Abstract
The kinetics of enantiomerization and dynamic thermodynamic resolution (DTR) of N-Boc-2-lithiopiperidine have been measured, revealing significant differences in enthalpy and entropy for these processes and a role for the achiral ligand TMEDA: the racemization and the DTR are catalytic and first order in [TMEDA], and this has implications for asymmetric synthesis with chiral organolithiums.
Substituted piperidines are key structures for the synthesis of many alkaloids and medicinal compounds. A direct method to access 2-substituted piperidines is by electrophilic quench of the corresponding 2-lithiated derivative.1 The racemic organolithium can be prepared most conveniently by proton abstraction, and a suitable method is to use N-tert-butoxycarbonyl-piperidine (N-Boc-piperidine) with sec-BuLi and N,N,N',N'-tetramethylethylenediamine, TMEDA.2 Unfortunately, the use of chiral ligands to effect an asymmetric deprotonation has met with limited success.3 An alternative approach is to use dynamic resolution4 and the first promising results for resolution of 2-lithiated piperidines have been reported recently.5 For high yields in this chemistry, carbanion inversion is required in the presence of the chiral ligand. Therefore it is crucial to have an understanding of the barriers to this inversion. This paper describes quantitative kinetic data for the enantiomerization of N-Boc-2-lithiopiperidine in the presence of TMEDA. In addition we have determined the activation parameters for dynamic thermodynamic resolution (DTR) of N-Boc-2-lithiopiperidine in the presence of a chiral ligand. This study has revealed a significant role for the ligand TMEDA in the rate of both enantiomerization and dynamic resolution.
In previous work, we determined the enantiomerization barrier of several lithiated pyrrolidines.6 The barrier to enantiomerization for N-Boc-2-lithiopiperidine 2 was determined in the same way, starting with the enantioenriched stannane 1 (prepared by DTR)5 (Scheme 1). Transmetallation at −78 °C in the presence of 1 equiv. of TMEDA, then equilibration at temperatures from −50 to −30 °C, followed by cooling to −78 °C and electrophilic quench with TMSCl gave N-Boc-2-trimethylsilylpiperidine 3.3
Scheme 1.
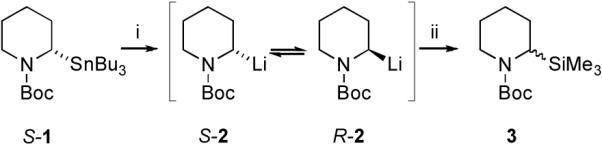
i, n-BuLi (1.1 equiv.), Et2O, TMEDA (1 equiv.), −78 °C, 1 h then warm to −50, −40 or −30 °C for various times; ii, −78 °C, TMSCl.
Under these conditions, the enantiomer ratio (er) of the silane reflects the ratio of organolithium enantiomers R-2 and S-2.7 These experiments gave rise to good first order plots for the loss of the major enantiomer over time from which an Eyring plot led to the determination of the activation parameters (Table 1 entry 1 and Supplementary Information†). Transmetallation of the stannane 1 (er 80:20) in Et2O in the absence of any ligand gave (after addition of TMSCl) a low yield of racemic silane 3 even at low temperature, which precluded kinetic measurements of racemization in the absence of any ligands.
Table 1.
Thermodynamic parameters for inversion of 2
| Entry | Description | ΔH‡ (kcal/mol) | ΔS‡ (cal/mol·K) |
|---|---|---|---|
| 1 | Enantiomerization of 2 with 1 equiv. TMEDA | 20.0 ± 1.7 | 12.0 ± 7.1 |
| 2 | DTR of 2 with 1 equiv. 4 | 17.0 ± 0.9 | −14.5 ± 3.2 |
| 3 | DTR of 2 with 4 and 2 equiv. TMEDA | 5.2 ± 0.8 | −53.2 ± 2.9 |
The rate of enantiomerization with varying amounts of TMEDA was measured at −40 °C (see Supplementary Information†). The linear correlation of kenant vs. [TMEDA] reveals a first-order dependence on [TMEDA].
Of significance for asymmetric synthesis, we carried out kinetic studies of the resolution of organolithium 2 in the presence of the chiral ligand 4 (Scheme 2). This ligand is known to promote dynamic resolution of 2 at −40 °C, under thermodynamic control, with a preference for one diastereomeric complex (S-2·4:R-2·4 = 77:23).5b Interestingly, in the absence of TMEDA, DTR was not observed at this temperature. However, at higher temperatures it was possible to obtain reasonable kinetic data. Hence, DTR of 2 by 4 at −10, 2, 10 and 20 °C gave, in moderate yields, the silane S-3 with increasing enantioenrichment over time, to a maximum er of 77:23. An Eyring plot (see Supplementary Information†) revealed activation parameters shown in Table 1 entry 2. Similar experiments with 2 equiv. of TMEDA added, conducted at −30, −20, −7, and −2 °C yielded the thermodynamic parameters listed in Table 1 entry 3.
