Abstract
BACKGROUND
Patent foramen ovale (PFO) is significantly associated with cryptogenic stroke (CS). However, even in patients with CS, a PFO can be an incidental finding. We sought to estimate the probability that a PFO in a patient with CS is incidental.
METHODS
A systematic search identified 23 case-control studies examining the prevalence of PFO in patients with CS versus controls with stroke of known-cause. Using simple assumptions and Bayes’ theorem we calculated the probability a PFO is incidental in patients with CS. Random effects meta-analyses estimated the odds ratio (OR) of a PFO in CS versus controls in different age populations, with or without atrial septal aneurysms (ASA), and were used to summarize across studies the probability that a PFO in CS is incidental.
RESULTS
The summary OR (95% confidence limits) for PFO in CS versus controls was 2.9 (CI 2.1, 4.0). The corresponding ORs for young and old patients (< or ≥ 55 years) were 5.1 (3.3, 7.8) and 2.0 (1.0, 3.7), respectively. The corresponding probabilities that a PFO in patients with CS is incidental were 33% (28%, 39%) in age-inclusive studies, 20% (16%, 25%) in younger patients, and 48% (34%, 66%) in older patients. These probabilities were much lower when an ASA was present.
CONCLUSIONS
In patients with otherwise CS, approximately a third of discovered PFOs are likely to be incidental, and hence not benefit from closure. This probability is sensitive to patient characteristics such as age and the presence of an ASA, suggesting the importance of patient-selection in therapeutic decision-making.
Keywords: Patent foramen ovale, Risk factors for stroke, Secondary stroke prevention
INTRODUCTION
Despite extensive workup, the cause of stroke remains unknown in approximately one third of patients. Such strokes are classified as cryptogenic stroke (CS).1 Several studies have shown a significant association between CS and the presence of a patent foramen ovale (PFO), suggesting that paradoxical emboli (i.e. emboli crossing from the venous to arterial circulation through a PFO) may be an important cause of CS.2 Thus, many physicians recommend PFO closure in patients with CS for secondary stroke prevention.
While the logic supporting PFO closure in a patient with CS seems compelling, the risk of stroke recurrence in patients with CS and PFO appears to be low, with an average annualized risk across studies of ~2%.3, 4 Further, it is well appreciated that PFO is a common and generally benign finding present on autopsy in ~25% of the population.3, 5, 6 Thus, while CS may be associated with PFO, some may be incidental, even in the setting of CS. Presumably, closure of an incidental PFO would expose patients to procedural and device-related risks without benefit, since the cause of the CS remains unaddressed. Thus, for any given patient, the possibility of benefit is dependent on the risk of stroke recurrence conditional on the probability that the index stroke is attributable to the PFO.
Despite an extensive literature on the association of PFO and CS, prior studies have not addressed a critical question relevant to both clinical practice and design of PFO closure trials; namely: what is the probability that a PFO in a patient with CS is an incidental finding, rather than the culprit? In the present study, we systematically review and summarize the evidence associating PFO with CS in case-control studies. Based on this evidence, we use Bayes’ theorem to estimate the probability that a PFO in a patient with CS is incidental in age-inclusive studies, and in studies limited to either younger or older patients.
