Abstract
OBJECTIVE
Traumatic brain injury (TBI) causes elevation of matrix metalloproteinases (MMPs), which are associated with neuroinflammation, blood-brain barrier (BBB) disruption, hemorrhage and cell death. We hypothesized that patients with TBI have an increase in MMPs in the ventricular cerebrospinal fluid (CSF) and plasma.
METHODS
Patients with TBI and a ventricular catheter were entered into the study. Samples of CSF and plasma were collected at the time of catheter placement, and 24 and 72 hrs after admission. Seven TBI patients were entered into the study with six having complete data for analysis. Only patients that had a known time of insult that fell within a six hour window from initial insult to ventriculostomy were accepted into the study. Control CSF came from ventricular fluid in patients undergoing shunt placement for normal pressure hydrocephalus (NPH). Both MMP-2 and MMP-9 were measured with gelatin zymography and MMP-3 with Western immunoblotting.
RESULTS
We found a significant elevation in the levels of the latent form of MMP-9 (92-kDa) in the CSF obtained at the time of arrival (TOA) (p<0.05). Elevated levels of MMP-2 were detected in plasma at 72 hours, but not in the CSF. Using albumin from both CSF and blood, we calculated the MMP-9 index, which was significantly elevated in the CSF, indicating endogenous MMP production. Western immunoblots showed increased levels of MMP-3 in CSF at all times measured, while MMP-3 was not detected in the CSF of NPH.
CONCLUSIONS
We show that MMPs are elevated in CSF of TBI patients. Although the number of patients was small, the results were robust and clearly demonstrated elevations of MMP-3 and MMP-9 in ventricular CSF in TBI patients compared to controls. While these preliminary results will need to be replicated, we propose that MMPs may be important in BBB opening and hemorrhage secondary to brain injury in patients.
Keywords: Traumatic brain injury, matrix metalloproteinases, cerebrospinal fluid, ventriculostomy
Introduction
Traumatic brain injury (TBI) in the United States is a major public health problem, resulting in severe disability in an estimated 80,000 to 90,000 people (14). Studies suggest that an inflammatory response around the site of injury leads to progressive grades of cerebral injury. Several animal models have been used to study the process of TBI, including the fluid-percussion model and the weight drop models. The initial insult damages neurons and blood vessels with secondary injury occurring as a result of peri-infarct ischemia and a cytotoxic neuroinflammatory cascade (9). The ensuing inflammation leads to an increase in matrix metalloproteinases (MMPs) with an increase in the permeability of the blood-brain barrier (BBB) (19, 32). These studies in animal models suggest that MMPs may play a role in human TBI.
The MMPs are a gene family of 26 enzymes that are important in normal growth, development, and wound healing, but also cause BBB disruption, hemorrhage, and cell death in a number of neurological diseases in which neuroinflammation plays a significant role (33). The MMPs are secreted as latent enzymes and the activity of the MMPs is tightly controlled to prevent ECM degradation. (27). Gelatinase A (MMP-2) and gelatinase B (MMP-9), contribute to BBB damage. In addition, we showed that stromelysin-1 (MMP-3) degrades basal lamina and tight junction proteins of the BBB (12). MMP-12, which has been detected in humans following traumatic spinal cord injury, may also be a factor in traumatic brain injury (5). Recently, Vilalta and colleagues observed significantly elevated levels of proMMP-2 and -9 in the plasma and brain extracellular fluid of patients with moderate or severe TBI (30). We hypothesized that TBI patients would have elevated levels of MMPs in the cerebrospinal fluid (CSF). To test the hypothesis we measured CSF and plasma levels of MMP-2, MMP-9, MMP-3, and metalloelastase (MMP-12) in patients with severe TBI. Control MMP levels in CSF and plasma were obtained from ventricular fluid of patients being treated for normal pressure hydrocephalus (NPH) with ventriculoperitoneal shunt (VPS).