Scheme 2.
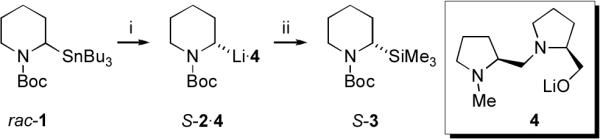
i, n-BuLi (1.2 equiv.), Et2O, alone or with 2 equiv. TMEDA, −78 °C, 1 h then 4 (1 equiv.) then warm to temperatures listed in text and Supporting Information for various times; ii, cool to −78 °C, TMSCl.
Examination of the enthalpic and entropic contributions to the free energies for inversion in the enantiomerization and resolution reveal interesting differences, indicative of different mechanisms in each case. A plot of ΔG‡ vs. temperature for the three systems studied is illustrated in Fig. 1. These findings indicate a role for TMEDA in both the enantiomerization and the DTR. Particularly intriguing is the effect of TMEDA on the DTR. The large negative entropy in Table 1 entry 3 indicates significant order in the transition state of the rate-determining step (rds) for the DTR process. Note, however, that the rds need not be the carbanion inversion (see below).
Fig. 1.
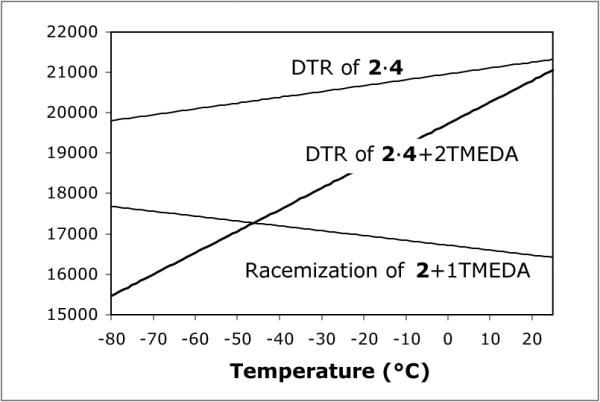
The relationship between ΔG‡ and temperature for the three systems studied (Table 1). For the DTRs, the barriers are for R-2 → S-2.
To further probe this role, the DTR of 2 by 4 was studied with varying amounts of TMEDA at −10 °C. Fig. 2 shows first order rate constants for DTR using 1 equivalent of 4, plotted against [TMEDA] from zero to one equivalent of TMEDA. The linear correlation suggests that the DTR of 2 by 4 is first order in the achiral ligand TMEDA. The intercept of 3.8(±0.8)×10−5 sec−1 indicates a competing DTR having a zero-order dependence on TMEDA (i.e., DTR not catalyzed by TMEDA), and is in agreement with the experimental value of 3.3(±0.2)×10−5 sec−1 at −10 °C (see Supplementary Information†). The rate constant of the DTR in Et2O at −10 °C in the presence of 1.0 equiv. TMEDA, k = 3.2(±0.2)×10−4 s−1, is approximately ten times that in the absence of TMEDA.
Fig. 2.
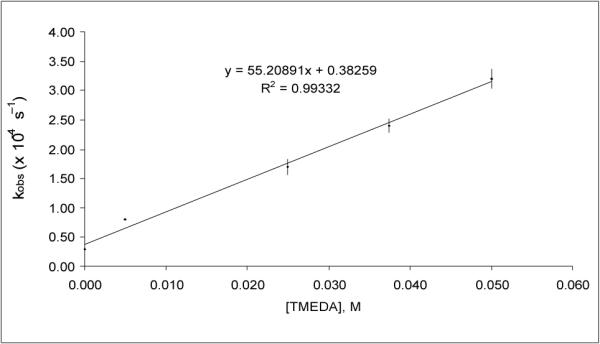
Observed rate constants for DTR of 2 by 4 at −10 °C vs [TMEDA].
Scheme 3 illustrates equilibria that could exist in the dynamic resolution, including a catalytic role for TMEDA in the DTR which is consistent with the kinetics. Parallel pathways of dynamic resolution are implicated, one of which is catalyzed by TMEDA. This could occur through mixed aggregates such as 2·4·TMEDA or through the simple 2·TMEDA complex. We know that k2, the rate constant for the uncatalyzed dynamic resolution (Scheme 3), is slow relative to the catalyzed process, but the rds of the latter is not known. Structural details of the components of this reaction are unknown at present. Each species is drawn as a monomer for simplicity. TMEDA is normally bidentate,8 and lithiated β-aminoalkoxides are chelated and form dimeric or tetrameric mixed aggregates with simple alkyllithiums.9 However, the degree of chelation and aggregation of diaminoalkoxides such as 4 is unknown, so we have not speculated on possible structural details of mixed aggregates at this time.
