Abstract
Axon pathfinding by localized expression of guidance molecules is critical for the proper development of the nervous system. In this report, we present a well-defined spatially patterned gene expression system to investigate neurite guidance in vitro. Nonviral gene delivery was patterned by combining substrate-mediated gene delivery with soft lithography techniques, and the amount of protein produced at the region of localized expression was varied by altering the vector concentration and the width of the pattern, highlighting the flexibility of the system. A neuronal coculture model was used to investigate responses to spatial patterns of nerve growth factor (NGF) expression. The soluble NGF gradient elicited a guidance cue, and the degree of guidance was governed by the distance a neuron was cultured from the pattern and the time between accessory cell and neuron seedings. A portion of the diffusible NGF bound to the culture surface in the extracellular space, and the surface-associated NGF supported neuron survival and neurite outgrowth. However, the surface-bound NGF gradient alone did not elicit a guidance signal, and in fact masked the guidance cue by soluble NGF gradients. Mathematical modeling of NGF diffusion was used to predict the concentration gradients, and both the absolute and fractional gradients capable of guiding neurites produced by patterned gene expression differed substantially from the values obtained with existing engineered protein gradients. Spatially patterned gene expression provides a versatile tool to investigate the factors that may promote neurite guidance.
Keywords: gradient, guidance, neurotrophic factor, substrate mediated gene delivery, dorsal root ganglia (DRG)
Cellular migration to a desired target is essential for proper cell function during morphogenesis, maintenance, and wound repair (Singer and Kupfer, 1986). Directed migration of leukocytes (immune responses) (Devreotes and Zigmond, 1988), endothelial cells (angiogenesis) (Gillitzer and Goebeler, 2001), and fibroblasts (wound healing) (Postlethwaite and Seyer, 1991) result from gradients formed by localized gene expression of guidance molecules by cells positioned at a target. Similarly, the proper development and function of the nervous system is dependent on axon guidance to the intended synaptic target. Guidance cues expressed by target cells have been implicated in pathfinding of circumferential, commissural, and longitudinal axons in the spinal cord and brain (Serafini et al., 1996; MacLennan et al., 1997). The leading edge of an axon, the growth cone, is responsible for guidance cue detection in the extracellular environment and subsequently directs axons along a specific path (Song and Poo, 1999). Upon binding to growth cone surface receptors, guidance signals are transduced by cytoplasmic signaling pathways, leading to cytoskeletal rearrangement and directed growth (Song and Poo, 2001).
Axonal guidance molecules are permissive or inhibitory, and they exist as soluble factors that diffuse throughout the extracellular space (chemotactic) or non-diffusible factors presented by cells in the microenvironment or extracellular matrix (haptotactic). Groups of functionally specialized cells within target tissues, such as the midline (Colamarino and Tessier-Lavigne, 1995), express diffusible guidance molecules and concentration gradients arise from this localized gene expression. Although the importance of chemotaxis in the developing nervous system is widely accepted, the first gradient of a diffusible guidance factor, netrin-1, was only recently visualized directly in the embryonic chick, rat, and mouse spinal cords (Kennedy et al., 2006). Nondiffusible factors such as proteoglycans (Snow et al., 1991) or cell adhesion molecules (Cohen et al., 1998) are differentially expressed by neuronal and nonneuronal cells and establish a path for axons. Importantly, diffusible factors may also associate with the tissue through ligand binding or nonspecific interactions and elicit a haptotactic signal (Deiner et al., 1997).
Because gradients are difficult to visualize in vivo and neuronal responses are complex, in vitro assays have been developed to investigate neurite guidance. In vitro gradient cultures have led to the discovery of cell-surface receptors on growth cones responsible for gradient recognition (Ming et al., 1999) and partial understanding of the intracellular events that lead to cytoskeletal rearrangement of the neurite shaft and oriented growth (Dent and Gertler, 2003). However, existing in vitro assays have specific drawbacks. Cocultures consisting of neurons and either target tissue (Chamley et al., 1973) or a cluster of cells expressing recombinant guidance molecules (Kennedy et al., 1994) aim to recapitulate natural gradients. However, the systems are variable, and the gradients are not well defined. In contrast, engineered protein gradients within agarose are well defined (Cao and Shoichet, 2001), but gradients formed by source/sink chambers may not adequately represent gradients formed by localized expression of diffusible molecules. Moreover, most in vitro guidance assays are acellular (Gundersen and Barrett, 1979), incorporating only the neuron of interest, and the ability of diffusible molecules to associate with nonneuronal cells and their extracellular matrix is often ignored (Heron et al., 2007).
In this report, we use a novel, well-characterized patterned gene delivery system to create tunable concentration gradients and investigate directed neurite extension. We hypothesized that gradients formed by spatially controlled delivery of genes encoding guidance molecules within a complex cellular environment would better mimic gradients that are formed naturally. The ability of diffusible chemotactic factors to bind to the cell culture surface and the subsequent effects on neurite guidance were investigated. Experimentally observed neurite guidance was correlated to predicted concentration gradients, which facilitated comparison to gradients fabricated by existing guidance assays.
MATERIALS AND METHODS
Plasmids and Reagents
Plasmid was purified from bacterial culture with Qiagen (Santa Clara, CA) reagents and stored in Tris-EDTA buffer at −20°C. The plasmid pEGFP-Luc has EGFP (enhanced green fluorescent protein) in the vector backbone with a cytomegalovirus promoter and was purchased from Clontech (Mountain View, CA). The plasmid pNGF has full-length mouse nerve growth factor (NGF) in the RK5 vector backbone with a cytomegalovirus promoter and was a gift from Dr. Hiroshi Nomoto (Gifu Pharmaceutical University, Japan). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and media components from Invitrogen (Carlsbad, CA) unless otherwise specified.
HEK293T Cell Culture
The human embryonic kidney cell line HEK293T was purchased from the American Type Culture Collection (ATCC, Manassas, VA). HEK293T cells were maintained in T-75 flasks with media change every 48 hr and passage every 60 hr in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin, and 1% sodium pyruvate at 37°C and 5% CO2. For all assays, tissue culture polystyrene wells were precoated with poly-L-lysine (MW 30,000–70,000) by incubating a 0.01% solution in wells for 1 hr.