PATIENTS AND METHODS
The University of New Mexico Human Research Review Committee approved the study. Patients were selected if the time of injury could be ascertained to have occurred within a 6 hour window from insult to ventriculostomy, which was placed for diagnostic and therapeutic purposes. Patients’ families gave informed consent for the use of the CSF and blood; there were no additional risks associated with the study beyond standard patient care. Seven TBI patients with a severe head injury, defined as Glasgow coma scale (GCS) ≤8 on admission, and a ventricular catheter were enrolled in the study (29). One patient with incomplete data was excluded from the data analysis. Four NPH patients that had VPS placement as part of their treatment gave informed consent for the collection of CSF from the ventricular catheter (Table 1). Three paired CSF and plasma samples were collected: at time of ventriculostomy, 24, and 72 hours following arrival. The NPH patients had paired CSF and plasma collected at the time of VPS placement. Plasma samples were collected into tubes with sodium heparin. After collection, the samples of CSF and plasma were centrifuged at 3000 rpm for 10 minutes at 4oC and the supernatant was collected, aliquoted, and stored at −80°C.
TABLE 1.
Patient Characteristics
| Glasgow Outcome Score | Ranchos Los Amigos Cognitive Functioning Scale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Age/Gender | Marshall | GCS-A, 72h, DC | ICPI/ICPF/ICPH | 1Mo | 3Mo | 6Mo | 1Mo | 3Mo | 6Mo | Radiologic Findings |
| ‡1 | 21M | 2 | 6T, 10T, 15 | 14, 12, 48d2 | M | G | G | 9 | 10 | 10 | SAH |
| 2 | 20M | 2 | 7T, 7T, 12 | 16, 14, 37d2 | M | M | G | 8 | 9 | 10 | Scattered ICH |
| 3 | 19M | 2 | 7T, 8T, 14 | 12, 18, 18d3 | M | G | G | 8 | 9 | 9 | ICH |
| 4 | 63M | 3 | 6T, 8T, 10T | 11, 12, 20d4 | S | † | † | 5 | † | † | EDH |
| *5 | 28M | 3 | 7T, 7T, 14 | 29, 20, 30d2 | S | G | G | 7 | 10 | 10 | SDH |
| 6 | 81M | 2 | 3T, 8T, 10T | 12, 4, 25d10 | P | P | P | 3 | 3 | 3 | SAH, IVH |
| 7 | 49M | 2 | 3T, 3T, 10T | 8, 14, 14d3 | S | S | S | 4 | 4 | 4 | DAI |
| NPH1 | 76M | ||||||||||
| NPH2 | 77M | ||||||||||
| NPH3 | 77F | ||||||||||
| NPH4 | 77M | ||||||||||
Abbreviations: Pt, Patient; NPH, Normal Pressure Hydrocephalus Control Patient; GCS, Glasgow Coma Scale (T, intubated; A, arrival; DC, discharge); ICP, Initial Intracranial Pressure in mmHg (I, Initial; F, Final; H, Highest ICP indicated by day); Mo, Month; G, Good; M, Moderate; S, Severe; P, Poor; SAH, Subarachnoid Hemorrhage; ICH, Intracerebral Hemorrhage; EDH Epidural Hematoma; SDH, Subdural Hematoma; IVH, Intraventricular Hemorrhage; DAI, Diffuse Axonal Injury;
Patient 5 was excluded.
Patient expired due to myocardial infarction.
Patient developed an infection after the course of the study.
Initial evaluation
Each patient received an initial clinical assessment, including history of the trauma, time of occurrence, and results of the condition before arriving in the hospital, demographics, relevant past medical and surgical history, medications, and family and social history. Physical examination was performed, which included the Glasgow Coma Scale (GCS) score, cranial nerve testing, motor and sensory examination, and an initial traumatic survey. Additional information was obtained after admission when the patient was stable. Basic laboratory data was obtained, including a chemistry panel, complete blood count, coagulation studies, and a urine drug screen. All patients had a computed tomography (CT) scan of the head to assess the volume, and evolution of the injury.
Indications for intervention
Patients were entered into the study if the GCS was less than 13, a definable contusion and/or intraventricular hemorrhage was found on neuroimaging, and a ventriculostomy was medically indicated. Patients were excluded from the study if they had a penetrating head injury, non-traumatic head injuries, or underlying intracranial lesion or disease. Patients that arrived without a known duration from time of injury, or if the injury occurred more than six hours prior to admission were not enrolled in the study.
Samples of CSF were obtained from the ventricular catheters and analyzed for cell count with differential, protein, albumin, glucose, gram stain with culture, and MMP levels. Similarly, blood was drawn to measure serum albumin and plasma MMP levels.