METHODS
Probability that PFO is incidental in CS
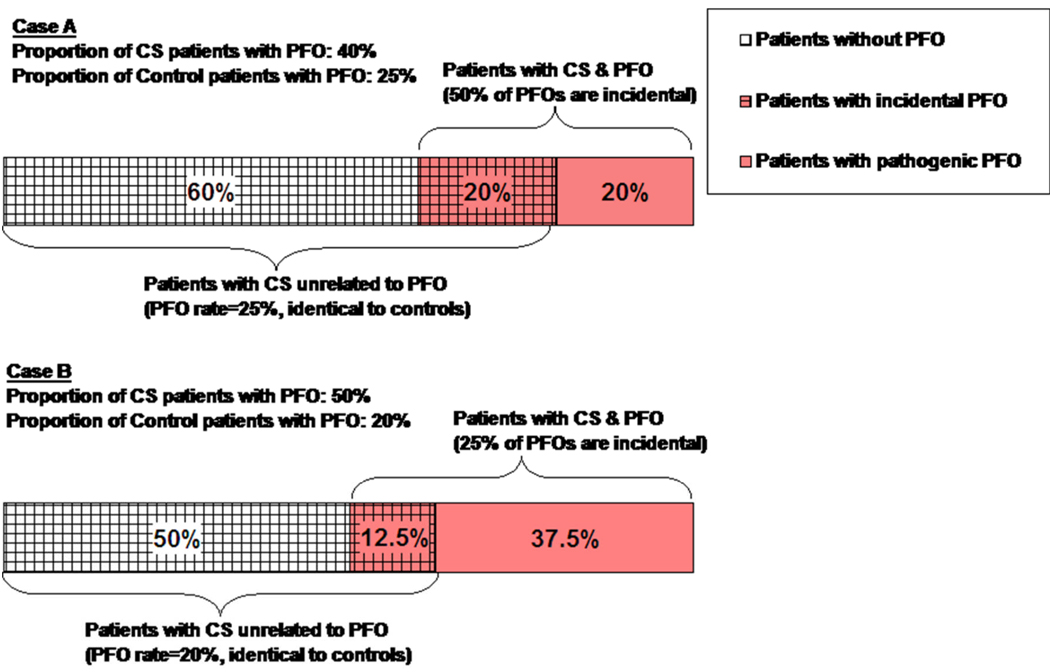
As shown in Figure 1, the proportion of incidental versus pathogenic PFOs in patients with CS can be calculated based on PFO prevalence in CS patients compared to controls. In Case A, PFO prevalence is 40% in the CS population and 25% in the control group. The corresponding figure indicates that, under these conditions, 50% of PFOs detected in CS patients would be incidental. This is based on the assumption that CS patients who have strokes from causes unrelated to PFO will have the same PFO prevalence as the control group (in this case 25%). If the PFO prevalence in CS patients were increased slightly to 50% and the PFO prevalence among control patients were decreased to 20% (as in Case B), then the rate of incidental PFOs among patients with CS and PFO would decrease to only 25%, as shown in Figure 1.
Figure 1. Proportion of CS Patients without PFO, with incidental PFO and with pathogenic PFO.
This figure shows how the proportion of incidental versus pathogenic PFO in patients with CS can be calculated based on the prevalence of PFO in CS patients and in controls. Case A shows that when the prevalence of PFO in the CS population is 40% and the prevalence of PFO in the control group is 25%, then 50% of PFOs discovered in CS patients would be incidental. This is based on the assumption that CS patients who have strokes from causes unrelated to PFO will have the same PFO prevalence as the control group (in this case 25%). If the PFO prevalence in CS patients were increased slightly to 50% and the PFO prevalence among control patients were decreased, then the rate of incidental PFOs among patients with CS and PFO would decrease to only 25% (Case B).
This simple conceptual framework is a direct application of Bayes’ theorem, as detailed in appendix Figure 1. To apply Bayes’ theorem, as in the prior examples, we made two assumptions: 1) if not for those strokes attributable to PFO, the prevalence of PFO would be similar in patients with CS compared to controls; and 2) CS in patients without a detected PFO is not caused by an undetected PFO. As shown in appendix Figure 1, these assumptions permit the calculation of the probability that a PFO is incidental based only on the prevalence of PFO in CS cases and in controls according to the following equation:
This equation can be applied to case-control studies, since all the terms on the left side of the equation are known.
Literature search and study selection
We sought to include all published case-control studies examining the association of PFO and CS. Our primary analysis was based on studies comparing the prevalence of PFO in cases with CS versus controls with ischemic stroke of determined cause, since these represent similar patient groups. A sensitivity analysis was also performed based on case-control studies comparing the prevalence of PFO in CS cases versus non-stroke controls. Studies were identified based on the most recently published systematic review of PFO and stroke, and complemented with an updated search of MEDLINE to cover the period from 1998 (2 years prior to publication of the most recent systematic review) to June 2008.2 The search used the following terms patent foramen ovale, atrial septal aneurysm, and right-to-left shunt.
Data extraction
Full manuscripts of eligible studies were reviewed and data directly extracted into electronic data tables. For each study, we extracted: first author, journal, publication year, mean age, number of cases and controls with corresponding numbers of patients with PFO, with or without atrial septal aneurysm (ASA), and modality used to diagnose PFO (e.g. transesophageal echocardiogram (TEE) versus transcranial Doppler). For studies that were included in the most recent systematic review, data were extracted by a single investigator and validated against the numbers reported in the published review.2 For more recent studies not included in the prior systematic review, independent dual data extraction was performed, and differences resolved by consensus.
