Abstract
Prior evidence suggests that the health and longevity benefits of antiretroviral therapy (ART) for persons living with AIDS (PLWAs) have not been equally distributed across racial/ethnic groups in the United States. Notably, black PLWAs tend to fare worse than their counterparts. We examine the role of neighborhood socioeconomic context on racial/ethnic differences in AIDS treatment and survival in San Francisco. The study population encompassed 4211 San Francisco residents diagnosed with AIDS between 1996 and 2001. Vital status was reported through 2006. Census data were used to define neighborhood-level indicators of income, housing, demographics, employment and education. Cox proportional hazards models were employed in multivariate analyses of survival times. Compared to whites, blacks had a significant 1.4 greater mortality hazard ratio (HR), which decreased after accounting for ART initiation. PLWAs in the lowest socioeconomic neighborhoods had a significant HR of 1.4 relative to those in higher socioeconomic neighborhoods, independent of race/ethnicity. The neighborhood association decreased after accounting for ART initiation. Path analysis was used to explore causal pathways to ART initiation. Racial/ethnic differences in neighborhood residence accounted for 19-22% of the 1.6-1.8 black-white relative odds ratio (ROR) and 14-15% of the 1.3-1.4 Latino-white ROR for delayed or no treatment. Our findings illuminate the independent and synergistic contributions of race and place on treatment disparities and highlight the need for future studies and interventions to address treatment initiation as well as neighborhood effects on treatment differences.
Keywords: Neighborhood environment, Racial/ethnic disparity, AIDS, USA, Race, Socioeconomic status, Antiretroviral therapy
1. Introduction
The introduction of highly active antiretroviral therapy in 1996 brought significant gains in health and survival among persons living with AIDS (PLWAs) in the US (Palella, Delaney, Moorman, Loveless, Fuhrer, Satten, et al., 1998). Unfortunately, the widespread availability of advanced treatment has been accompanied by growing racial/ethnic disparity in mortality and morbidity within the US (Curtis & Patrick, 1993; Hall, McDavid, Ling, & Sloggett, 2006). Nationally, white PLWAs experienced a 77% reduction in deaths between 1995 and 2000, compared to a 68% and 56% reduction among Latino and black PLWAs, respectively (Centers for Disease Control and Prevention, 2001). In San Francisco in 1996, the mortality rate among white PLWAs was 15 per 100, with black and Latino mortality rates 5% and 1% greater, respectively. Although the white mortality rate subsequently declined to 4 per 100 in 1999 and 2 per 100 by 2006, the Latino rate declined more rapidly. By 1999 the Latino mortality rate was 15% lower than the white rate, and by 2006 was 23% lower than the white rate. In contrast, the 1999 black mortality rate was 70% higher than the white rate, and the 2006 rate was over 2-times greater than the white rate (San Francisco Department of Public Health, 2000, 2003b, 2008).
It has been offered that neighborhood context can explain racial/ethnic disparities in AIDS mortality in San Francisco (McFarland, Chen, Hsu, Schwarcz, & Katz, 2003). Neighborhood context has been shown to have an independent effect on other measures of health and mortality (Do, Finch, Basurto-Davila, Bird, Escarce, & Lurie, 2008; Riva, Gauvin, & Barnett, 2007; Robert, 1999), and given the pervasiveness of residential segregation in the US, neighborhood effects are likely to compound race/ethnic disparities in health (Massey & Denton, 1993). In 2000, the City and County of San Francisco was 44% non-Latino white, 13% Asian, 12% Latino, and 8% black. However, 38% of Asians, 46% of Latinos and 54% of blacks would have had to relocate to another census tract in order to be evenly distributed with whites (Lopez, 2001). Yet, the relevance of neighborhood context to AIDS mortality in San Francisco is perplexing given the county’s historic commitment to providing comprehensive medical and supportive services to all PLWAs, regardless of ability to pay (San Francisco HIV Health Services Planning Council, 2005).
We theorize that racial/ethnic disparity in AIDS mortality is, in part, a consequence of neighborhood effects on treatment disparities. First, the physical and social stress associated with residence in disadvantaged neighborhoods may adversely affect morbidity and mortality independent of personal characteristics or provision of medical facilities. From a ‘contextual’ perspective the neighborhood environment itself places stress on the body and ability to effectively access resources (Ellen, Mijanovich, & Dillman, 2001; Gore-Felton & Koopman, 2008; Kirby & Kaneda, 2005). From a ‘compositional’ perspective, residents of disadvantaged neighborhoods may experience more stressors, such as material hardships and psychological distress, which can adversely affect treatment uptake and efficacy (Boardman, 2004; Ganz, 2000; Ironson, Balbin, Stieren, Detz, Fletcher, Schneiderman, et al., 2008). These ‘compositional’ and ‘contextual’ stress effects are difficult to disentangle empirically (Cummins, Curtis, Diez-Roux, & Macintyre, 2007). Second, provision of and access to treatment may be unequally distributed by neighborhood. Although the majority of HIV-specific care sites are situated near areas with the highest concentration of PLWAs, some of the poorest areas of San Francisco are furthest from providers (San Francisco Department of Public Health, 2003a). Additionally, residence in affluent neighborhoods may confer treatment and survival advantages by supporting individual and collective acquisition of resources (Wallace, 2003). Third, neighborhoods are important in fostering social networks that influence health-related behaviors, attitudes, and norms (Ellen et al., 2001; Kirby & Kaneda, 2005). HIV stigma and discrimination, coupled with limited anonymity in accessing HIV services relative to persons residing in more advantaged neighborhoods may reduce utilization of HIV/AIDS services among residents of disadvantaged neighborhoods (Lichtenstein, Hook, & Sharma, 2008; Wingwood, DiClemente, Mikhail, McCree, Davies, Hardin, et al., 2007).
Mortality disparities may also result from racial/ethnic differences independent of neighborhoods. African Americans may be more reluctant to utilize health-related services compared to others due to distrust of the medical system or racial biases of providers (Altice, Mostashari, & Friedland, 2001; Dovidio, Penner, Albrecht, Norton, Gaertner, & Shelton, 2008), HIV stigma and discrimination in black social networks (Lichtenstein et al., 2008; Wingwood et al., 2007), or differences in provider-patient interactions (King, Wong, Shapiro, Landon, & Cunningham, 2004; Wong, Cunningham, Shapiro, Andersen, Cleary, Duan, et al., 2004). Stress associated with racism may also reduce utilization and efficacy of ART (Williams & Williams-Morris, 2000). Fig. 1 provides a schematic representation of our theoretical model.
Fig. 1.