We obtained control CSF samples from patients undergoing VPS for NPH. The CSF was removed at the time of VPS placement for studies as mentioned above. Blood was drawn at the time of VPS placement for albumin and plasma MMP measurements. Patients were excluded if they were thought to have a condition that would lead to elevation of the MMPs, such as infection, bleeding, or tumor. All controls underwent clinical evaluation and neuroimaging to exclude these causes of the hydrocephalus. We recognize an inherent limitation in this study with not having paired CSF collected from NPH control patients beyond the initial placement for comparison to the TBI CSF at 24 and 72 hours. The procedures in this study were non-invasive beyond normal patient care and we did not feel the increased risk for infection was warranted. Of the TBI patients evaluated, Patient 1 developed an infection; however, its course was not within the time of sample collection (Table 1).
Gelatin-Substrate Zymography for Measurement of MMPs in Plasma and CSF
The levels of MMP-2 and MMP-9 in CSF and plasma were measured by gelatin-substrate zymography, using methods previously reported (2). Positive controls for human MMP-2 and MMP-9 were run on every gel to determine molecular weights. Additionally, to control for between gel differences, samples were run in duplicate across different gels and normalized to known amounts of MMP-2 and MMP-9 positive controls. Zones of proteolytic activity in zymograms appear as clear bands against a blue background. Dried gels were scanned (Duoscan scanner; AGFA Corporation, Ridgefield Park, NJ, USA), and analyzed using AlphaEase software (Alpha Innotech Corporation, San Leandro, CA, USA). Sample load was normalized to 10 μl of sample volume.
MMP Indexing
Previous studies in patients with multiple sclerosis have established that the high levels of MMP-9 in the CSF come from an endogenous source as opposed to delivery from blood. In the TBI patients, the elevated MMP levels detected could be from endogenous production by neural tissue or delivery from systemic circulation across a compromised BBB. The MMP source was determined by indexing the levels of MMP-2 and -9 in the CSF and the blood to those of albumin. In this regard, proteins transported from the blood to the CSF have a low index value, while those made in the brain have an elevated index (17). The MMP-2 and -9 indexes are analogous to the IgG index used in the diagnosis of multiple sclerosis. For this study, we measured MMPs in paired CSF and plasma by zymography and determined their index values to provide further insight into their sources following TBI.
The MMP index for MMP-2 and -9 was formed from the measured CSF and serum albumin levels and the densitometric results of the MMPs in the CSF and the plasma using the equation: Index = QMMP/QAlbumin, where QMMP = MMPCSF/MMPPlasma and QAlbumin = CSFAlbumin/BloodAlbumin (17).
Fluorometric MMP-3 and MMP-12 Enzymatic Activity
The fluorescence resonance energy transfer (FRET) peptide, 5-carboxyfluorescein (5-FAM)/QXL520 (catalog no. 60580; AnaSpec Incorporated, San Jose, CA, USA), which is specifically cleaved by both MMP-3 and MMP-12, was utilized to measure their activity in the CSF and plasma samples as previously described (6). The intact peptide’s [5-FAM-Arg-Pro-Lys-Pro-Val-Glu-Nva-Trp-Arg-Lys(QXL520)-NH2] fluorescence of 5-FAM is quenched by QXL520, however, following enzyme cleavage the fluorescence of 5-FAM is detected at excitation/emission wavelengths of 490/520 nm. The change in relative fluorescence units was monitored at 5 min intervals for 1 hr at room temperature using a luminescence spectrometer (Model LS55; PerkinElmer Instruments, Buckinghamshire, UK) connected to a computer running FL WinLab software.
Immunoblotting for MMP-3 and MMP-12
Equal volumes of sample were loaded on 10% acrylamide gels for MMP-3 and MMP-12 analysis under reducing conditions. Both a prestained and biotinylated protein marker were loaded (Cell Signaling Technology Incorporated, Danvers, MA, USA) along with positive controls for both recombinant human MMP-3 and MMP-12 (R&D Systems Incorporated, Minneapolis, MN, USA) Following transfer for 1 hr at 100 v, samples were detected with anti-MMP-3 antibody (0.1μg/ml goat polyclonal; R&D Systems Inc.). The blots were then stripped and re-probed with an anti-MMP-12 antibody (1:5000 rabbit polyclonal; Abcam Incorporated, Cambridge, MA, USA). The secondary antibodies included peroxidase-conjugated anti-biotin (1:2000; Cell Signaling Tech.) and peroxidase-conjugated donkey anti-goat and donkey anti-rabbit (1:40,000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The West Pico Kit (Pierce Biotechnology Incorporated, Rockford, IL, USA) was used for chemiluminescent detection of the samples. The bands were densitometrically analyzed and samples were normalized to equal load volumes of their respective positive controls.