Meta-analyses of case-control studies
We performed random-effects meta-analyses to estimate the summary odds-ratio (OR) and 95% confidence limits of having a PFO in patients with CS versus controls with ischemic stroke of determined cause.7 Separate meta-analyses were performed for studies that were age-inclusive (i.e. set no upper or lower age limit for inclusion), those that were limited to young patients (typically defined as < 55), and studies that were limited to older patients (typically ≥ 55 years or older). Heterogeneity between studies was tested using the Q statistic (considered significant at p < 0.10), and its extent quantified with I2.8 All meta-analyses were performed in Stata/SE 9.2 (StataCorp LP, College Station, TX) using the metan procedure..
Using our Bayes theorem-derived equation, we calculated the probability that a discovered PFO in a patient with CS is incidental for each individual study. A summary probability for all studies combined was calculated with confidence limits based on the natural log of each study’s probability that a PFO is incidental and its weight in a random-effects model.9
Sensitivity analyses
In separate sensitivity analyses, both the random-effects meta-analyses and the corresponding Bayesian estimation of the probability a PFO is incidental were repeated using studies comparing the prevalence of PFO in cases with CS versus controls without stroke. We further divided these studies into ones that used referred controls (i.e. subjects requiring evaluation for a clinical indication other than a stroke) or healthy controls (i.e. volunteers without a clinical indication for evaluation).