Patterned Reporter Gene Expression
Microfluidic networks were fabricated following methods described previously (Houchin-Ray et al., 2007). Briefly, photolithography techniques were used to fabricate topographically patterned molds. Polydimethylsiloxane, also referred to as Dow Corning Sylgard 184 Elastomer (a gift from Dow Corning Corporation, Midland, MI) was cured on the patterned molds, and after curing, ports were punched at both ends of the channels to fabricate microchannels with dimensions of 1.0, 0.5, or 0.25 mm wide × 10 mm long × 0.25 mm high. Microchannels were soaked for 1 hr in a 0.2% Pluronic L35 (a gift from BASF Corporation; Mount Olive, NJ) solution, rinsed, and dried in a sterile hood. To form lipoplexes, plasmid (pEGFP-Luc) in DMEM was complexed with Lipofectamine 2000 in DMEM (DNA:lipid 1:1 and vector concentration 2–10 ng/μl) by adding lipid to DNA, pipetting gently, and incubating 10 min. To pattern lipoplex deposition, microchannels were reversibly sealed to poly-L-lysine- coated wells, and complexes were injected in the microchannels. After 1 hr of deposition, complexes were removed from the microchannels, and the wells were rinsed with phosphate-buffered saline (PBS). HEK293T cells (4 × 104 cells/cm2) were seeded in wells with patterned lipoplexes and cultured for 48 hr under the conditions described above. Luciferase transgene levels were measured with the Luciferase Assay System (Promega, Madison, WI). After 48 hr of culture, cells were lysed and assayed with a luminometer (Turner Bio-systems) set for a 3-sec delay with signal integration for 10 sec. A standard curve was formed by using Luciferase standards and linear regression analysis to convert relative light units of each sample to moles. Transfection efficiency was defined as the number of transfected cells divided by the total number of cells. To determine total number of cells, cultures were stained with 5 μg/ml Hoechst 33258 (Molecular Probes, Eugene, OR). Cells producing the EGFP (green) and cell nuclei (blue) were imaged on a Leica inverted fluorescence microscope with a cooled CCD camera (Photometrics, Tucson, AZ) by MetaVue (Universal Imaging, Downingtown, PA) acquisition software. The number of transfected cells was determined by counting green positive cells in five random images per pattern, and the total number of cells in each image was established by counting cell nuclei. Production rate (p) of luciferase was calculated for each condition on the basis of luciferase levels and transfection efficiency, and reported in units of pmol/cell/min.
Patterned NGF Expression
Patterned NGF expression was achieved with methods described above and cultures were assessed to determine 1) total NGF secreted by transfected cells and 2) the location of secreted NGF (diffusible vs. surface associated). Lipoplexes were formed with a 7:3 mixture of pNGF (neurotrophic factor of interest) and pEGFP-Luc (to visualize the pattern) and a vector concentration of 10 ng/μl. Lipoplexes were injected into Pluronic-treated microchannels (1.0 mm width) and allowed to deposit for 1 hr. HEK293T cells were seeded as above and cultured for 24 or 48 hr. At the end of the culture period, the culture media and lysates were analyzed for NGF concentration with a ChemiKine NGF Sandwich enzyme-linked immunosorbent assay (ELISA) kit (Millipore, Billerica, MA). Cell lysates were prepared by incubating cultures in reporter lysis buffer (Promega) with protease inhibitors (aprotinin, antipain, benzamidine, and pepstatin A) for 20 min at −80°C. To assess surface-associated NGF, patterned NGF expression cultures were performed as described above. At the end of the culture period, HEK293T cells were fixed with 2% paraformaldehyde in PBS for 10 min. HEK293T cells were stained for NGF with anti-NGF-β (Millipore) and avidin–biotin complex–horseradish peroxidase immunostain techniques (Vector Labs; Burlingame, CA). After a 30-sec development, cells were washed with PBS and imaged with a Leica DM IL light microscope (Leica; Wetzlar, Germany) equipped with a Spot Insight 2 Megapixel Color Mosaic camera and Spot software (Spot Diagnostic Instruments; Sterling Heights, MI). Images were analyzed for immunostain intensity by Adobe Photoshop with a color threshold set on the basis of the negative control and normalized to the entire surface area occupied by cells.
Neurite Outgrowth and Guidance in Response to Patterned NGF Expression
A neuronal coculture model was used to investigate neurite outgrowth in response to spatial patterns of NGF expression. Patterned NGF expression cultures were performed as described above. At the end of the culture period (9, 24, or 48 hr), HEK293T cells were either rinsed with PBS (referred to as 9-hr, 24-hr, and 48-hr cultures) or fixed with 2% paraformaldehyde for 10 min to halt the secretion of diffusible NGF (referred to as 9-hr, 24-hr, and 48-hr surface NGF). Fixed HEK293T cultures were assessed for HEK293T survival with calcein AM and ethidium homodimer-1 stain. Additionally, 9-, 24-, and 48-hr surface NGF cultures were cultured for 24 hr after fixing, and the medium was assessed for the presence of soluble NGF with a ChemiKine NGF Sandwich ELISA kit (Millipore), to ensure that the production of soluble NGF was completely halted.
After HEK293T, cultures were either rinsed (9-hr, 24-hr, and 48-hr cultures) or fixed (9-hr, 24-hr, and 48-hr surface NGF), dorsal root ganglia (DRG) explants were isolated from 8-day chicken embryos (MI State University; East Lansing, MI) and seeded on live or fixed HEK293T cultures 0.5–2.5 mm from the center of the pattern of NGF expression (2 explants/well) by pipetting under a dissecting microscope. To assess the response by individual neurons, DRG explants were dissociated by using methods described previously (Houchin-Ray et al., 2007). Briefly, DRG explants were incubated for 30 minutes at 37°C in 0.25% trypsin (Worthington Biochemical; Lakewood, NJ), followed by trituration with fire-polished glass Pasteur pipettes to dissociate the ganglia. Nonneuronal and neuronal cells were separated by panning for 2 hr at 37°C. Neurons were seeded on HEK293T cultures (1 × 104 cells/cm2). At 24 hr after explant or dissociated neuron seeding, cultures were stained for the neuron-specific class III β-tubulin by incubating fixed cells in TUJ1 antibody (Covance, Berkely, CA) diluted in 5% normal goat serum (Vector Labs; Burlingame, CA) in PBS for 1 hr followed by incubation in tetrarhodamine isothiocyanate (TRITC)-conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch; West Grove, PA) in PBS for 30 min. Cocultures were imaged on a Leica inverted fluorescence microscope with a cooled CCD camera (Photometrics) by MetaVue (Universal Imaging) acquisition software. The distance an explant or dissociated neuron was located in relation to the pattern of expression was determined with the calipers function in MetaVue to measure the distance between the neuron cell body and the center of the pattern of expression (as determined by EGFP expression).
Neuronal responses to patterned NGF expression were evaluated in terms of neurite length in relation to the pattern of expression (explants) and neurite orientation in relation to the pattern of expression (dissociated neurons). To assay neurite length from explants, the explant was first divided in half along the vertical axis, parallel to the pattern of expression edge. Neurites extending from the half of the explant closest to the pattern edge were defined as toward the pattern of NGF expression, and neurites extending from the half of the explant farthest from the pattern edge were defined as away from the pattern. The tracing algorithm in the NeuronJ plugin for ImageJ (Meijering et al., 2004) was used to determine neurite length. At least 10 neurite lengths were measured for each explant half, averaged, and reported as a ratio of the two averages (Ltoward/Laway). To assay neurite orientation from individual neurons, the neuron cell body was halved similar to the procedure used for explants. The location at which the neurite initiated was first determined. Neurites extending from the half of the explant farthest from the pattern edge were used in the quantification of guidance because neurite guidance is defined as the redirection of neurites. The directional angle of the neurite relative to the pattern of expression was quantified by first drawing two lines: one line was drawn from the neuron cell body perpendicular to the pattern of expression edge, and the second line was drawn from the neuron cell soma to the neurite growth cone. An angle between the two lines was quantified and categorized in 30° increments. The values 0–90° or −(0–90°) define the two quadrants in the direction toward the pattern of expression, while neurites oriented 90–180° or −(90–180°) define the two quadrants in the opposite direction. Positive and negative quadrants were defined as symmetrical and equivalent and therefore were reported as only positive angles.