Statistical Analyses
Statistical analyses were performed using the software package, GraphPad Prism (v.4.03, San Diego, CA, USA). Non-parametric Kruskal-Wallis one-way analysis of variance tests were performed with Dunn’s multiple comparison post-tests; a Mann-Whitney two-tailed t-test was performed for the indexing data. Data are expressed as means ± standard error of the mean. Significance was set at P<0.05.
RESULTS
Table 1 shows the clinical characteristics of the TBI patients. Of the seven patients entered into the study, only six had complete data that could be used for the analyses. The Glasgow Coma Scale of all the patients ranged from 3T to 7T upon admission, 3T to 10T by 72 hours, and 10T to 15T at time of discharge from the neuroscience intensive care unit; Marshall Scores based on CT findings are indicated as well. The Glasgow Outcome Scale and Ranchos Los Amigos Cognitive Functioning Scale results are shown in the Table along with the CT findings. Intracranial pressures are indicated for times of initial ventriculostomy placement with a range of 8 to 29 mmHg, at ventriculostomy removal (4–20 mmHg), and for peak values (14 mmHg by day 3 and 48 mmHg by day 2) for all TBI patients listed. Because of the small number of patients, correlation of CSF results with clinical information was not feasible.
Zymography of the CSF and plasma showed the characteristic bands for MMP-2 and MMP-9, which are seen in patients with stroke and multiple sclerosis. The 92-kDa band for latent MMP-9 was present along with the 72-kDa band for latent MMP-2. Quantitative data from the densitometric measurements of the gels are shown in Figure 1. Matrix metalloproteinase-9 was present in all plasma samples and remained unaltered following TBI at all time points compared to NPH. However, CSF MMP-9 levels were significantly elevated at time of arrival (TOA) compared to NPH and remained elevated at 24 and 72 hours (Figure 1A, p=0.048). Matrix metalloproteinase-2 was significantly elevated in the plasma by 72 hours following TBI compared to NPH (Figure 1B, p=0.026). Levels for MMP-2 in the CSF were not significantly altered following TBI compared to NPH (Figure 1B).
Figure 1.
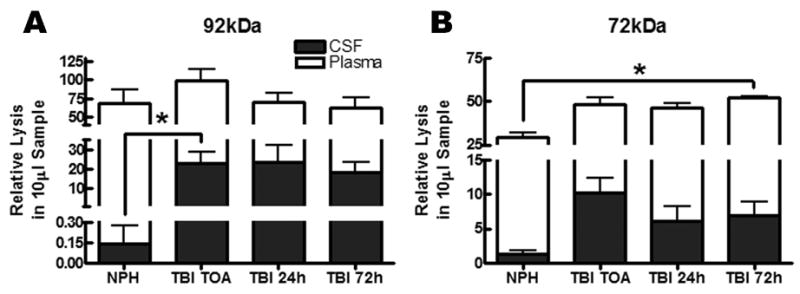
Levels of MMP-9 and MMP-2 for both cerebrospinal fluid (CSF) and plasma in normal pressure hydrocephalus (NPH) control patients and traumatic brain injured (TBI) patients from time of arrival to 72 hours as measured by zymography. (A) The pro-form of MMP-9 (92kDa) is present in the plasma for NPH and TBI patients at time of arrival (TOA), 24, and 72 hours. MMP-9 is only significantly elevated at TOA in CSF compared to NPH (p<0.05) with no change in plasma levels. (B) The pro-form of MMP-2 (72kDa) is up regulated by 72 hours (p<0.03) in the plasma for TBI but is not significantly elevated in the CSF following TBI.