RESULTS
Eligible studies
There were 17 age-inclusive case-control studies comparing PFO prevalence in patients with CS versus controls with ischemic stroke of known cause (Table 1), with a total of 1,154 cases with CS (of which 427 had a PFO) and 1,852 controls (of which 296 had a PFO).10–26 Of these studies, 5 reported separate analyses for younger and older patients.10, 12, 16, 20, 26 Six additional studies limited enrollment to young patients.27–32 This resulted in a total of 11 entries for young patients and 5 for old patients (Table 1). Fewer studies examined the association of PFO with concurrent ASA in cases with CS versus controls with ischemic stroke of known cause (2 age-inclusive, 2 young-patient, and 1 old-patient analyses).25, 26, 30 The diagnostic technique for PFO was TEE in the majority of studies (74%).
Table 1.
Case-control studies of patients with cryptogenic stroke versus controls with ischemic stroke of known-cause (age-inclusive analyses).
| Cases (PFO/n) |
Controls (PFO/n) |
Age Category | Diagnostic Technique |
|||
|---|---|---|---|---|---|---|
| Age-inclusive | Younger | Older | ||||
| Lechat 1988 | 20/41 | 4/19 | − | + (<55) | − | TTE |
| Webster 1988 | 19/34 | 1/6 | − | + (<40) | − | TTE |
| Jeanrenaud 1990 | 8/11 | 0/5 | − | − (<50) | − | TTE |
| Hausmann 1992 | 16/74 | 10/48 | + | + (<40) | + (≥40) | TTE |
| de Belder 1992 | 9/35 | 10/69 | + | − | − | TEE |
| Di Tullio 1992 | 19/45 | 7/101 | + | + (<55) | + (≥55) | TTE |