Mathematical Modeling of Concentration Gradients
Mathematical modeling of NGF diffusion was used to predict the concentration profile in the culture well. Equation 1 describes one-component diffusion in three dimensions in a continuous medium with a term for protein degradation:
| (1) |
where C is the concentration, D is the diffusivity of the protein, and k is the rate constant for protein degradation. The value for the diffusivity of NGF was obtained from published reports (D = 12 × 10−7 cm2/sec) (Stroh et al., 2003), and was used in Equation 2 to calculate the effective diffusivity that incorporated reversible binding of the ligand to the accessory cell surface:
| (2) |
where De is the effective diffusion constant and R is a dimensionless coefficient, calculated by the equation S = RC, where S is the amount of ligand bound to the surface and C is the amount of soluble NGF (Crank, 1975). The values of S and C were calculated from NGF ELISA data. The rate constant for protein degradation, k, was determined from the reported half-life of NGF (t½ = 4.5 hr) (Tria et al., 1994), (k = 0.0029 min−1).
The Crank-Nicolson implicit method was used to solve numerically the partial differential equation. The initial condition is a zero concentration throughout the culture (Equation 3).
| (3) |
The boundary conditions indicate a flux (q), within the pattern of transfected cells (Equation 4), and no flux boundary conditions elsewhere.
| (4) |
The flux, (q), was determined from the protein production rate (p) (pmol/cell/min), which was calculated from NGF ELISA data (pmol/min) while accounting for protein degradation and transfection efficiency within the region of patterned gene delivery (total number of transfected cells). The value q was calculated in terms of pmol/cm3/min on the basis of the assumption that the volume occupied by a transfected cell was 1,000 μm3. Note that the region of patterned transfection occurs within x = 0 to xpatt and y = 0 to ypatt. The boundaries in the x-direction (xmax) and y-direction (ymax) were defined as 12 mm, the approximate radius of a 12-well tissue culture well. The boundary in the z-direction (zmax) was set equal to 4 mm, the approximate height of the culture medium.
Concentration gradient analysis was performed in terms of the absolute concentration gradient (Equation 5) and the fractional concentration gradient (Equation 6):
| (5) |
| (6) |
Statistics
Statistical analysis was performed by JMP software (SAS Institute, Cary, NC). Comparative analyses were executed by one-way analysis of variance with Tukey post tests at a 95% confidence level. χ2 analysis was used to analyze categorical data. For all cocultures, the sample set size was n > 20 for explant experiments and n > 200 for neuron experiments for each condition analyzed.
RESULTS
Spatial Patterns of Reporter Gene Expression
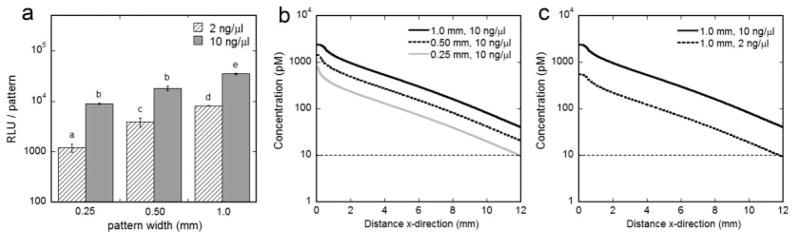
Previously developed techniques combining soft lithography and substrate-mediated gene delivery (Houchin-Ray et al., 2007) were used to pattern expression of the reporter gene luciferase, while varying pattern width (0.25–1 mm) and vector concentration (2–10 ng/μl). Relative protein expression levels increased with increasing pattern width and vector concentration (Fig. 1a). The corresponding protein production rates ranged from 6.0 × 10−8 pmol/cell/min (0.25 mm, 2 ng/μl) to 4.4 × 10−7 pmol/cell/min (1 mm, 10 ng/μl). According to the model prediction, the ligand concentration remains above zero for the longest distance with a pattern width of 1.0 mm (Fig. 1b) and a vector concentration of 10 ng/μl (Fig. 1c). This condition was used throughout for analysis of NGF production, binding, and neuronal response because it enables neurite guidance to be investigated over distances on the order of millimeters.
Fig. 1.
Spatially patterned gene expression. Quantification of luciferase transgene expression from patterned gene delivery, while varying vector concentration (2–10 ng/μl) and channel width (0.25–1.0 mm) (a). Values are reported as mean ± SEM, and statistically different values are marked by different letters (P < 0.05). Predicted concentration gradients from patterns of gene delivery (b,c). Note that x = 0 is the center of the pattern of expression. All data are plotted on a log scale.
Spatial Patterns of NGF Expression
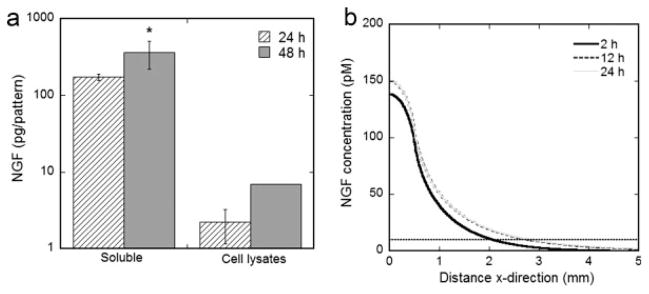
Both the amount of NGF secreted by transfected cells and the distribution of NGF were quantified in order to accurately predict NGF concentration profiles. The amount of soluble NGF in the culture medium was 173 and 364 pg/pattern for 24- and 48-hr cultures, respectively (Fig. 2a). Additional NGF was detected in the cell lysates and was 2.2 and 6.9 pg/pattern for 24-and 48-hr cultures (Fig. 2a). These results were used to calculate the NGF production rate (p = 4.2 × 10−10 pmol/cell/min) and effective diffusivity (De, NGF = 11.7 × 10−7 cm2/sec), which were inserted into the mathematical model to predict NGF concentration gradients from localized expression. The predicted NGF concentration profile indicates the NGF concentration was below the approximate maximum concentration for gradient detection (10–100 nM, 100 times the dissociation constant of the trkA and p75 receptors) (Tessier-Lavigne and Placzek, 1991; Goodhill, 1997) for all distances and times and remained above the estimated minimum concentration for gradient detection (1–10 pM, 1% the dissociation constant of the trkA and p75 receptors, 10 pM is marked by the dashed line) (Zigmond, 1981; Good-hill, 1997) at x < 2.0 mm for 2 hr, and x < 2.8 mm for 12 hr and 24 hr (Fig. 2b). The time points used in the mathematical model coincided with critical time points in the coculture experiments, where t = 0 hr corresponded with the time of neuron cell seeding in which the culture medium was exchanged and the soluble NGF concentration is CNGF = 0 pmol/ml, and t = 24 hr corresponded with the time of experimental assay (Fig. 3).