Calculation of the MMP index suggested endogenous MMP-9 synthesis (Figure 2A, p=0.019). The MMP-2 index data was not significant with data spread above and below those of the controls. The data indicate that some patients with the higher index values may have had an endogenous source of MMP-2 production (Figure 2B).
Figure 2.
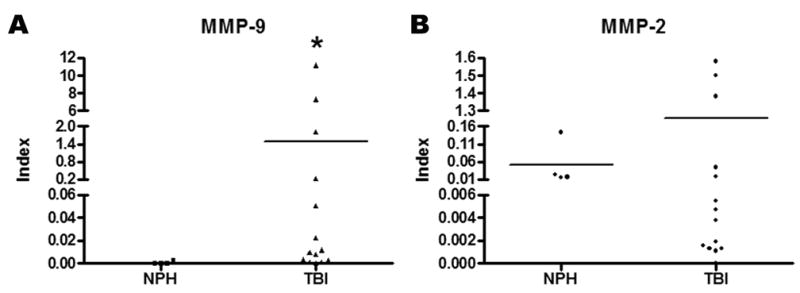
MMP indexing for the evaluation of endogenous brain MMP production as reflected in the cerebrospinal fluid (CSF) for time of arrival, 24, and 72 hours following TBI compared to NPH controls. (A) The MMP-9 index is statistically elevated over NPH (p<0.02) and suggests intrathecal production. (B) The index for MMP-2 is not significantly different from NPH. The data spread for MMP-2 is suggestive that some patients may have endogenous production.
The fluorogenic assay, which detects activity of both MMP-3 and MMP-12, showed similar activity in plasma and CSF for TBI and NPH (Figure 3A, B). The failure to detect active MMP-3 in the samples was further corroborated by the immunoblots as no detectable lower molecular weight active bands were measured. When analyzed by Western immunoblot, which detects both the latent zymogen and active form, only the latent form of MMP-3 was detectable; MMP-12 was not present in any of the samples analyzed (Figure 4A). The latent form of MMP-3 was significantly elevated in the CSF of TBI patients at all time points following trauma compared to NPH controls (p=0.04; Figure 4B). MMP-3 was detected in neither CSF nor plasma for NPH patients. The presence of MMP-3 during the acute stages of TBI in the CSF suggests a potential inflammatory role for this protease following trauma.
Figure 3.
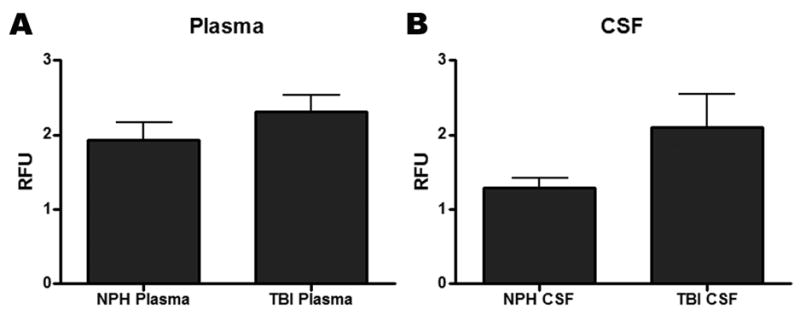
Analysis for the relative fluorescent unit (RFU) activity of MMP-3 and/or MMP-12 using a FRET peptide that is nonspecifically cleaved by both metalloproteinases. No significant differences in activity were observed for (A) plasma or for (B) cerebrospinal fluid (CSF) when comparing normal pressure hydrocephalus (NPH) to the pooled samples of the traumatic brain injury patients (TBI).
Figure 4.