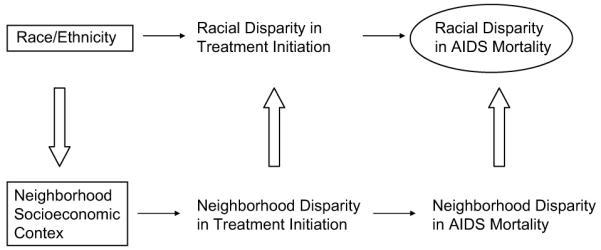
Schematic representation of the pathways linking race/ethnicity and neighborhood context to racial/ethnic disparities in AIDS mortality.
It is worth noting that Latinos may present an anomaly to this framework. Although Latinos have higher poverty rates than non-Latino whites, they tend to exhibit lower mortality rates in numerous contexts including AIDS mortality. The reasons for this ‘Latino paradox’ continue to be debated (Abraido-Lanza, Dohrenwend, Ng-Mak, & Turner, 1999; Palloni & Arias, 2004), and may include very ill immigrants returning to their country of birth. We acknowledge that a more complex causal model of racial/ethnic disparities in AIDS mortality may be necessary to fully understand Latino differences.
In this study we examine three hypotheses concerning the contribution of race/ethnicity and residence to disparities in AIDS mortality and treatment. First, racial/ethnic disparity in mortality is due to residential segregation and the socioeconomic status of neighborhoods, which affects treatment initiation through at least one of three mechanisms theorized above: stressors, access and social networks. Second, neighborhood socioeconomic context affects AIDS mortality independent of treatment initiation due to other neighborhood effects on general health. Third, racial/ethnic differences in treatment occur independently of neighborhood effects due either to cultural/social norms regarding treatment or racial/ethnic differences in provider-client interactions.
2. Methods
This study assesses the role of neighborhood socioeconomic context on racial/ethnic disparities in (1) AIDS survival and (2) antiretroviral therapy (ART) initiation.
2.1. Study population
Our study population was all individuals reported with AIDS to the San Francisco Department of Public Health (SFDPH) from January 1, 1996 through December 31, 2000, and followed through December 31, 2006. We restrict our analyses to persons diagnosed with AIDS prior to January 1, 2001 in order to improve assessment of racial/ethnic disparities in survival and treatment, as such disparities do not become apparent until two years after AIDS diagnosis (San Francisco Department of Public Health, 2008). SFDPH AIDS surveillance data are gathered using both active (e.g., review of hospital and laboratory reports) and passive (e.g., direct provider reports) surveillance, as described in detail elsewhere (Hsu, Vittinghoff, Katz, & Schwarcz, 2001). Evaluations have found the SFDPH AIDS case reporting to be greater than 95% complete (Schwarcz, Hsu, Parisi, & Katz, 1999).
In our analysis of AIDS survival cases were restricted to persons (i) 15 years old or older, (ii) with a known residence within the county or otherwise identified as homeless and diagnosed in the county, (iii) surviving at least 30 days from diagnosis of AIDS, and (iv) dying from specified disease-related causes. Following previous literature, we exclude persons who died within 30 days of diagnosis as they may reflect persons who were previously diagnosed but unreported (Nash, Katyal, & Shah, 2005). As our focus is on health outcomes that are readily addressed by medical providers we limit our analyses to disease-related deaths and exclude other causes of death such as overdose, suicide, homicide, mental illness, unspecified or not reported causes. In our analysis of ART initiation we follow similar restrictions except that persons are included regardless of the cause of death. All individual-level data were deidentified.
2.2. Variables
2.2.1. Outcome variables
Death was the primary outcome variable for our analysis of disparity in AIDS survival. Although cause of death (COD) was initially classified into two groups—(i) HIV/AIDS -related deaths (AIDS), and (ii) other disease-related deaths not due to HIV/AIDS, such as cerebrovascular disease, chronic obstructive lung disease, kidney infection, viral hepatitis and others (Palella, Baker, Moorman, Chmiel, Wood, Brooks et al., 2006)—there was little difference in parameter estimates by COD. We therefore present results for all disease-related COD.
ART initiation was the primary outcome variable for our analysis of disparity in ART initiation. ART initiation was calculated as the date of AIDS diagnosis minus the date of the individual began ART. When used as an outcome variable ART initiation was grouped into three categories: initiating ART (i) prior to diagnosis or up to 59 days after diagnosis, (ii) 60 days or more after diagnosis, and (iii) never.
2.2.2. Predictor variables
Our principal predictor variables were race/ethnicity and neighborhood context. Race/ethnicity was based on the SFDPH data definitions reclassified into five groups: white, African American (black), Latino, Asian/Pacific Islander, and Other/Unknown.
Exploratory factor analysis (EFA) was used to construct neighborhood socioeconomic context (NSEC) scores for 76 neighborhoods. Neighborhood-level data were constructed from the 2000 US Census Summary File 3 (U.S. Census Bureau, 2002). In addition to San Francisco, Alameda County—a large and diverse county situated east of San Francisco—was included in development of the summary neighborhood score in order to enhance variability, reliability and precision in measuring and defining neighborhood socioeconomic context. All relevant variables were aggregated from the block group to the neighborhood level. San Francisco neighborhoods (j = 23) were defined by combining US census tract boundaries within 23 contiguous areas that approximate local, conventional usage and place names and guided by real estate boundaries, history, and tourist guide books as previously reported (San Francisco Department of Public Health, 2003a). Alameda County neighborhoods were defined by zip code (j = 53).
Neighborhood socioeconomic indicators considered in construction of the summary measure included: proportion of residents by race/ethnicity; proportion of residents by annual household income level; log per capita income; proportion of residents by age range; proportion male; proportion of males in the labor force; proportion of employed males in the labor force; proportion of each gender group in specified education levels; and log median household value in US dollars. Stepwise exclusion of neighborhood indicators was conducted until all remaining indicators demonstrated a loading on the single factor greater than an absolute value of 0.6. The |0.6| criterion was chosen to select only those variables that were moderately or strongly associated with the underlying factor. The final variables were converted to z-scores, weighted by the factor loadings, and summed to determine final scores. NSEC scores were constructed for the San Francisco homeless population by taking the average of all NSEC scores that fell under one standard deviation below the county mean. This rationale assumes that the mobile homeless population is likely to be distributed across these neighborhoods. The final NSEC scores ranged from -17 to 14 (N (0, 7.6)). Quartile ranges were used to identify four NSEC levels: low (-17 to -5), moderate-low (-5 to -.1) moderate-high (0 to 4.9) and high (5 or greater). EFA was conducted in R version 2.5.1 using the factanal package.