Fig. 2.
NGF expression by spatially patterned gene delivery. Quantification of NGF production at 24 and 48 hr after HEK293T seeding on patterned pNGF lipoplex deposition, plotted on a log scale (a). NGF was detected both soluble in the culture medium and associated with cell lysates. Values are reported as mean ± SEM. *Statistically different at P < 0.05. Mathematical model predictions for NGF concentration profiles at 2, 12, and 24 hr (b). Note that x = 0 is the center of the pattern of expression.
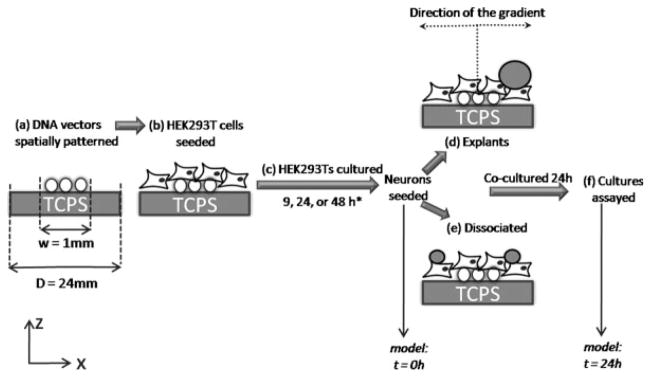
Fig. 3.
Schematic of the coculture system. A schematic outlining the steps involved in coculturing DRG neurons on spatially patterned gene expression. DNA vectors encoding NGF were deposited to specific regions of a tissue culture polystyrene (TCPS) surface (a). HEK293T cells were seeded onto the tissue culture surface with immobilized DNA vectors (b) and cultured for a range of times (9, 24, and 48 hr) (c). *For surface-associated NGF cultures, HEK293T cells were fixed at the end of the culture period to halt NGF secretion. DRG neurons were seeded on top of the HEK293T cultures as entire explants (d) or dissociated neurons (e) (marking the t = 0 hr time point for the mathematical model) and cultured for 24 hr. At the end of the culture period (marking the t = 24 hr time point for the mathematical model), the cocultures were fixed and assayed for neuronal response (f). It is important to note that the schematic only represents the x- and z-planes, but the actual cultures involve a y-dimension, in which the length of the pattern of expression is y = 10 mm. Schematic is not drawn to scale.
Neurite Outgrowth and Guidance by Spatial Patterns of NGF Expression
The ability of spatial patterns of NGF expression to induce neurite outgrowth and guidance was investigated by coculturing DRG explants or dissociated DRG neurons on HEK293T cells with patterned NGF expression, while varying the time between HEK293T cell seeding and DRG seeding (9, 24, and 48 hr), referred to hereafter as 9-hr, 24-hr, and 48-hr cultures (Fig. 3). A minimum time of 9 hr was investigated in order to ensure that transfected cells were producing and secreting NGF at the time the explants or dissociated neurons were seeded. It is important to note that we have previously shown that HEK293T cells alone, without pNGF transfection, do not support neuron survival or neurite extension (Houchin-Ray et al., 2007). The HEK293T cell line was therefore chosen for these studies because the neuronal responses observed can be directly attributed to patterns of NGF expression.
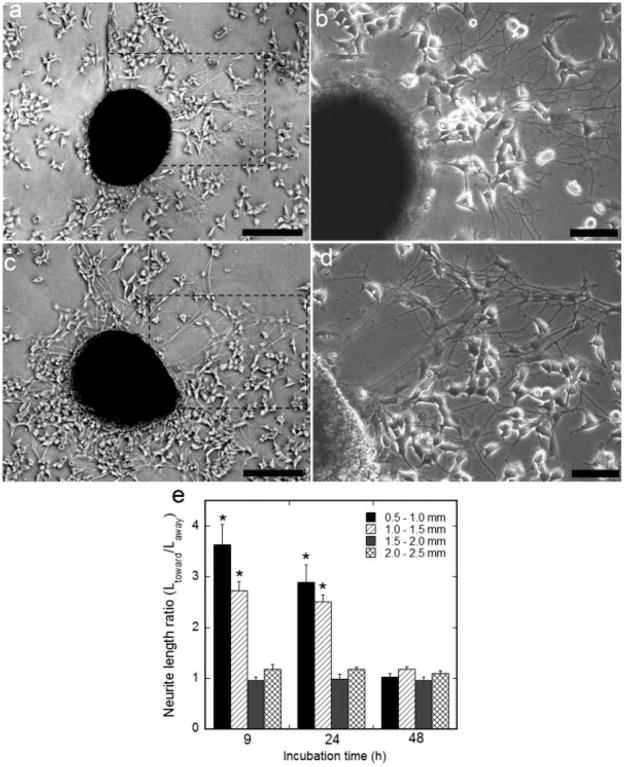
DRG explants seeded 0.5–2.5 mm outside the center of NGF expression on 9-hr, 24-hr, and 48-hr cultures exhibited neuron survival and neurite outgrowth. DRG explants seeded on 9-hr and 24-hr cultures exhibited longer neurite lengths toward patterns of NGF expression (Fig. 4a–d). Quantitative evaluation of neurite length indicated a higher ratio of neurite length toward vs. away from the pattern of expression for 9 hr (3.6 ± 0.4) and 24 hr (2.9 ± 0.3) cultures, compared with 48 hr (Fig. 4e, P < 0.05) for explants seeded 0.5–1.0 mm from the pattern center. Importantly, neurite length was also dependent on the distance from the pattern of expression the explant was cultured. A higher ratio of neurite length toward the pattern of expression vs. neurite length away from the pattern of expression was observed with explants seeded 1.0–1.5 mm from the pattern center on 9 hr (2.7 ± 0.2) and 24 hr (2.5 ± 0.1) cultures, with a significant decrease in neurite length ratio when explants were seeded 1.5–2.5 mm from the pattern center (Fig. 4e, P < 0.05). Greater neurite length toward the pattern of expression may suggest the presence of a guidance cue because neurites directed by an extracellular guidance factor make few navigational errors and rarely pause (Dent and Gertler, 2003). However, to investigate further the presence of a guidance cue, neurite orientation from dissociated DRG neurons was investigated.
Fig. 4.