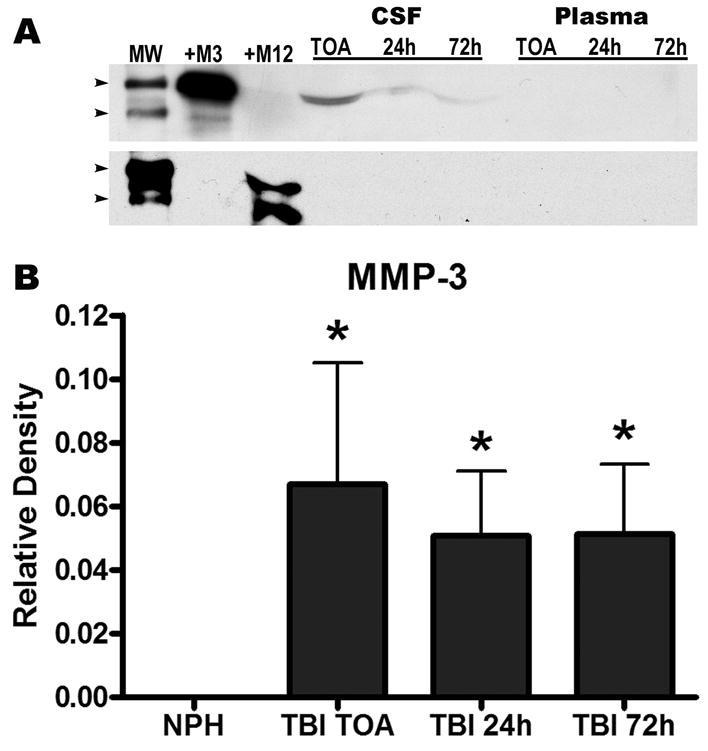
Western immunoblotting for MMP-3 and MMP-12 in plasma and cerebrospinal fluid (CSF) for normal pressure hydrocephalus (NPH) and traumatic brain injured (TBI) patients. (A) The same blot probed for MMP-3 (top blot) then stripped and re-probed for MMP-12 (lower blot). A molecular weight (MW) ladder indicates the 60 and 50kDa regions (arrowheads). Recombinant human MMP-3 (+M3) and MMP-12 (+M12) positive controls were loaded and indicate the zymogen forms as approximately 55kDa; lower molecular weight species are present in both positive controls. The zymogen of MMP-3 was only detectable in the CSF of TBI patients and was not detected in plasma or NPH samples; MMP-12 was not detected in any of the samples. (B) Densitometric analysis of the Western blots for MMP-3 indicates significantly elevated levels at all time points for TBI CSF compared to NPH (p<0.04).
DISCUSSION
We found increased levels of MMP-9 by gelatin zymography and MMP-3 by Western immunoblot in the ventricular CSF of patients with severe TBI. Both MMP-3 and MMP-9, which are inducible enzymes, were significantly increased compared to control NPH CSF. The constitutively expressed MMP-2 failed to show a significant increase following TBI at all time points measured. Neither MMP-3 nor MMP-9 was detected in the control CSF, indicating that the increased levels in the TBI patients were a result of the trauma. Our results, which are the first to demonstrate MMPs in the CSF after TBI in humans, are consistent with animal TBI studies demonstrating elevated MMP-3 and MMP-9 in brain tissue after injury. Since MMPs are elevated in a number of neuroinflammatory conditions, where they contribute to the secondary injury, these data suggest that MMPs may contribute to tissue damage secondary to an inflammatory response in TBI.
MMPs have critical roles in regulating the ECM that make them important under normal and pathological conditions. They are synthesized in a latent form and can be either stored in inflammatory cells, secreted, tethered to ECM proteins, or the plasma membrane (8). To prevent unwanted tissue damage, the activity of the MMPs is tightly controlled (27). Regulation occurs at multiple levels, including gene expression, post-translational processing, compartmentalization, and inhibition with endogenous tissue inhibitors of matrix metalloproteinases (TIMPs) (10). Several MMPs are constitutively expressed, including, MMP-2, which is normally found in brain and CSF. During inflammation other inducible MMPs are expressed (7, 10, 27, 34). Transcriptional control of the gelatinases differs for constitutive and inducible enzymes. Constitutively expressed MMP-2 has an activator protein-2 (AP-2) site and an SP-1 site in the gene promoter region. While the inducible MMPs, such as MMP-3 and -9 contain an AP-1 site. In addition, MMP-9 contains a nuclear factor-κB (NF-κB) site, adding to its induction during an inflammatory reaction (22). Promoters in the inducible gene respond to immediate early gene products, c-Fos and c-Jun, and to various cytokines, such as interleukin-1β and tumor necrosis factor-α (23).