Scheme 3.
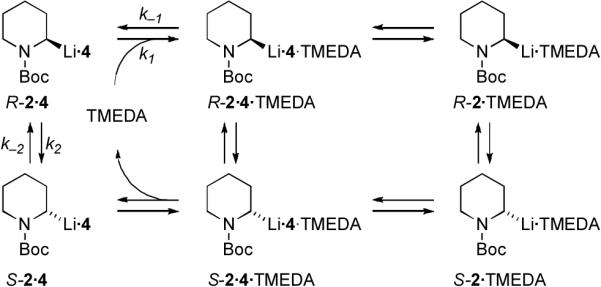
Possible equilibria and catalytic cycle for the DTR.
As TMEDA has a significant role in the DTR, we tested ligands other than TMEDA for this process. Racemic 2 was generated by tin–lithium exchange in the presence of the chiral ligand 5 and the additional ligand. After 90 min at −40 °C the mixture was cooled to −78 °C prior to addition of TMSCl. With one equivalent of TMEDA, chiral ligand 5 promotes DTR to give silane 3, er 80:20 (Table 2, entry 1).5a Entries 2 and 3 indicate that dynamic resolution is operative using various amounts of TMEDA. However, without TMEDA at this temperature a low yield of racemic silane 3 was produced (entry 4). Et3N and (–)-sparteine 1 gave racemic silane 3 (entries 5–6). Pentamethyldiethylenetriamine (PMDTA) and the lithium alkoxide of N,N-dimethylaminoethanol gave low selectivity (entries 7 and 8), 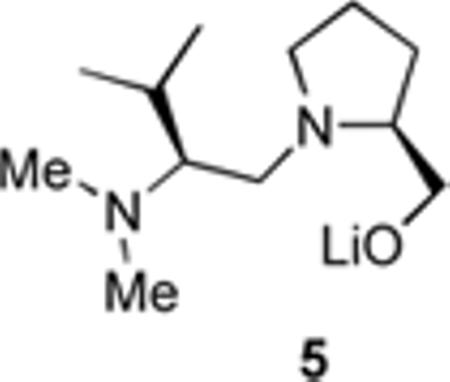 suggesting only slow turnover in the catalytic cycle. More closely related to TMEDA is the ligand tetraethylethylenediamine (TEEDA), which gave a low yield of the silane 3 with slightly enhanced er (entry 9). From Table 2 it is clear that, of the ligands studied, TMEDA has the most beneficial influence on overall yield and selectivity.
suggesting only slow turnover in the catalytic cycle. More closely related to TMEDA is the ligand tetraethylethylenediamine (TEEDA), which gave a low yield of the silane 3 with slightly enhanced er (entry 9). From Table 2 it is clear that, of the ligands studied, TMEDA has the most beneficial influence on overall yield and selectivity.
Table 2.
Dynamic resolution of 2·5 at −40 °C for 90 min
| Entry | Additional ligand | Yield (%) 3 | er (S:R) |
|---|---|---|---|
| 1 | TMEDA | 73 | 80:20 |
| 2 | TMEDA (0.1 equiv.) | 68 | 80:20 |
| 3 | TMEDA (2 equiv.) | 59 | 77:23 |
| 4 | none | <10 | 50:50 |
| 5 | Et3N | 11 | 50:50 |
| 6 | (−)-sparteine 1 | 27 | 50:50 |
| 7 | PMDTA | 56 | 59:41 |
| 8 | Me2NCH2CH2OLi | 31 | 53:47 |
| 9 | TEEDA | 8 | 83:17 |
In summary, the thermodynamic parameters for racemization of N-Boc-2-lithiopiperidine (2·TMEDA) and resolution of N-Boc-2-lithiopiperidine (2·4 and 2·4·TMEDA) are significantly different. Indeed, the resolution of 2·4·TMEDA is mostly entropy controlled. We have found parallel pathways for the DTR, the slower of which is zero order in TMEDA, whereas the faster one has a first order dependence in TMEDA. Other ligands are less effective for the asymmetric substitution of N-Boc-2-lithiopiperidine. The results reported here have implications for dynamic resolution chemistry and it will be important to determine how widespread is the requirement for added achiral ligands in such processes.
Supplementary Material
Acknowledgments
We thank the EPSRC (EP/E012272/1) and the National Science Foundation (CHE 0616352). Core facilities were funded by the University of Sheffield, the National Institutes of Health (R15569) and the Arkansas Biosciences Institute. The authors are grateful to Professors David Collum and Nick Williams for helpful advice on interpretation of the kinetic data.