Neurite outgrowth from explants seeded on patterned NGF expression. DRG explants were cocultured on patterned NGF expression. Neurites extending toward the pattern of NGF expression exhibited longer lengths compared with neurites extending away from the pattern, with explants cultured 9 hr (a,b) and 24 hr (c,d) after HEK293T seeding. The pattern of expression is to the right of the cultured explant (a,c), and a zoom panel for each explant displays the neurites in detail (b,d). Quantification of neurite length, represented as the ratio of the average neurite length toward vs. away from the pattern of expression, while varying the incubation time between cell seedings (9, 24, and 48 hr) and the distance from the pattern center (e). Values are reported as mean ± SEM. *Statistically different at P < 0.05. Scale bars = 250 μm in a,c; 100 μm in b,d.
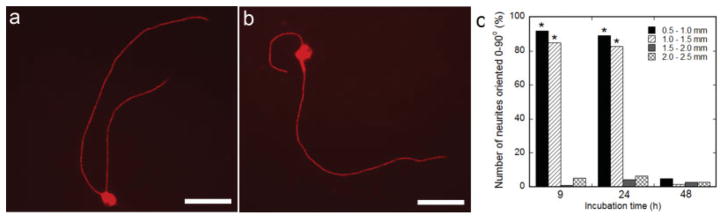
Dissociated DRG neurons seeded 0.5–2.5 mm outside the center of NGF expression on 9-hr, 24-hr, and 48-hr cultures exhibited neuron survival and neurite outgrowth. The percentage of neurites that initiated from the neuron and toward the pattern was not significantly different than 50% in all conditions (data not shown). Dissociated neurons seeded on 9-hr and 24-hr cultures exhibited neurite guidance toward the pattern of NGF expression (Fig. 5a,b). Quantitative analysis of neurite guidance was performed by evaluating neurite orientation relative to the pattern of expression for neurites that had initiated away from the pattern of expression, which would require a turning response by the axons. Neurons seeded on 9-hr and 24-hr cultures extended a significantly higher percent of neurites 0–90° relative to the pattern of expression (92% and 89%, respectively) compared with 48-hr cultures (5%) (Fig. 5c, P < 0.05), when neurons were positioned 0.5– 1.0 mm from the pattern of expression. A higher percentage of neurites extended 0–90° relative to the pattern of expression for neurons seeded on 9-hr (85%) and 24-hr (83%) cultures with neurons 1.0–1.5 mm from the pattern center, with a significant decrease in percentage of neurites 0–90° relative to the pattern of expression when neurons were located 1.5–2.5 mm from the pattern center (Fig. 5c, P < 0.05).
Fig. 5.
Neurite orientation from dissociated neurons seeded on patterned NGF expression. Dissociated DRG neurons were cocultured on patterned NGF expression. Neurites initiated away from the pattern of NGF expression exhibited guidance toward the pattern, with DRGs cultured 9 hr (a) and 24 hr (b) after HEK293T seeding (neurons and neurites were stained red with TRITC-labeled secondary antibody). The pattern of expression is to the right of the neuron (a,b). Quantification of neurite orientation, represented as the orientation angle in relation to the pattern while varying the incubation time between cell seedings (9, 24, and 48 hr) and the distance from the pattern center (c). Values are reported as mean ± SEM. *Statistically significantly different at P < 0.05. Scale bars = 50 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Characterization of Surface-associated NGF
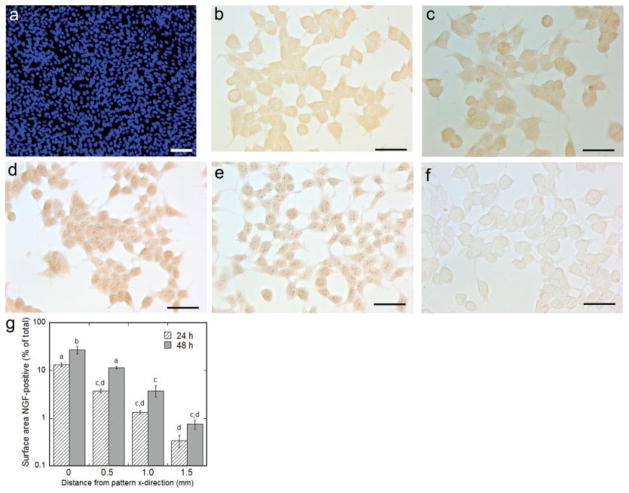
NGF has been shown to nonspecifically associate with the extracellular matrix and cell surface proteoglycans, and this surface-associated NGF may contribute to the different neuronal responses observed for the range of accessory cell culture times. We subsequently investigated whether NGF associated with the accessory cell surface and/or extracellular matrix in the coculture model. It is important to note that HEK293T cells were confluent before immunocytochemistry (ICC), as determined by Hoechst staining of live cells (Fig. 6a), and the representative images of ICC do not reflect the cell confluency as a result of cell loss during ICC. Immunocytochemistry revealed that NGF was localized to the surface at the center of patterned expression (Fig. 6b,d) 0.5 mm from the pattern center (Fig. 6c,e) and 1.5 mm from the pattern center (not shown) for 24- and 48-hr accessory cell cultures compared with the negative control (no pNGF) (Fig. 6f). Quantification of immunostain intensity indicated the percentage of NGF-positive surface area decreased with increasing distance from the pattern (Fig. 6g, P < 0.05). Additionally, the percentage of NGF-positive surface area was higher after 48-hr accessory cell culture compared with 24 hr (Fig. 6g, P < 0.05).
Fig. 6.
Immunocytochemistry for the detection of surface-associated NGF. The accessory cell culture was confluent before ICC (a). The accessory cell culture surface stains positive for NGF at the site of patterned gene delivery for 24 and 48 hr culture, respectively (b,d), and 0.5 mm from the center of the pattern for 24 and 48 hr culture, respectively (c,e), compared with negative control (no pNGF delivery) (f). Quantification of immunostain intensity vs. distance from the pattern center, plotted on a log scale (g). Values are reported as mean ± SEM. *Statistically significantly different at P < 0.05. Scale bars = 100 μm in a; 50 μm in b–e. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Neurite Outgrowth and Guidance by Surface-associated NGF
The role of surface-associated NGF in promoting and directing neurite outgrowth from patterned NGF expression was investigated by coculturing neurons on pNGF transfected accessory cells that had been fixed (Fig. 3). Fixation halts NGF production and removes soluble NGF while retaining NGF in an active form that is associated with the surface. Live/dead assay of fixed HEK293T cells confirmed the fixing procedure rendered all HEK293T cells dead (data not shown). Additionally, analysis of the culture medium after HEK293T fixation and 24-hr culture revealed no traces of soluble NGF (data not shown), indicating that neuronal responses observed on the surface NGF cultures were a result of surface-associated NGF. DRG explants or dissociated neurons were cultured on patterned NGF expression with fixed accessory cells 9, 24, and 48 hr after HEK293T seeding. The cocultures on fixed accessory cells are referred to hereafter as 9-hr, 24-hr, and 48-hr surface NGF.