The proteolysis of the ECM by MMPs leads to vasogenic edema, hemorrhage, and cell death by attacking the basal lamina around blood vessels and disrupting the tight junctions between endothelial cells (18). MMPs are secreted as latent enzymes that undergo activation by other proteinases, autocatalytic processing, S-nitrosylation of the thiol group, or activation by urokinase-mediated plasmin generation (11, 24, 31). In stroke, MMP-9 is elevated in the brain and blood of patients at the greatest risk of hemorrhage; it is also found to be elevated in the blood of patients with intracerebral hemorrhage (1, 3, 21, 26).
In a recent study of arterial and jugular blood in patients with TBI, elevated levels of MMP-9 were found on the day of admission, which correlated with the inflammatory marker, interleukin-6; they also showed that hypothermia returned the elevated levels to normal (28). The findings of elevated MMP-9 levels in TBI patient’s jugular blood and the increased risk for hemorrhage further support a role for MMPs in these injury processes.
Mice deficient in MMP-9 have reduced BBB disruption and attenuated edema and infarct volume when subjected to transient focal cerebral ischemia (4). A recent study utilizing the cortical contusion model in rats showed that MMP-9 contributes to BBB disruption and brain edema; both of which were attenuated by treatment with the broad-spectrum MMP inhibitor, GM6001 (25). MMP-9 knockout mice also exhibited a significant decrease in motor deficits after trauma suggestive of its role in injury following injury (32). Expression of MMP-3 was associated with BBB opening in LPS-induced neuroinflammation (20). Knockout of the MMP-3 gene resulted in reduced disruption of the BBB with fewer infiltrating neutrophils (12). Additionally, the findings of elevated MMP-3 mRNA and protein in the hippocampus of rat after TBI are indicative of a possible role for MMPs in the injury process of cerebral trauma (13).
Matrix metalloproteinases are found in CSF in neuroinflammation (16). The source of the MMPs detected in the CSF is controversial. MMPs are produced by circulating leukocytes, including neutrophils and lymphocytes, which invade the brain during inflammation. However, neural cells are also responsible for their production. Endogenous production of MMPs in the central nervous system was shown in patients with multiple sclerosis. This finding was determined by indexing the levels of MMP-9 in the CSF to that in the plasma, utilizing albumin in both compartments as a reference, and creating an index similar to that of the IgG index in multiple sclerosis (17). Using this same approach, we found that the MMP-9 index was increased, suggesting that the MMP-9 production was the result of endogenous production by cells in the central nervous system. Intrathecal MMP-9 production was further supported by the absence of a statistical increase over basal levels of MMP-9 in the plasma at the time the CSF levels were increased. However, since MMP-9 was detected in the blood, some spill-over into the CSF cannot be ruled out. In other conditions, such as bacterial meningitis, there is a closer relationship for the MMPs in the brain to those in the blood, and it cannot be ruled out that the white blood cells may be a partial source of MMPs seen in the CSF (15). Intrathecal MMP-3 production is indicated since it was only detected in the CSF of the TBI patients and was not detected in the blood of the TBI patients or in the blood or CSF of NPH controls.
Our results obtained in this small sample of patients will need to be replicated in larger cohorts, but the robustness of the data clearly implicates both MMP-3 and MMP-9 in the pathophysiology of TBI in humans. We found some variability in the timing of the MMP elevations in the different patients that will need further study, but the highest levels of MMP-9 were found in the initial samples of CSF obtained at the time of arrival. We propose that the MMPs identified in the CSF may be indicative of disruption of the BBB. Animal studies have shown a benefit of broad-spectrum MMP inhibitors in the treatment of TBI, and there was protection with knockout of MMP-9. If further studies correlate elevated levels of MMPs in the CSF with disruption of the BBB in humans with TBI, then treatment trials with MMP inhibitors may be warranted.
Acknowledgments
Supported by grants from UNM HSC CTSC Research Core Pilot Project Award in Clinical and Translational Research and DHHS/PHS/NIH/NCRR/GCRC Grant# MO1RR00997 and NIH grants to GAR (RO1 NS045847 and RO1 NS052305).
Lori Kirby was instrumental as the clinical coordinator for this study. Additionally, the nurses and other allied health personnel were paramount for the research in the neuroscience and surgical critical care units.