| Di Tullio 1993 | 9/19 | 10/25 | + | − | − | TEE |
| Cabanes 1993 | 36/64 | 7/36 | − | + (<55) | − | TEE |
| Ranoux 1993 | 31/54 | 1/14 | − | + (<55) | − | TEE |
| Homma 1994 | 16/36 | 7/38 | + | − | − | TEE |
| Albers 1994 | 3/25 | 39/120 | + | − | − | TEE |
| Jones 1994 | 14/71 | 21/149 | + | + (<50) | + (≥50) | TEE |
| Job 1994 | 27/41 | 11/33 | − | + (≤45) | − | TEE |
| Klotzsch 1994 | 31/40 | 19/71 | + | − | − | TEE |
| Zahn 1995 | 50/118 | 18/70 | + | − | − | TEE |
| Schminke 1995 | 33/60 | 8/40 | + | − | − | TCD |
| Yeung 1996 | 43/116 | 17/94 | + | + (<50) | + (≤50) | TCD |
| Petty 1997 | 22/55 | 15/61 | + | − | − | TEE |
| Roijer 1997 | 17/67 | 3/54 | + | − | − | TEE |
| Serena 1998 | 30/53 | 38/150 | + | − | − | TCD |
| Steiner 1998 | 19/42 | 12/53 | + | − | − | TEE |
| Kanda 1998 | 19/71 | 30/433 | + | − | − | TEE |
| Handke 2007 | 77/227 | 34/276 | + | + (<55) | + (≤55) | TEE |
Association of PFO with CS
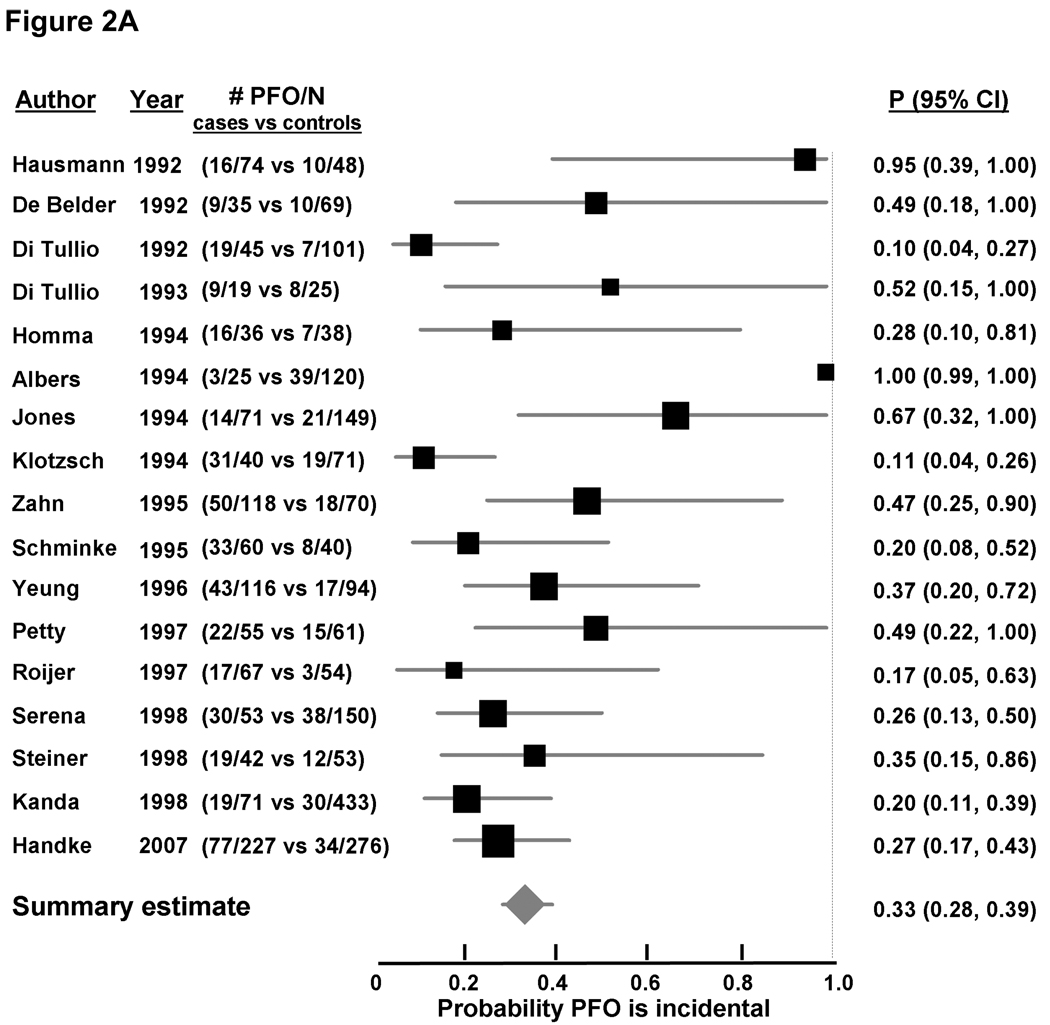
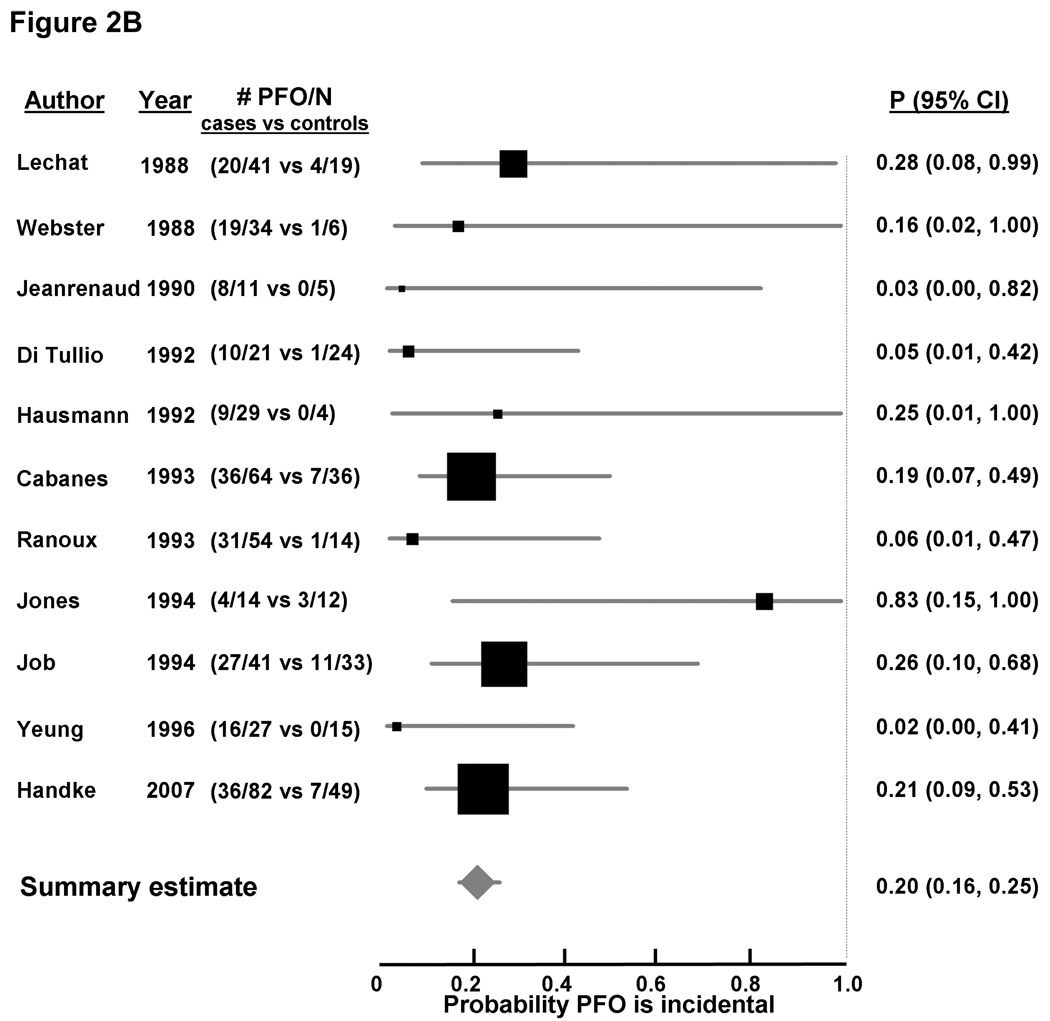
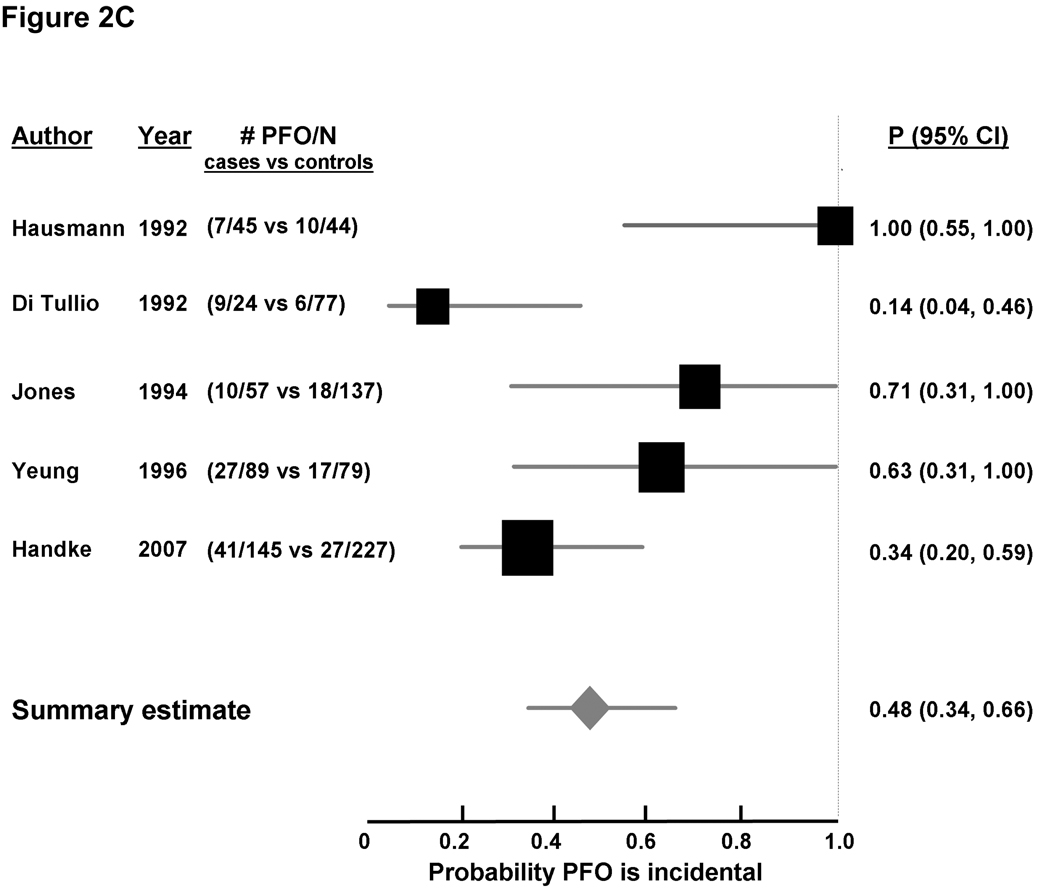
Random-effects meta-analysis of age-inclusive studies showed a significant association between PFO and CS (OR 2.9 [2.1, 4.0], Figure 2A appendix), with significant heterogeneity among studies (I2 0.63, p < 0.0001). A stronger significant association between CS and PFO was observed in studies enrolling younger patients (OR 5.1, [3.3, 7.8], Figure 2B appendix), without significant heterogeneity among studies (I2 0.0, p = 0.47). Conversely, the association between PFO and CS in studies enrolling older patients was weaker but did reach