Footnotes
Electronic Supplementary Information (ESI) available: kinetic data for racemization and dynamic resolution. See DOI: 10.1039/b000000x/
Notes and references
- 1.See, for example Coldham I, Leonori D. Org. Lett. 2008;10:3923. doi: 10.1021/ol801579r. Wolckenhauer SA, Rychnovsky SD. Tetrahedron. 2005;61:3371. Stead D, O'Brien P, Sanderson AJ. Org. Lett. 2005;7:4459. doi: 10.1021/ol0516869. Ma D-W, Ma N. Tetrahedron Lett. 2003;44:3963. Xiao D, Lavey BJ, Palani A, Wang C, Aslanian RG, Kozlowski JA, Shih N-Y, McPhail AT, Randolph GP, Lachowicz JE, Duffy RA. Tetrahedron Lett. 2005;46:7653. Dieter RK, Chen N, Yu H, Nice LE, Gore VK. J. Org. Chem. 2005;70:2109. doi: 10.1021/jo0481405. Girard N, Hurvois J-P. Tetrahedron Lett. 2007;48:4097. Lim SH, Ma S, Beak P. J. Org. Chem. 2001;66:9056. doi: 10.1021/jo0108865. Gawley RE. Curr. Org. Chem. 1997;1:71.
- 2.Beak P, Lee WK. J. Org. Chem. 1993;58:1109. [Google Scholar]
- 3.a) Bailey WF, Beak P, Kerrick ST, Ma S, Wiberg KB. J. Am. Chem. Soc. 2002;124:1889. doi: 10.1021/ja012169y. [DOI] [PubMed] [Google Scholar]; b) McGrath MJ, Bilke JL, O'Brien P. Chem. Commun. 2006:2607. doi: 10.1039/b603804m. [DOI] [PubMed] [Google Scholar]; c) Coldham I, O'Brien P, Patel JJ, Raimbault S, Sanderson AJ, Stead D, Whittaker DTE. Tetrahedron: Asym. 2007;18:2113. [Google Scholar]
- 4.a) Beak P, Anderson DR, Curtis MD, Laumer JM, Pippel DJ, Weisenburger GA. Acc. Chem. Res. 2000;33:715. doi: 10.1021/ar000077s. [DOI] [PubMed] [Google Scholar]; b) Lee WK, Park YS, Beak P. Acc. Chem. Res. 2009;42:224. doi: 10.1021/ar8000662. [DOI] [PubMed] [Google Scholar]; c) Coldham I, Dufour S, Haxell TFN, Patel JJ, Sanchez-Jimenez G. J. Am. Chem. Soc. 2006;128:10943. doi: 10.1021/ja061963m. For a discussion of dynamic resolution and its application to 2-lithiated pyrrolidines. [DOI] [PubMed] [Google Scholar]
- 5.a) Coldham I, Raimbault S, Chovatia PT, Patel JJ, Leonori D, Sheikh NS, Whittaker DTE. Chem. Commun. 2008:4174. doi: 10.1039/b810988e. [DOI] [PubMed] [Google Scholar]; b) Coldham I, Patel JJ, Raimbault S, Whittaker DTE. Chem. Commun. 2007:4534. doi: 10.1039/b710066c. [DOI] [PubMed] [Google Scholar]
- 6.a) Yousaf TI, Williams RL, Coldham I, Gawley RE. Chem. Commun. 2008:97. doi: 10.1039/b714302h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ashweek NJ, Brandt P, Coldham I, Dufour S, Gawley RE, Haeffner F, Klein R, Sanchez-Jimenez G. J. Am. Chem. Soc. 2005;127:449. doi: 10.1021/ja048090l. [DOI] [PubMed] [Google Scholar]; c) Beng TK, Yousaf TI, Coldham I, Gawley RE. J. Am. Chem. Soc. 2009;131:6908. doi: 10.1021/ja900755a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enantiomer ratio (er), normalized as a fraction or percent, is the preferred expression of enantiopurity in kinetic analysis. See: Gawley RE. J. Org. Chem. 2006;71:2411. doi: 10.1021/jo052554w. J. Org. Chem. 2008;73:6470. corrigendum.
- 8.a) Waldmüller D, Kotsatos BJ, Nichols MA, Williard PG. J. Am. Chem. Soc. 1997;119:5479. [Google Scholar]; b) Fraenkel G, Liu H. J. Am. Chem. Soc. 2004;126:5202. doi: 10.1021/ja030285q. [DOI] [PubMed] [Google Scholar]
- 9.a) Sun X, Winemiller MD, Xiang BS, Collum DB. J. Am. Chem. Soc. 2001;123:8039. doi: 10.1021/ja010136c. [DOI] [PubMed] [Google Scholar]; b) Granander J, Sott R, Hilmersson G. Chem. Eur. J. 2006;12:4191. doi: 10.1002/chem.200501371. [DOI] [PubMed] [Google Scholar]; c) Khartabil HK, Gros PC, Fort Y, Ruiz-López MF. J. Org. Chem. 2008;73:9393. doi: 10.1021/jo8019434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