References
- 1.Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, varez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99:65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]
- 2.Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, Stetler-Stevenson WG, Rosenberg GA. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke. 2004;35:e159–e162. doi: 10.1161/01.STR.0000127420.10990.76. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Sabin J, Delgado P, Abilleira S, Molina C, Arenillas J, Ribo M, Santana A, Quintana M, Monasterio J, Montaner J. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: Relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 4.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA. Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury. BMC Neurol. 2007;7:17. doi: 10.1186/1471-2377-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA. Cyclooxygenase Inhibition Limits Blood-Brain Barrier Disruption following Intracerebral Injection of Tumor Necrosis Factor-{alpha} in the Rat. J Pharmacol Exp Ther. 2007;323:488–498. doi: 10.1124/jpet.107.127035. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 9.Gaetz M. The neurophysiology of brain injury. Clin Neurophysiol. 2004;115:4–18. doi: 10.1016/s1388-2457(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 10.Gottschall PE, Deb S. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. [Review] Neuroimmunomodulation. 1996;3:69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- 11.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 12.Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Fillmore HL, Reeves TM, Phillips LL. Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Experimental Neurology. 2005;192:60–72. doi: 10.1016/j.expneurol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Langlois JA, Rutland-Brown W, Thomas KE. Emergency Department Visits, Hospitalizations, and Deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2006. Traumatic Brain Injury in the United States; pp. 1–70. [Google Scholar]
- 15.Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Hollander GA. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis. 2000;31:80–84. doi: 10.1086/313922. [DOI] [PubMed] [Google Scholar]
- 16.Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Brain Res Rev. 2001;36:249–257. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 17.Liuzzi GM, Trojano M, Fanelli M, Avolio C, Fasano A, Livrea P, Riccio P. Intrathecal synthesis of matrix metalloproteinase-9 in patients with multiple sclerosis: implication for pathogenesis. Mult Scler. 2002;8:222–228. doi: 10.1191/1352458502ms800oa. [DOI] [PubMed] [Google Scholar]
- 18.Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: Role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- 19.Morita-Fujimura Y, Fujimura M, Gasche Y, Copin JC, Chan PH. Overexpression of copper and zinc superoxide dismutase in transgenic mice prevents the induction and activation of matrix metalloproteinases after cold injury-induced brain trauma. J Cereb Blood Flow Metab. 2000;20:130–138. doi: 10.1097/00004647-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Mun-Bryce S, Lukes A, Wallace J, Lukes-Marx M, Rosenberg GA. Stromelysin-1 and gelatinase A are upregulated before TNF-alpha in LPS-stimulated neuroinflammation. Brain Res. 2002;933:42–49. doi: 10.1016/s0006-8993(02)02303-x. [DOI] [PubMed] [Google Scholar]
- 21.Rosell A, Ortega-Aznar A, varez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg GA. Matrix metalloproteinases in brain injury. Journal of Neurotrauma. 1995;12:833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg GA. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist. 2002;8:586–595. doi: 10.1177/1073858402238517. [DOI] [PubMed] [Google Scholar]
- 24.Rozanov DV, Strongin AY. Membrane type-1 matrix metalloproteinase functions as a proprotein self-convertase. Expression of the latent zymogen in Pichia pastoris, autolytic activation, and the peptide sequence of the cleavage forms. J Biol Chem. 2003;278:8257–8260. doi: 10.1074/jbc.M213246200. [DOI] [PubMed] [Google Scholar]
- 25.Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl. 2006;96:130–133. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- 26.Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Davalos A. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36:86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]
- 27.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. [Review] [250 refs] Annual Review of Cell & Developmental Biology. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suehiro E, Fujisawa H, Akimura T, Ishihara H, Kajiwara K, Kato S, Fujii M, Yamashita S, Maekawa T, Suzuki M. Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma. 2004;21:1706–1711. doi: 10.1089/neu.2004.21.1706. [DOI] [PubMed] [Google Scholar]
- 29.van BB, Odding E, Maas AI, Ribbers GM, Bergen MP, Stam HJ. Traumatic brain injury: classification of initial severity and determination of functional outcome. Disabil Rehabil. 2003;25:9–18. doi: 10.1080/dre.25.1.9.18. [DOI] [PubMed] [Google Scholar]
- 30.Vilalta A, Sahuquillo J, Rosell A, Poca MA, Riveiro M, Montaner J. Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med. 2008;34:1384–1392. doi: 10.1007/s00134-008-1056-1. [DOI] [PubMed] [Google Scholar]
- 31.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 34.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]