2.2.3. Confounding, mediating and explanatory variables
In our AIDS survival models potential confounding and explanatory variables included transmission category (how HIV infection was acquired), health insurance status, ART initiation, age and CD4 count. Transmission categories were defined following surveillance data classification, with the exception that injection drug users (IDU) include both heterosexual and homosexual IDUs.
Although HIV care is available to all PLWAs in San Francisco regardless of ability to pay, health insurance may have important implications for the type and quality of care PLWAs are able to access as well as utilization rates. Some persons who do not have employer-provided or privately acquired insurance will have access to public insurance if they are US citizens and have low income, a disability or are elderly. Other US residents will have no insurance. Health insurance status at diagnosis was classified as private, public, none or unknown. Insurance type at diagnosis is only a relative proxy of insurance and health care access, since the type of insurance may change over time for PLWAs.
Delays in treatment were estimated using the timing of ART initiation. When used as an explanatory variable in the survival models ART initiation was grouped into five categories: (i) more than one year prior to diagnosis, (ii) within one year prior to diagnosis, (iii) between 0 and 59 days after diagnosis, (iv) 60 days or more after diagnosis, and (v) never.
The health status variables were age and adjusted CD4. Age at diagnosis is used as a proxy of age-related health status, and was categorized into 15-24, 25-34, 35-39, 40-44, 45-49, and older than 49 years old. CD4 counts provide an indication of the strength of the immune system and the progression of HIV, with lower counts representing weaker immune systems and greater AIDS progression. CD4 counts were categorized into five levels: <100, 100-199, 200-349, 350-499, and greater than 499 cells/mm3. Time is an important confounder in using CD4 as a measure of health at AIDS diagnosis, since PLWAs differ in the time at which their CD4 was reported relative to their AIDS diagnosis. To account for the time confounding, CD4 counts were adjusted by the timing of CD4 collection, which was calculated as the date of AIDS diagnosis minus the date of CD4 reported nearest to diagnosis. CD4 timing was grouped into four categories: (i) more than one month prior to diagnosis, (ii) within one month prior to AIDS diagnosis, (iii) 0-30 days after diagnosis, and (iv) more than 30 days after diagnosis. Linear regression of the log CD4 count on the timing categories was used to adjust CD4 counts to expected values at date of AIDS diagnosis. Details on CD4 adjustment are available from the authors upon request.
In our analysis of ART initiation we include insurance status, injection drug use, homelessness, and treatment probability scores (TPS) as potential mediators or confounders. Insurance status and injection drug use are defined above. A case is classified as homeless if, at the time of HIV or AIDS diagnosis, the medical record states that the patient is homeless or the patient’s address is one of the following: (i) a known homeless shelter, (ii) a health care clinic, or (iii) a free postal address not connected to a residence (‘general delivery’).
TPS were constructed to account for the fact that the timing of ART initiation may be influenced by standard guidelines based on CD4 count. The discretionary relationship between treatment timing and race/ethnicity could be confounded at the population level if there are group differences in presenting CD4. TPS are population-based counterfactual scores of the probability of initiating treatment at a certain time given CD4 count and the time between diagnosis and measurement of CD4. A multinomial logistic regression (using the R nnet package) of treatment times, CD4 level, timing of reported CD4 and the interaction of the two was used to construct TPS. Predicted probabilities were based on the estimated coefficients from the regression model and derived using the predict function in R. TPS scores for delayed (≥60 days) and no treatment were used in the path models. The TPS were grouped into low, middle and high based on tercile distributions. The low, middle and high ranges of TPS for delayed ART initiation were .083-.218, .219-.230, and .231-.501; and for no ART initiation were .000-.127, .128-.142, and .143-.375. Details on TPS construction are available from the authors upon request.
2.3. Analyses
2.3.1. AIDS survival
Cox proportional hazard models were used to assess the association between 5-year survival and race/ethnicity, neighborhood context, and treatment initiation after accounting for potential confounders. Predictor variables were entered into the model in a stepwise fashion. The order of inclusion was (i) race/ethnicity and transmission category, (ii) neighborhood socioeconomic context, (iii) health status (age and adjusted CD4) and (iv) health care (insurance and ART initiation). Kaplan-Meier curves were also used to visually assess racial/ethnic differences in five-year survival. Differences in survival by racial/ethnic group were determined by way of Mantel-Haenszel tests of equality. Survival analyses were conducted in R 2.5.1 using the survival package.
2.3.2. ART initiation
Causal models of ART initiation, classified as early, delayed or never, were explored using path analysis. The initial ‘saturated’ path model (Model One) tested the assumption that treatment is influenced directly by race/ethnicity (RACE), injection drug use (IDU), homelessness (HMLESS), neighborhood socioeconomic context (NSEC), insurance status (INSURE) and a treatment probability score (TPS) for delayed or no treatment. Model One also postulated that (a) RACE directly affects IDU, HMLESS, NSEC, INSURE and TPS; (b) IDU affects HMLESS, NSEC, INSURE and TPS; (c) HMLESS affects INSURE and TPS; (d) INSURE affects NSEC and TPS; and (e) NSEC affects TPS. A schematic of the saturated model is presented in Fig. 2.
Fig. 2.
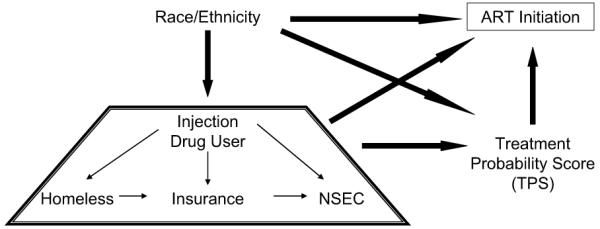
Schematic representation of initial (saturated) treatment initiation path model tested. Each item in the trapezoid is hypothesized to be independently affected by race/ethnicity and to independently affect TPS and ART initiation.
Five alternative models were developed and tested for adequacy of fit against this initial model and each other. Model Two assumed that a person’s TPS is not influenced by other factors. The TPS independence assumption was maintained in each of the remaining four models, which posited that RACE does not directly influence ART initiation (Model Three); that neither RACE nor NSEC directly influences ART initiation (Model Four); that IDU does not directly affect ART initiation (Model Five); and that RACE does not directly affect a persons’ likelihood of IDU (Model Six). Because all models incorporate only categorical variables, maximum likelihood was used to estimate path coefficients, which also allowed for interpretation of coefficients as relative odds. Model fit was compared using the Bayesian information criterion (BIC), where for nested models lower values indicate better fit. Path analyses were performed in Mplus version 4.21. Predicted relative odds ratios of delayed or no treatment for black and Latino PLWAs compared to white PLWAs were calculated from the path coefficients and associated thresholds.