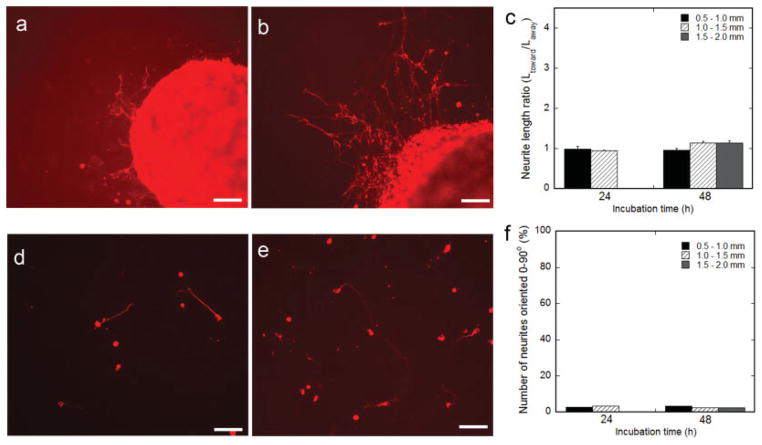
DRG explant survival was dependent on the time between cell seeding and the distance an explant was cultured from the pattern center. Explants did not survive when seeded on 9-hr surface NGF. Explants survived when seeded 0–1.5 mm from the pattern center on 24-hr surface NGF, and 0–2 mm from the pattern center on the 48-hr surface NGF (Fig. 7a,b). Surface NGF cultures supported neurite extension, but the neurite length did not vary with position in relation to the pattern of expression. The ratio of neurite length toward vs. away from the pattern of expression on 24-hr and 48-hr surface NGF was statistically 1 in all conditions (Fig. 7c).
Fig. 7.
Neurite outgrowth and guidance by surface-associated NGF. DRG explants (a–c) or dissociated DRG neurons (d–f) were cocultured on patterned NGF expression directly after HEK293T fixation (to halt production of NGF) at 24 hr (a,d) and 48 hr (b,e) after HEK293T seeding (neurons and neurites were stained red with TRITC-labeled secondary antibody). Quantification of neurite length, represented as the ratio of the average neurite length toward vs. away from the pattern of expression (c), and neurite orientation (f), while varying the incubation time between cell seedings (9, 24, and 48 hr) and the distance from the pattern center (0.5–2.0 mm). Values are reported as mean ± SEM. Scale bars = 100 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Neuron survival and neurite outgrowth from dissociated DRG neurons were also supported by 24-hr and 48-hr surface NGF cultures (Fig. 7d,e). Similar to explants, dissociated neurons survived when seeded 0–1.5 mm from the pattern center on 24-hr surface NGF and 0–2 mm from the pattern center on the 48-hr surface NGF. The percentage of neurites initiated toward the pattern was not significantly different than 50% in all conditions (data not shown). Dissociated DRG neurons did not exhibit neurite guidance when seeded on 24-hr and 48-hr surface NGF. In all conditions, less than 5% of neurites that had initiated away from the pattern of expression were oriented 0–90° relative to the pattern (Fig. 7f). Importantly, 9-hr, 24-hr, and 48-hr surface NGF cultures supplemented with soluble NGF in the culture medium did not demonstrate directed neurite outgrowth from dissociated neurons (data not shown).
DISCUSSION
In this article, we present a well-defined system of patterned gene expression to investigate neurite guidance in response to concentration gradients formed by localized secretion of NGF. The protein production rate and the resulting concentration gradient were varied by altering pattern width and vector concentration. Patterned gene expression guided neurite outgrowth, with guidance dependent on the distance a neuron was cultured from the pattern and the time between cell seedings. Portions of NGF secreted from patterned expression nonspecifically associated with the surface, which supported neuron survival and neurite outgrowth. Surface-associated NGF alone, however, did not elicit a guidance signal. This is the first report that defines concentration gradients produced by spatial patterns of gene expression necessary for successful neurite guidance. Moreover, these results demonstrate the importance of considering diffusible factor binding in the characterization of gradients that guide neurites. These findings underscore the broad impact a patterned gene expression system may have on the investigation of guidance cues in many tissue development scenarios, as well as in the regeneration of tissues with complex architectures.
The distance a neuron was cultured from the pattern of expression influenced neurite guidance for 9-hr and 24-hr cultures. Guidance was observed when neurons were cultured 0.5–1.5 mm from the center of patterned NGF expression. On the basis of knowledge for bacteria and leukocyte chemotaxis (Devreotes and Zigmond, 1988), it has been suggested that neurite guidance by chemotactic mechanisms is governed by the mean chemoattractant concentration (Goodhill, 1997). If the concentration drops below 1% of the dissociation constant, few of the receptors will be bound at any given time, yielding little difference in binding and the inability of the growth cone to detect the gradient (Goodhill, 1997). The dissociation constant of NGF is 1 nM for p75 and 0.1 nM for trkA (Tse et al., 2007); therefore, the minimal concentration of NGF required for growth cone gradient detection is between 1 and 10 pM. The mathematical model predicts a total concentration of NGF dropped below 10 pM at x = 1.5 mm at t = 2 hr, and maintained concentrations close to the minimal concentration at x = 1.5 mm (C = 39 pM at t = 12 hr, and C = 62 pM at t = 24 hr) for the 24-hr time course. This approach to a minimal NGF concentration for guidance detection may partially explain the correlation between guidance and distance.
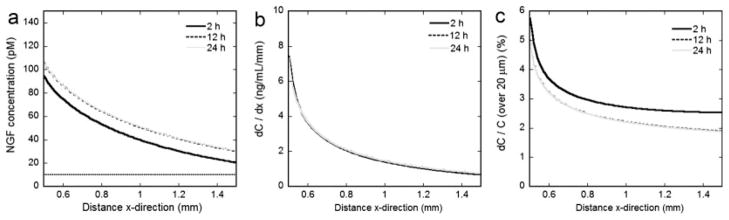
The predicted concentration profiles (Fig. 8a) were subsequently used to calculate the absolute and fractional concentration gradients, both of which have been implicated in neurite guidance for the regions in which guidance was observed (x = 0.5–1.5 mm). The absolute concentration gradient ranged from 7.5 to 0.7 ng/ml/mm at x = 0.5–1.5 mm over the 24-hr time course (Fig. 8b). The fractional concentration gradient over the width of the growth cone (~20 μm) between x = 0.5–1.5 mm ranged 6.0–2.5%, 5.0–2.0%, and 5.0–2.0% for 2, 12, and 24 hr, respectively (Fig. 8c).
Fig. 8.
Predicted NGF concentration gradients. The predicted concentration profiles (a), absolute concentration gradients (b), and fractional concentration gradients (c) within the region of observed neurite guidance (x = 0.5–1.5 mm) for t = 2, 12, and 24 hr.
