiemann D, Lehmann G, Ulfig N, Veh RW, Schwarz JR, Gago H. Hypertension. 2004;43:891–896. doi: 10.1161/01.HYP.0000121882.42731.6b. [DOI] [PubMed] [Google Scholar]
- 7.Jiang F, Li CG, Rand MJ. Eur. J. Pharmacol. 1997;340:181–186. doi: 10.1016/s0014-2999(97)01381-2. [DOI] [PubMed] [Google Scholar]
- 8.Weil M, Abeles R, Nachmany A, Gold V, Michael E. Cell Death Differ. 2004;11:361–363. doi: 10.1038/sj.cdd.4401371. [DOI] [PubMed] [Google Scholar]
- 9.Bauer JA. Med. Hypotheses. 1998;51:65–67. doi: 10.1016/s0306-9877(98)90256-0. [DOI] [PubMed] [Google Scholar]
- 10.Firth RA, Hill HAO, Pratt JM, Thorp RG, Williams RJP. J. Chem. Soc. A. 1969:381–386. [Google Scholar]
- 11.Wolak M, Stochel G, Hamza M, van Eldik R. Inorg. Chem. 2000;39:2018–2019. doi: 10.1021/ic991266d. [DOI] [PubMed] [Google Scholar]
- 12.Zheng D, Birke RL. J. Am. Chem. Soc. 2001;123:4637–4638. doi: 10.1021/ja015682k. [DOI] [PubMed] [Google Scholar]
- 13.Randaccio L, Geremia S, Nardin G, Wuerges J. Coord. Chem. Rev. 2006;250:1332–1350. [Google Scholar]
- 14.Selcuki C, van Eldik R, Clark T. Inorg. Chem. 2004;43:2828–2833. doi: 10.1021/ic0347945. [DOI] [PubMed] [Google Scholar]
- 15.Zheng D, Yan L, Birke RL. Inorg. Chem. 2002;41:2548–2555. doi: 10.1021/ic010802a. [DOI] [PubMed] [Google Scholar]
- 16.Wolak M, Zahl A, Schneppensieper T, Stochel G, van Eldik R. J. Am. Chem. Soc. 2001;123:9780–9791. doi: 10.1021/ja010530a. [DOI] [PubMed] [Google Scholar]
- 17.Wolak M, Stochel G, van Eldik R. Inorg. Chem. 2006;45:1367–1379. doi: 10.1021/ic051300q. [DOI] [PubMed] [Google Scholar]
- 18.Crystal data for NOCbl. Empirical formula: C62H89N14O15PCo; Mr=1359 gmol−1; orthorhombic; space group P212121; a=15.930, b=21.390, c=24.066 Å; V=8200.3(1) Å3; Z=4; absorption coefficient=0.29 mm−1; F(000)=3912; limiting indices: −18≤h≤18, −26≤k≤26, −29≤l≤29; reflections collected/unique: 39700/17159; Rmerge and Rsym: 0.035, 0.041; refinement method: full-matrix least squares on F2; data/restraints/parameters: 15872/0/1036; GOF on F2: 0.975; R factors (I>4σI): R1=0.0964, wR2=0.2786; R factor (all data): R1=0.1061; largest difference peak and hole: +0.53 and −0.46 e Å−3. CCDC-642210 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19.Bultitude J, Larkworthy LF, Mason J, Povey DC, Sandell B. Inorg. Chem. 1984;23:3629–3633. [Google Scholar]
- 20.Wiberg N, Holleman AF. Inorganic Chemistry. 34th ed. Walter de Gruyter; Berlin: 2001. p. 1582. [Google Scholar]
- 21.Scheidt WR, Hoard JL. J. Am. Chem. Soc. 1973;95:8281–8288. doi: 10.1021/ja00806a013. [DOI] [PubMed] [Google Scholar]
- 22.Richter-Addo GB, Hodge SJ, Yi GB, Khan MA, Ma T, Van Caemelbecke E, Guo N, Kadish KM. Inorg. Chem. 1996;35:6530–6538. doi: 10.1021/ic960031o. [DOI] [PubMed] [Google Scholar]
- 23.Ellison MK, Scheidt WR. Inorg. Chem. 1998;37:382–383. doi: 10.1021/ic971109j. [DOI] [PubMed] [Google Scholar]
- 24.Franz KJ, Doerrer LH, Spingler B, Lippard SJ. Inorg. Chem. 2001;40:3774–3780. doi: 10.1021/ic010181l. [DOI] [PubMed] [Google Scholar]
- 25.Garau G, Geremia S, Marzilli LG, Nardin G, Randaccio L, Tauzher G. Acta Crystallogr. Sect. B. 2003;59:51–59. doi: 10.1107/s0108768102019353. [DOI] [PubMed] [Google Scholar]
- 26.Perry BP, Fernandes MA, Brown KL, Zou X, Valente EJ, Marques HM. Eur. J. Inorg. Biochem. 2003:2095–2107. [Google Scholar]
- 27.Goodwin J, Kurtikyan T, Standard J, Walsh R, Zheng B, Parmley D, Howard J, Green S, Mardyukov A, Przybyla DE. Inorg. Chem. 2005;44:2215–2223. doi: 10.1021/ic048701a. [DOI] [PubMed] [Google Scholar]
- 28.Champloy F, Gruber K, Jogl G, Kratky C. J. Synchrotron Radiat. 2000;7:267–273. doi: 10.1107/S0909049500006336. [DOI] [PubMed] [Google Scholar]
- 29.Suarez-Moreira E, Hannibal L, Smith CA, Chavez RA, Jacobsen DW, Brasch NE. Dalton Trans. 2006:5269–5277. doi: 10.1039/b610158e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.