3. Results
In San Francisco there were a total of 4211 PLWAs aged 15 and older who were diagnosed from 1996 through 2000. The racial/ethnic composition was: 61% white, 19% black, 14% Latino, and 4% Asian. Over 28% of cases had died by the end of 2006. Based on the exclusion criteria for analysis of AIDS survival, 3866 cases (92%) were retained. Given the exclusion criteria for analysis of ART initiation, 3901 cases (93%) were retained.
3.1. Neighborhood socioeconomic context scale
Descriptive statistics and initial and final factor loadings for the population-level variables tested in the neighborhood level EFA are presented in Table 1. Per capita income, percent of households with income above $100,000 and the percent of white residents demonstrated substantially high loadings on the factor. Consequently, the NSEC score should be thought of primarily as an indicator of neighborhood economic affluence. Neighborhoods were grouped into low, moderate-low, moderate-high and high based on NSEC quartiles as described above.
Table 1.
Descriptive statistics and factor loadings for neighborhood-level variables tested in construction of the socioeconomic context score, San Francisco and Alameda Counties, 2000
| Mean | Standard deviation | Factor loadingsa | |
|---|---|---|---|
| Income & Wealth | |||
| % Low income (<$20,000) | 17.9 | 11.7 | -0.643 |
| % Moderate income ($20,000-49,000) | 27.2 | 78.3 | -0.761 |
| % Middle income ($50,000-99,000) | 31.1 | 74.4 | Ref |
| % High income ($100,000) | 23.8 | 14.4 | 0.866 |
| Income per capita ($) | 31338 | 14855 | 0.978 |
| Housing | |||
| % Homeowners | 49.5 | 23.6 | (0.318) |
| Median value of housing units ($) | 356137 | 178464 | 0.851 |
| Race/Ethnicity | |||
| % White | 44.7 | 21.3 | 0.876 |
| % Black | 13.2 | 14.9 | Ref |
| % Latino | 15.5 | 11.2 | -0.649 |
| % Asian | 21.2 | 12.9 | (-0.151) |
| Age profile | |||
| % <18 | Ref | ||
| % 18-24 | 10.2 | 8.18 | (-0.322) |
| % 25-59 | 55.3 | 7.84 | 0.609 |
| % 60+ | 14.8 | 4.78 | (0.206) |
| Employment & Education (males)b | |||
| % In labor force | 71.3 | 7.65 | 0.760 |
| % Of labor force employed | 94.3 | 3.3 | 0.785 |
| % High school or lower | 15.6 | 7.3 | Ref |
| % Some college/2-year degree | 24.6 | 6.5 | (-0.440) |
| % Bachelor of Science/Bachelor of Arts degree | 24.6 | 9.8 | 0.810 |
| % Advanced degree | 19.2 | 1.4 | 0.757 |
| Proportion variance | 0.617 | ||
| χ2 | 377.28 | ||
| df | 54 | ||
| p | <.0001 |
Loadings from final factor model presented except for items in parentheses, which represent initial factors loadings excluded from model. ‘Ref’ indicates reference group.
Female employment and education indicators included in initial factor analysis, but were excluded given redundancy with male employment and education loadings. Proportion of male in population excluded because of overidentification.
3.2. AIDS survival
Table 2 provides descriptive statistics of the key individual-level variables used in the survival analysis. Fig. 3 presents the Kaplan-Meier 5-year survival curves by race/ethnicity. Whites, blacks and Latinos follow similar survival profiles until approximately two years when survival among Latinos begins to out-pace that of their ethnic counterparts, and the black survival rate drops significantly relative to others (Mantel-Haenszel χ2 21.3, df (2), p < .001). These patterns were consistent when cause of death was separated into AIDS-related and other disease-related deaths.
Table 2.
Survival status by clinical predictors, PLWA, San Francisco, 1996-2006
| Cause of death |
||||
|---|---|---|---|---|
| AIDS | Other | Censored | Excluded | |
| N | 494 | 142 | 3230 | 345 |
| Survival (mean days (standard deviation)) | 768 (532) | 847 (528) | 1819 (44) | 943 (767) |
| CD4 count (mean cells/mm3 (SD)) | 133 (130) | 172 (123) | 184 (143) | 162 (133) |
| Time to CD4 report | ||||
| >30 days prior to diagnosis | 3.5% | 7.0% | 3.4% | 4.6% |
| 30-1 day prior to diagnosis | 79.5 | 81.8 | 84.3 | 80.2 |
| 0-30 days after diagnosis | 7.4 | 8.4 | 8.2 | 10.7 |
| >30 days after diagnosis | 9.6 | 2.8 | 4.0 | 4.0 |
| Time to ART treatment | ||||
| >1 year prior to diagnosis | 18.5 | 21.0 | 25.8 | 19.1 |
| 1-0 years prior to diagnosis | 11.7 | 12.6 | 13.6 | 13.9 |
| 0-60 days after diagnosis | 21.2 | 20.3 | 27.5 | 13.6 |
| >60 days after diagnosis | 21.6 | 19.6 | 23.9 | 15.6 |
| Never | 26.9 | 26.6 | 9.2 | 37.7 |
| Race | ||||
| White | 60.8 | 53.8 | 62.2 | 57.8 |
| Black | 23.2 | 31.5 | 16.9 | 24.3 |
| Latino | 11.5 | 11.9 | 15.2 | 13.5 |
| Asian/Pacific Islander | 3.3 | 2.1 | 4.7 | 3.1 |
| Other/Unknown | 1.2 | 0.7 | 0.9 | 1.2 |
| Gender | ||||
| Male | 87.1 | 87.4 | 90.4 | 85.8 |
| Female | 9.0 | 9.8 | 6.8 | 9.8 |
| Transgender | 3.8 | 2.8 | 2.7 | 4.3 |
| Age at diagnosis | ||||
| 15-24 years | 2.1 | 0.7 | 2.3 | 2.0 |
| 25-34 | 21.6 | 9.8 | 30.0 | 21.2 |
| 35-39 | 20.1 | 16.8 | 24.4 | 23.5 |
| 40-44 | 18.9 | 15.4 | 18.8 | 19.1 |
| 45-49 | 17.3 | 22.4 | 13.4 | 14.8 |
| 50+ | 19.9 | 35.0 | 11.1 | 19.4 |
| Transmission category | ||||
| Men who have sex with men (MSM) | 55.9 | 47.6 | 68.8 | 51.9 |
| MSM & injection drug user (IDU) | 20.8 | 23.8 | 15.4 | 18.8 |
| IDU | 19.1 | 24.5 | 11.1 | 23.8 |
| Health insurance at AIDS diagnosis | ||||
| Private | 27.5 | 31.5 | 43.7 | 27.8 |
| Public | 23.9 | 30.1 | 15.5 | 29.3 |
| None | 44.9 | 37.8 | 37.2 | 38.8 |
| Unknown | 3.6 | 0.7 | 3.4 | 4.0 |
| Homeless | 14.2 | 14.1 | 8.5 | 20.2 |
| Neighborhood socioeconomic context | ||||
| Low | 42.0 | 44.4 | 29.1 | 48.4 |
| Moderate-Low | 11.1 | 13.4 | 13.9 | 8.4 |
| Moderate-High | 22.8 | 24.6 | 27.6 | 22.5 |
| High | 24.0 | 17.6 | 29.3 | 20.6 |
Fig. 3.