The absolute and fractional concentration gradients formed by patterned gene expression were discrepant with what has been previously shown to guide neurites by gradients engineered with purified proteins. The absolute concentration gradient produced by patterned expression (7.5–0.7 ng/ml/mm) was well below the suggested requirement for neurite guidance with engineered NGF gradients in agarose (133 ng/ml/mm) (Cao and Shoichet, 2003). However, it has been suggested that the critical chemorepulsive information to pathfinding Ti1 pioneer growth cones within the developing grasshopper limb is governed by the Sema 2a fractional gradient and not the absolute gradient (Isbister et al., 2003). Fractional gradients produced by patterned expression were primarily steeper (6.0–2.0%) than engineered protein gradients that have previously been shown to guide neurites (3.3, 0.80, and 0.45%) (Cao and Shoichet, 2001). Interestingly, these findings coincide with observations from leukocyte chemotaxis that lower mean concentrations require steeper fractional gradients to elicit a guidance cue (Devreotes and Zigmond, 1988).
The soluble NGF gradients from patterned expression provided a guidance signal for neurite outgrowth, while surface-associated NGF contributed to neuron survival and neurite outgrowth, but did not direct extension. Noteworthy is that the guidance cue by soluble NGF gradients was abolished when neurons were seeded 48 hr after accessory cells. Because the culture medium was exchanged before neurons were seeded and NGF production rates were similar for the 48-hr period, the predicted soluble NGF gradients were similar for 9 hr, 24 hr, and 48 hr cultures, and therefore, the lack of a guidance signal could not be explained by differences in soluble gradients. As a result, the ability of NGF to associate with the surface, and in turn, influence neurite outgrowth and guidance was investigated. Surface-associated NGF after 24 and 48 hr accessory cell cultures supported neuron survival and neurite outgrowth. Importantly, surface-associated NGF alone did not elicit a guidance cue. Previously, immobilized NGF within poly(2-hydroxyethylmethacrylate) gels guided neurites (Moore et al., 2006). Predictions for neurite guidance by haptotactic mechanisms suggest a need for steeper concentration gradients when chemotactic factors are adsorbed to a substrate (Goodhill and Urbach, 1999). The surface bound NGF gradients formed by diffusion from localized expression and association with the surface were not sufficient to elicit a haptotactic guidance signal. Moreover, the presentation of NGF after adsorption to the surface may differ from NGF encapsulated within synthetic hydrogels, and in turn may alter the haptotactic capability of immobilized NGF. Because surface-associated NGF alone did not elicit a guidance signal, and the amount of bound NGF was significantly higher after 48 hr of accessory cell culture compared with 24 hr, surface-associated NGF may contribute to the disappearance of the guidance signal during the 48-hr culture. The relatively high surface concentration in the 48-hr culture may mask the soluble concentration gradient, rendering the soluble NGF gradient ineffective at guiding neurites.
The binding of diffusible factors to the extracellular matrix has been implicated in cell migration and differentiation during morphogenesis (The et al., 1999), with heparin-binding growth factors binding to ECM proteoglycans (Ramirez and Rifkin, 2003). Although NGF has a low affinity for heparan sulfate, the incorporation of NGF into a hydrogel loaded with heparin reduced the ligand diffusion rate and slowed release (Sakiyama-Elbert and Hubbell, 2000), suggesting that NGF may interact with accessory cell proteoglycans in the patterned expression system presented here. Furthermore, NGF is a sticky protein and has been shown to nonspecifically adsorb to various surfaces (Pearce et al., 1973). Previously, NGF bound to nitrocellulose paper (Pettmann et al., 1988) or adsorbed to poly-L-glutamate surfaces (Gundersen, 1985) supported neuron survival and neurite extension, substantiating that nonspecifically adsorbed NGF retains activity.
Patterned gene delivery may be useful in repairing damaged nerves, as axon guidance across a lesion and to a target is essential for functional recovery. Nerve guidance bridges have been designed to present topographical guidance cues (Yang et al., 2005), and have shown promise in both peripheral nervous system and central nervous system injury models (Xu et al., 1999; Hadlock et al., 2000). The central nervous system, however, is not a conducive environment for regeneration, and at the onset of injury, is plagued by widespread changes in gene expression, many of which involve guidance cues and their receptors (Curinga and Smith, 2008). Spatially patterned gene delivery within spinal cord bridges provides a novel method to alter gene expression at the injury site and present guidance signals over an extended period of time. Cells genetically modified to express neurotrophic factors transplanted at a spinal injury site stimulated axonal outgrowth and supported functional recovery after injury (Mitsui et al., 2005), while injections of viral vectors encoding NGF stimulated axon outgrowth and navigation from transplanted DRG neurons (Ziemba et al., 2008). Collectively, these studies demonstrate the promise for gene delivery in spinal cord regeneration strategies.
Patterned gene expression to create concentration gradients may offer significant advantages relative to existing in vitro neurite guidance assays. Gradients formed by localized secretion of diffusible factors within a complex cellular environment are similar to the mechanisms hypothesized to form chemotactic gradients naturally. Although the accessory cell type used in this manuscript is not located in the nervous system, the secreted protein can bind to extracellular matrix molecules and to the cell surface proteoglycans, which can influence the gradient that is formed and the immobilized protein may stimulate cellular responses. Moreover, the concentration gradients achieved by patterned gene expression extend length scales on the order of 1 mm, far greater than lengths achieved with pipette assays (Lohof et al., 1992), and comparable to the maximum length of chemotactic gradients during growth cone guidance (Goodhill, 1997) and morphogenesis (Crick, 1970). Although target tissue cultures represent the natural formation of gradients, the cultures and resultant concentration profiles have inherent variability and are difficult to characterize. We present a well-characterized system in terms of protein production from the source, protein binding to the surface, and predicted concentration gradients from patterns of expression. The protein production rates can be varied by simple changes in system conditions, and nonviral DNA delivery strategies provide the flexibility to investigate any therapeutic factor by readily exchanging plasmids without altering the delivery system. This flexibility enables the investigation of other diffusible guidance factors with different concentration requirements for guidance, and can be used to study migration of other cell types (e.g., fibroblasts) in response to localized expression of guidance factors.
In summary, patterned gene expression produced soluble NGF gradients capable of guiding neurite growth, with the extent of guidance dependent on the distance neurons were cultured from the pattern and the amount of NGF bound to the surface. The patterned expression system presented here allowed for comparison to existing in vitro gradient assays, and we have demonstrated that absolute and fractional gradients formed by localized expression within a cellular environment that direct neurite outgrowth differ from engineered gradients with purified proteins in an acellular environment. Spatially patterned gene expression provides a well-defined and tunable method to investigate cellular responses to gradients of guidance molecules and may be applied to the regeneration of tissues with complex architectures.
Acknowledgments
We thank Dr. Tatiana Segura (University of California Los Angeles), Dr. Angela K. Pannier (University of Nebraska), and Laura De Laporte (Northwestern University) for helpful scientific discussions, and the laboratory of Dr. Andrew T. Dudley for assistance with supplies. Photolithography was performed at the Materials Processing and Crystal Growth core facility (Northwestern University). Financial support for this research was provided by grants from NIH (R01 GM066830, R01 EB005678, LDS) and NSF (Graduate Research Fellowship, THR).