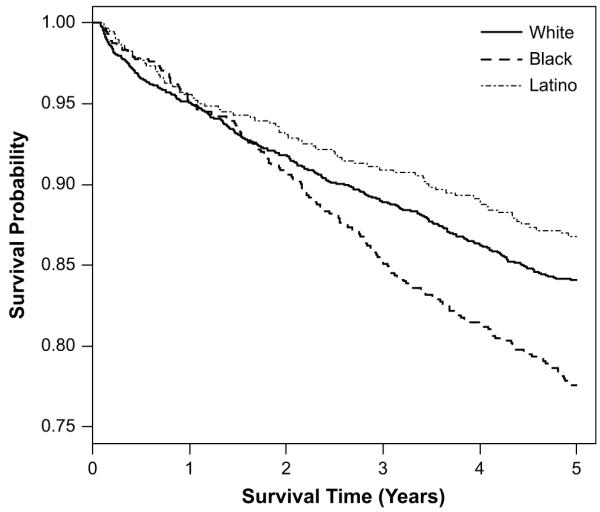
Comparison of 5-year survival curves for black, white and Latino persons diagnosed with AIDS between 1996 and 2001, San Francisco, CA.
When considering race/ethnicity alone the black-white hazard ratio (HR) is 1.4. However, after accounting for injection drug use, the black HR dropped to 1.2 (Table 3). When neighborhood NSEC was included into the model, the black coefficient approached one and lost significance. Neighborhood differences in survival, which are largely attributable to residence in lower NSEC neighborhoods, lost significance after accounting for never having received treatment. Thus, the associations between race, NSEC and treatment are important in understanding differences in survival.
Table 3.
Hazard ratios (HR) for all disease mortality among persons with AIDS, San Francisco, CA (N = 3771)
| (1)b |
(2) |
(3) |
(4) |
|||||
|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Race/Ethnicitya | ||||||||
| White (Reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 1.24* | (1.02, 1.51) | 1.14 | (0.93, 1.34) | 0.97 | (0.79, 1.20) | 0.88 | (0.72, 1.08) |
| Latino | 0.82 | (0.64, 1.06) | 0.79 | (0.62, 1.01) | 0.82 | (0.63, 1.06) | 0.77 | (0.59, 1.00) |
| Asian | 0.69 | (0.43, 1.11) | 0.68 | (0.42, 1.08) | 0.64 | (0.40, 1.04) | 0.69 | (0.43, 1.12) |
| Risk transmission category | ||||||||
| Men who have sex with men (Reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Injection drug user | 1.63*** | (1.37, 1.94) | 1.49*** | (1.25, 1.78) | 1.64*** | (1.37, 1.96) | 1.39** | (1.16, 1.68) |
| Neighborhood socioeconomic context | ||||||||
| Low (Reference) | 1.00 | 1.00 | 1.00 | |||||
| Moderate-Low [-5,0) | 0.74* | (0.57, 0.97) | 0.70* | (0.53, 0.93) | 0.77 | (0.59, 1.02) | ||
| Moderate-High [0,5) | 0.71** | (0.58, 0.88) | 0.71** | (0.58, 0.88) | 0.84 | (0.67, 1.04) | ||
| High [5,15) | 0.71** | (0.57, 0.89) | 0.69** | (0.55, 0.87) | 0.85 | (0.67, 1.07) | ||
| Age at diagnosis | ||||||||
| 15-40 (Reference) | 1.00 | 1.00 | ||||||
| 40-44 | 1.23 | (0.96, 1.57) | 1.22 | (0.97, 1.53) | ||||
| 45-49 | 1.90*** | (1.52, 2.37) | 2.04*** | (1.63, 2.54) | ||||
| 50+ | 2.66*** | (2.16, 3.28) | 2.65*** | (2.14, 3.28) | ||||
| Adjusted CD4 (ln) | 0.71*** | (0.66, 0.76) | 0.70*** | (0.65, 0.75) | ||||
| Insurance | ||||||||
| None (Reference) | 1.00 | |||||||
| Public | 1.18 | (0.96, 1.45) | ||||||
| Private | 0.65*** | (0.52, 0.80) | ||||||
| ART initiation | ||||||||
| >1 year prior to diagnosis (Reference) | 1.00 | |||||||
| 1-365 days prior to diagnosis | 1.10 | (0.82, 1.48) | ||||||
| 0-60 days after diagnosis | 0.88 | (0.68, 1.14) | ||||||
| >60 days after diagnosis | 1.01 | (0.78, 1.30) | ||||||
| Never | 3.09*** | (2.41, 3.95) | ||||||
| Likelihood ratio | 60 | 8 df; 0.000 | 74 | 8 df; 0.000 | 248 | 12 df; 0.000 | 401 | 18 df; 0.000 |
p < .05
p < .01
p < .001.
Other/unknown racial category included in all models but not significant and not presented.
Female, transgender, and heterosexual included in model but not significant.
3.3. ART initiation
In order to investigate the relationship between race/ethnicity and treatment initiation we constructed six alternative causal path analytic models. Model fit, as determined by BIC, was worse for the initial saturated model (BIC = 48,600 on 67 parameters) and was best for Model Six (BIC = 26,986 on 43 parameters), where TPS was associated with ART initiation independent of other factors, and race/ethnicity was not associated with injection drug use.
The path coefficients for Model Six were converted to odds ratios and are listed in Table 4. The association between the treatment propensity score (TPS) and ART initiation was significant and followed the anticipated direction: those who were more likely to have delayed or no treatment given their CD4 profile (count and timing) demonstrated greater odds of delayed or no ART initiation. Nevertheless, after accounting for the TPS and other factors, direct effects on the odds of ART initiation persisted for NSEC. Those residing in higher NSEC neighborhoods were less likely to have delayed or no ART initiation relative to residents of lower NSEC neighborhoods. Put differently, holding other pathways constant, there was a 40% greater odds of no treatment if one resided in the lowest NSEC compared to residents in the highest NSEC.
Table 4.
Path odds ratios (95% confidence intervals) for antiretroviral treatment models, San Francisco, CA (N = 3905).
| Homeless | Public Insurance | Uninsured | NSECa | ARTb | |
|---|---|---|---|---|---|
| Blackc | 2.34 (2.05, 2.66) | 1.69 (1.52, 1.88) | 1.48 (1.35, 1.62) | 0.40 (0.37, 0.44) | 1.26 (1.16, 1.38) |
| Latinoc | 1.99 (1.68, 2.35) | 0.87 (0.75, 1.00) | 2.90 (2.63, 3.20) | 0.56 (0.51, 0.61) | 1.00 (0.91, 1.11) |
| Asian/PIc | 0.32 (0.17, 0.57) | 0.68 (0.52, 0.89) | 1.77 (1.51, 2.09) | 0.65 (0.56, 0.74) | 0.76 (0.64, 0.91) |
| Injection drug user | 8.28 (7.27, 9.43) | 4.03 (3.67, 4.43) | 1.81 (1.68, 1.96) | 0.36 (0.33, 0.39) | 1.20 (1.11, 1.31) |
| Homeless | 0.83 (0.72, 0.95) | 3.04 (2.68, 3.46) | 1.63 (1.45, 1.83) | ||
| Public insuranced | 0.45 (0.41, 0.50) | 0.92 (0.83, 1.03) | |||
| Uninsuredd | 0.43 (0.40, 0.46) | 1.44 (1.33, 1.57) | |||
| NSEC | 0.90 (0.88, 0.93) | ||||
| TPS: Delayede | 1.25 (1.19, 1.3) | ||||
| TPS: Nevere | 1.18 (1.13, 1.23) | ||||
| R-Square | 0.288 | 0.111 | 0.674 | 0.788 | 0.652 |
Neighborhood Socioeconomic Context Score: low (reference), moderate-low, moderate-high or high.
ART initiation: prior to 60 days after diagnosis (Reference), 60+ days after diagnosis, or never.
Reference: White.
Reference: Insured.
Treatment probability scores of delayed treatment or no treatment: low (Reference), medium or high.
As anticipated, race/ethnicity was significantly associated with residence. Compared to white PLWAs, the relative odds of residing in a higher NSEC were 60% lower for blacks and 44% lower for Latinos independent of other factors. After accounting for all pathways, the odds that a white PLWA would reside in the highest NSEC was 0.4 and the odds of residing in the lowest PLWA was 0.3. The odds or residing in the highest and lowest NSEC, respectively, were 0.2 and 0.7 for Latinos, and 0.2 and 0.9 for blacks. Thus, compared to whites, the odds of residing in the lowest NSEC were twice as great for Latinos and nearly three-times greater for blacks, after accounting for all pathways.
Despite the association between residence and race/ethnicity, blacks demonstrated a differential direct effect on ART initiation compared to whites. Fig. 4 highlights the direct and indirect pathways through which black race influences ART initiation. For clarity, pathways not associated with black race and treatment initiation have been omitted from the figure. As evident in Fig. 4, the odds of delayed or no treatment were 30% higher for blacks relative to whites. There was no direct difference in the odds of delayed or no treatment for Latinos relative to whites (Table 4).
Fig. 4.
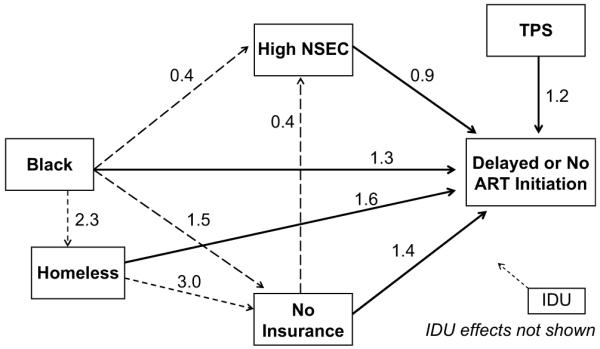
Estimated odds of delayed or no treatment among black PLWAs relative to whites from the final path analytic model on treatment initiation, San Francisco, CA, 1996 to 2006. Solid black lines indicate significant direct effects on ART initiation. Dashed lines indicate significant mediating pathways between black race and delayed or no treatment. For clarity, pathways with injection drug users (IDU) on homelessness, insurance, NSEC and treatment are not shown.
After accounting for all pathways, the relative odds ratio (ROR) of delayed ART initiation relative to whites was 1.3 for Latinos and 1.6 for blacks. The ROR for no ART initiation relative to whites was 1.4 for Latinos and 1.8 for blacks. Fig. 5 presents the predicted ROR for blacks and Latinos relative to whites when the coefficients for the race-homelessness and race-NSEC paths were set to zero (i.e., when black and Latino PLWAs have the same probability as white PLWAs of being homeless or residing in particular NSEC neighborhoods). Removing racial/ethnic differences in the probability of being homeless had little effect on the ROR for ART initiation. However, when racial/ethnic differences in NSEC were removed a more pronounced reduction in the ROR of delayed or no ART initiation was evident. The Latino-white predicted ROR for delayed and no treatment declined by 14-15%. The black-white predicted ROR for delayed treatment and no treatment declined by 19% and 22% when racial/ethnic differences in NSEC were removed. Nevertheless, the black-white ROR remained considerably large, reflecting the direct effect of black race on ART initiation.
Fig. 5.
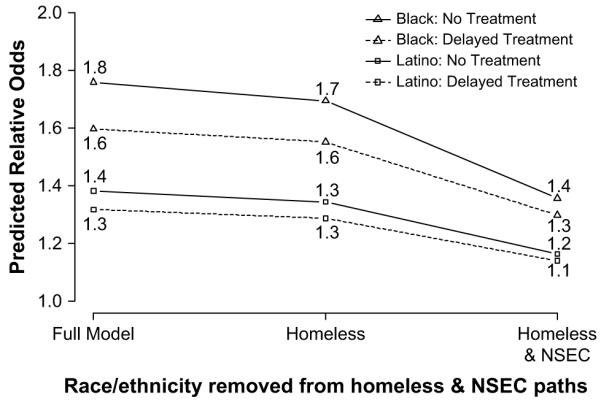
Comparison of the predicted relative odds of delayed or no treatment for blacks and Latinos compared to white PLWAs when race/ethnicity effects are removed from homelessness and neighborhood socioeconomic context (NSEC) in the treatment initiation path model, San Francisco, CA, 1996-2006.
It is worth noting that, the Latino-white disparity in ART initiation can be similarly explained by way of insurance status. Latinos have a nearly three-times higher odds of being uninsured compared to whites (Table 4). When race/ethnicity effects on insurance status are removed from the model, the Latino-white predicted ROR declined by 12% for delayed treatment and 14% for no treatment. In contrast, removing race/ethnicity effects on insurance reduced the black-white predicted ROR by 5% for delayed treatment and 6% for no treatment.