References
- Cao X, Shoichet MS. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience. 2001;103:831– 840. doi: 10.1016/s0306-4522(01)00029-x. [DOI] [PubMed] [Google Scholar]
- Cao X, Shoichet MS. Investigating the synergistic effect of combined neurotrophic factor concentration gradients to guide axonal growth. Neuroscience. 2003;122:381–389. doi: 10.1016/j.neuroscience.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Chamley JH, Goller I, Burnstoc G. Selective growth of sympathetic-nerve fibers to explants of normally densely innervated autonomic effector organs in tissue-culture. Dev Biol. 1973;31:362–379. doi: 10.1016/0012-1606(73)90272-8. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Taylor JSH, Scott LB, Guillery RW, Soriano P, Furley AJW. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1998;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annu Rev Neurosci. 1995;18:497–529. doi: 10.1146/annurev.ne.18.030195.002433. [DOI] [PubMed] [Google Scholar]
- Crank J. The mathematics of diffusion. 2. Oxford, NY: Oxford UP, Inc.; 1975. p. 424. [Google Scholar]
- Crick F. Diffusion in embryogenesis. Nature. 1970;225(5231):420. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- Curinga G, Smith GM. Molecular/genetic manipulation of extrinsic axon guidance factors for CNS repair and regeneration. Exp Neurol. 2008;209:333–342. doi: 10.1016/j.expneurol.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells—a focus on leukocytes and dictyostelium. Annu Rev Cell Biol. 1988;4:649– 686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukocyte Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- Goodhill GJ. Diffusion in axon guidance. Eur J Neurosci. 1997;9:1414–1421. doi: 10.1111/j.1460-9568.1997.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Goodhill GJ, Urbach JS. Theoretical analysis of gradient detection by growth cones. J Neurobiol. 1999;41:230–241. doi: 10.1002/(sici)1097-4695(19991105)41:2<230::aid-neu6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gundersen RW. Sensory neurite growth cone guidance by substrate adsorbed nerve growth-factor. J Neurosci Res. 1985;13(1–2):199–212. doi: 10.1002/jnr.490130114. [DOI] [PubMed] [Google Scholar]
- Gundersen RW, Barrett JN. Neuronal chemotaxis: chick dorsal-root axons turn toward high concentrations of nerve growth factor. Science. 1979;206(4422):1079–1080. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6:119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- Heron PM, Sutton BM, Curinga GM, Smith GM, Snow DM. Localized gene expression of axon guidance molecules in neuronal co-cultures. J Neurosci Methods. 2007;159:203–214. doi: 10.1016/j.jneumeth.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Houchin-Ray T, Whittlesey KJ, Shea LD. Spatially patterned gene delivery for localized neuron survival and neurite extension. Mol Ther. 2007;15:705–712. doi: 10.1038/mt.sj.6300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbister CM, Mackenzie PJ, To KCW, O’Connor TP. Gradient steepness influences the pathfinding decisions of neuronal growth cones in vivo. J Neurosci. 2003;23:193–202. doi: 10.1523/JNEUROSCI.23-01-00193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, Delatorre JR, Tessierlavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal-cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan AJ, McLaurin DL, Marks L, Vinson EN, Pfeifer M, Szulc SV, Heaton MB, Lee N. Immunohistochemical localization of netrin-1 in the embryonic chick nervous system. J Neurosci. 1997;17:5466– 5479. doi: 10.1523/JNEUROSCI.17-14-05466.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JCF, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58A:167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- Ming GI, Song HJ, Berninger B, Inagaki N, Tessier-Lavigne M, Poo MM. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Fischer I, Shurnsky JS, Murray M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol. 2005;194:410–431. doi: 10.1016/j.expneurol.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Moore K, Macsween M, Shoichet M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006;12:267–278. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- Pearce FL, Banthorp Dv, Cook JM, Vernon CA. Adsorption of nerve growth-factor onto surfaces—implications for assay in tissue-culture. Eur J Biochem. 1973;32:569–575. doi: 10.1111/j.1432-1033.1973.tb02643.x. [DOI] [PubMed] [Google Scholar]
- Pettmann B, Manthorpe M, Powell JA, Varon S. Biological-activities of nerve growth-factor bound to nitrocellulose paper by Western blotting. J Neurosci. 1988;8:3624–3632. doi: 10.1523/JNEUROSCI.08-10-03624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite AE, Seyer JM. Fibroblast chemotaxis induction by human recombinant interleukin-4—identification by synthetic peptide analysis of 2 chemotactic domains residing in amino-acid-sequences 70– 88 and 89–122. J Clin Invest. 1991;87:2147–2152. doi: 10.1172/JCI115247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. Cell signaling events: a view from the matrix. Matrix Biol. 2003;22:101–107. doi: 10.1016/s0945-053x(03)00002-7. [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Snow DM, Watanabe M, Letourneau PC, Silver J. A chondroitin sulfate proteoglycan may influence the direction of retinal ganglion cell outgrowth. Development. 1991;113:1473–1485. doi: 10.1242/dev.113.4.1473. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM. The cell biology of neuronal navigation. Nature Cell Biology. 2001;3:E81–E88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- Stroh M, Zipfel WR, Williams RM, Webb WW, Saltzman WM. Diffusion of nerve growth factor in rat striatum as determined by multi-photon microscopy. Biophys J. 2003;85:581–588. doi: 10.1016/S0006-3495(03)74502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M. Target attraction—are developing axons guided by chemotropism. Trends Neurosci. 1991;14:303–310. doi: 10.1016/0166-2236(91)90142-h. [DOI] [PubMed] [Google Scholar]
- The I, Bellaiche Y, Perrimon N. Hedgehog movement is regulated through tout velu–dependent synthesis of a heparan sulfate proteoglycan. Mol Cell. 1999;4:633–639. doi: 10.1016/s1097-2765(00)80214-2. [DOI] [PubMed] [Google Scholar]
- Tria MA, Fusco M, Vantini G, Mariot R. Pharmacokinetics of nerve growth factor (NGF) following different routes of administration to adult rats. Exp Neurol. 1994;127:178–183. doi: 10.1006/exnr.1994.1093. [DOI] [PubMed] [Google Scholar]
- Tse THZ, Chan BP, Chan CM, Lam J. Mathematical modeling of guided neurite extension in an engineered conduit with multiple concentration gradients of nerve growth factor (NGF) Ann Biomed Eng. 2007;35:1561–1572. doi: 10.1007/s10439-007-9328-4. [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhang SX, Li H, Aebischer P, Bunge MB. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, De Laporte L, Rives CB, Jang JH, Lin WC, Shull KR, Shea LD. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104:433–446. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH. Consequences of chemotactic peptide receptor modulation for leukocyte orientation. J Cell Biol. 1981;88:644–647. doi: 10.1083/jcb.88.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]