4. Discussion
Despite the presumed comprehensiveness of San Francisco’s HIV care delivery system, racial and ethnic differences in ART use and AIDS mortality persisted through 2006. Our first hypothesis was that racial/ethnic disparities in AIDS mortality are due to residential segregation and the socioeconomic context of neighborhoods. Indeed, we found that the mortality disparity between black PLWAs and others was negated after accounting for neighborhood socioeconomic status. The neighborhood effect on mortality rates did not demonstrate a gradient by NSEC. Instead, the relative hazard of mortality was consistently higher for residents of the lowest NSEC compared to residents of other NSEC levels. We next examined whether the effect of NSEC on AIDS mortality could be understood in part through disparities in treatment. This appears to be the case. In the proportional hazards models, the effect of neighborhood context on AIDS mortality diminished after accounting for differences in ART initiation, and our path analytic model produced a significant NSEC gradient effect on ART initiation. However, the relative contribution of NSEC to racial/ethnic differences in ART initiation varied. While NSEC strongly contributed to the predicted odds of delayed or no treatment for black PLWAS, lack of insurance was a more important influence than NSEC on the predicted odds among Latinos. Unfortunately, we are unable to identify which of the theorized neighborhood pathways—stressors, access or social networks—is most important in supporting such disparities. Future work should turn to an examination of these proximal mediating pathways.
We found no evidence to support our second hypothesis of a residual neighborhood effect on AIDS mortality independent of ART initiation. The role of the lower NSEC on mortality disparities appears to be entirely attributed to disparities in never receiving treatment. Thus, an independent ‘contextual’ neighborhood effect on mortality is likely not influencing AIDS mortality disparities in San Francisco.
We did find support for our third hypothesis that racial/ethnic differences in treatment initiation occur independently of neighborhood context. Blacks demonstrated significantly greater odds of delayed or no treatment relative to other groups even after accounting for their increased likelihood of residing in lower NSEC neighborhoods. No direct effect was evident for Latinos. In the absence of other information it is difficult to assess whether the direct racial/ethnic effects are the results of differences in cultural/social norms or provider-client interactions. It is also possible that the differences are the result of unmeasured confounders such as mental illness and substance use. More studies are needed to assess the relative importance of each of these proposed pathways. Nevertheless, the results suggest that improving case management activities and patient’s treatment knowledge and self-efficacy, and reducing provider-patient racial biases are potentially useful approaches to reducing racial/ethnic treatment disparities.
Although these analyses have concerned themselves with the influence of residence on racial/ethnic patterns in treatment, it is important to highlight the role of injection drug use on ART initiation. Injection drug use is a significant predictor of delayed or no treatment and higher mortality. When all pathways are accounted for, the relative odds of no treatment is 1.6 for IDU compared to non-IDU. Although race and ethnicity are not causally linked to injection drug use, roughly 55% of black PLWAs were reported to be IDU compared to 22-25% of white and Latino PLWAs. These differences are likely to compound aggregate racial/ethnic treatment initiation disparities.
Although every effort was made to address potential biases in the study, several limitations remain. First, the measure of socioeconomic context is limited and does not incorporate indicators of social resources and networks. Neighborhood affluence is not a full proxy for other contextual measures, such as social capital, service and resource availability, and neighborhood quality, which have also been proposed as important influences on health and health-seeking behavior. Second, the excluded AIDS cases, which were largely comprised of persons who died within 30 days of AIDS diagnosis had substantially higher rates of homelessness. We are likely, then, to underestimate the importance of homelessness to mortality. Since the primary focus of the study was on the role neighborhood context, these results should not significantly bias our findings. However, they do highlight the importance of considering homelessness as well as neighborhood context in future analyses.
Third, the proportionate hazard and path analytic models included neighborhood context at the level of the individual instead of allowing for area variability through methods such as multilevel modeling. Given that there were a total of 23 San Francisco neighborhoods and only 14 of these neighborhoods had 100 or more PLWAs, there would have been insufficient power to assess area variability. Nonetheless, we acknowledge that inference regarding neighborhood effects may be biased due to inability to capture variation within and between neighborhoods, and potential correlated errors between individuals.
Fourth, the study relied on AIDS surveillance data, and thus several additional individual-level variables of interest such as substance use, mental health, treatment adherence, stress, health self-efficacy, and social supports were not available. These may lead to substantial omitted variable bias in the results. To the extent that these factors mediate racial/ethnic and neighborhood differences in survival and treatment, however, they support rather than nullify our central findings. Capturing these proximal measures will be an important component of future work on treatment disparities.
Fifth, errors in the reported dates for treatment, diagnosis and CD4 measurement could lead to substantial bias in the effect estimates if these differences are systematically related to particular groups. Finally, although racial/ethnic and neighborhood disparities exist in multiple areas in the US, the study findings cannot be generalized beyond the study population of San Francisco. San Francisco may represent a special case, particularly with respect to residence, health care provision, and the demographics of the epidemic.
5. Conclusion
The establishment of a universal case-management AIDS service delivery system, while likely meeting the needs of a great many individuals, fails to achieve universal coverage in practice. Neighborhood and race/ethnicity effects are prominent determinants of treatment disparities. Future studies should examine proximal pathways by which neighborhood context and race/ethnicity influence treatment initiation. These proximal measures should assess the role of stressors, access and social networks on neighborhood differences, as well as racial/ethnic differences in provider-patient interactions, service utilization and HIV stigma and discrimination.
References
- Abraido-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: A test of the “salmon bias” and healthy migrant hypotheses. American Journal of Public Health. 1999;89(10):1543–1548. doi: 10.2105/ajph.89.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. Journal of Acquired Immune Deficiency Syndrome. 2001;28(1):47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- Boardman JD. Stress and physical health: the role of neighborhoods as mediating and moderating mechanisms. Social Science & Medicine. 2004;58(12):2473–2483. doi: 10.1016/j.socscimed.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . HIV/AIDS surveillance report. Department of Health and Human Services; 2001. [Google Scholar]
- Cummins S, Curtis S, Diez-Roux AV, Macintyre S. Understanding and representing ‘place’ in health research: A relational approach. Social Science & Medicine. 2007;65(9):1825–1838. doi: 10.1016/j.socscimed.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Curtis JR, Patrick DL. Race and survival time with AIDS: a synthesis of the literature. Am J Public Health. 1993;83(10):1425–1428. doi: 10.2105/ajph.83.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DP, Finch BK, Basurto-Davila R, Bird C, Escarce J, Lurie N. Does place explain racial health disparities? Quantifying the contribution of residential context to the Black/white health gap in the United States. Social Science & Medicine. 2008;67(8):1258–1268. doi: 10.1016/j.socscimed.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: the implications of psychological processes for understanding racial disparities in health and health care. Social Science & Medicine. 2008;67:478–486. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Ellen IG, Mijanovich T, Dillman K-N. Neighborhood effects on health: exploring the links and assessing the evidence. Journal of Urban Affairs. 2001;23(34):391–408. [Google Scholar]
- Ganz M. The relationship between external threats and smoking in central Harlem. American Journal of Public Health. 2000;90(3):367–371. doi: 10.2105/ajph.90.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore-Felton C, Koopman C. Behavioral mediation of the relationship between psychosocial factors and HIV disease progression. Psychosomatic Medicine. 2008;70(5):569–574. doi: 10.1097/PSY.0b013e318177353e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HI, McDavid K, Ling Q, Sloggett A. Determinants of progression to AIDS or death after HIV diagnosis, United States, 1996 to 2001. Annals of Epidemiology. 2006;16(11):824–833. doi: 10.1016/j.annepidem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Hsu LC, Vittinghoff E, Katz MH, Schwarcz SK. Predictors of use of highly active antiretroviral therapy (HAART) among persons with AIDS in San Francisco, 1996-1999. Journal of Acquired Immune Deficiency Syndrome. 2001;28(4):345–350. doi: 10.1097/00126334-200112010-00007. [DOI] [PubMed] [Google Scholar]
- Ironson G, Balbin E, Stieren E, Detz K, Fletcher MA, Schneiderman N, et al. Perceived stress and norepinephrine predict the effectiveness of response to protease inhibitors in HIV. International Journal of Behavioral Medicine. 2008;15(3):221–226. doi: 10.1080/10705500802219606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WD, Wong MD, Shapiro MF, Landon BE, Cunningham WE. Does racial concordance between HIV-positive patients and their physicians affect the time to receipt of protease inhibitors? J Gen Intern Med. 2004;19(11):1146–1153. doi: 10.1111/j.1525-1497.2004.30443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. Journal of Health and Social Behavior. 2005;46(1):15–31. doi: 10.1177/002214650504600103. [DOI] [PubMed] [Google Scholar]
- Lichtenstein B, Hook EW, Sharma AK. Public tolerance, private pain: stigma and sexually transmitted infections in the American Deep South. Culture, Health & Sexuality. 2008;7(1):43–57. doi: 10.1080/13691050412331271416. [DOI] [PubMed] [Google Scholar]
- Lopez A. Racial/ethnic diversity and residential segregation in the San Francisco Bay Area CCSRE Race and Ethnicity in California: Demographics report series. Center for Comparative Studies in Race and Ethnicity, Stanford University; Stanford, CA: 2001. [Google Scholar]
- Massey DS, Denton NA. American apartheid: Segregation and the making of the underclass. Harvard University Press; Cambridge, MA: 1993. [Google Scholar]
- McFarland W, Chen S, Hsu L, Schwarcz S, Katz M. Low socioeconomic status is associated with a higher rate of death in the era of highly active antiretroviral therapy, San Francisco. Journal of Acquired Immune Deficiency Syndrome. 2003;33(1):96–103. doi: 10.1097/00126334-200305010-00014. [DOI] [PubMed] [Google Scholar]
- Nash D, Katyal M, Shah S. Trends in predictors of death due to HIV-related causes among persons living with AIDS in New York City: 1993-2001. Journal of Urban Health. 2005;82(4):584–600. doi: 10.1093/jurban/jti123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ, Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. Journal of Acquired Immune Deficiency Syndrome. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Palloni A, Arias E. Paradox lost: Explaining the Hispanic adult mortality advantage. Demography. 2004;41(3):385–415. doi: 10.1353/dem.2004.0024. [DOI] [PubMed] [Google Scholar]
- Riva M, Gauvin L, Barnett TA. Toward the next generation of research into small area effects on health: a synthesis of multilevel investigations published since July 1998. Journal of Epidemiology and Community Health. 2007;61(10):853–861. doi: 10.1136/jech.2006.050740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert SA. Socioeconomic position and health: The independent contribution of community socioeconomic context. Annual Review of Sociology. 1999;25:489–516. [Google Scholar]
- San Francisco Department of Public Health . HIV/AIDS Statistics & Epidemiology Section. 2000. HIV/AIDS epidemiology annual report 1999. [Google Scholar]
- San Francisco Department of Public Health . HIV/AIDS Statistics & Epidemiology Section 146. 2003a. Atlas of HIV/AIDS in San Francisco 1981-2000. [Google Scholar]
- San Francisco Department of Public Health . HIV/AIDS Statistics & Epidemiology Section. 2003b. HIV/AIDS epidemiology annual report 2002. [Google Scholar]
- San Francisco Department of Public Health . HIV/AIDS Statistics & Epidemiology Section. 2008. HIV/AIDS epidemiology annual report 2007. [Google Scholar]
- San Francisco HIV Health Services Planning Council . San Francisco, CA eligible metropolitan area 2006-2009 comprehensive HIV health services plan. 2005. [Google Scholar]
- Schwarcz SK, Hsu LC, Parisi MK, Katz MH. The impact of the 1993 AIDS case definition on the completeness and timeliness of AIDS surveillance. AIDS. 1999;13(9):1109–1114. doi: 10.1097/00002030-199906180-00015. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau . Census 2000 Summary File 3. California: 2002. [Google Scholar]
- Wallace RG. AIDS in the HAART era: New York’s heterogeneous geography. Socal Science and Medicine. 2003;56(6):1155–1171. doi: 10.1016/s0277-9536(02)00121-1. [DOI] [PubMed] [Google Scholar]
- Williams DR, Williams-Morris R. Racism and mental health: the African American experience. Ethnicity & Health. 2000;5(34):243–268. doi: 10.1080/713667453. [DOI] [PubMed] [Google Scholar]
- Wingwood GM, DiClemente RJ, Mikhail I, McCree DH, Davies SL, Hardin JW, et al. HIV discrimination and the health of women living with HIV. Women & Health. 2007;46(23):99–112. doi: 10.1300/J013v46n02_07. [DOI] [PubMed] [Google Scholar]
- Wong MD, Cunningham WE, Shapiro MF, Andersen RM, Cleary PD, Duan N, et al. Disparities in HIV treatment and physician attitudes about delaying protease inhibitors for nonadherent patients. Journal of General Internal Medicine. 2004;19(4):366–374. doi: 10.1111/j.1525-1497.2004.30429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