statistical significance (OR 2.0, [>.0, 3.7], Figure 2C appendix), with significant between-study heterogeneity (I2 0.67, p = 0.018). While fewer studies examined the association of PFO and concomitant ASA with CS compared to ischemic stroke of known cause, that association was quite strong; the pooled OR was 8.9 [1.2, 64.0] for the two age-inclusive studies; 11.3 [2.6, 48.9] for the two studies examining younger patients, and 3.9 [1.8, 8.5] in the analysis in older patients.
Probability that PFO is incidental in CS
In age-inclusive studies, the proportion of patients with PFO among CS cases ranged from 12% to 78% (median 40%, interquartile range 26% to 46%), and the corresponding proportion among controls with ischemic stroke of known cause ranged from 6% to 33% (median 20%, interquartile range 14% to 25%). In studies of younger patients, the proportion of patients with PFO ranged from 29% to 73% (median 56%, interquartile range 45% to 59%) among cases and 0% to 33% (median 14%, interquartile range 1% to 21%) among controls. The corresponding proportions in studies of older patients ranged from 16% to 38% (median 28%, interquartile range 17% to 32%) in cases and 8% to 23% (median 13%, interquartile range 11% to 22%) in controls.
Using Bayes’ theorem as described above, the summary probability that a PFO is incidental in patients with CS was 33% [28%, 39%] in age-inclusive studies (Figure 2A). The corresponding probability was 20% [16%, 25%] for younger patients (Figure 2B), and 48% [34%, 66%] for older patients (Figure 2C). A PFO was less likely an incidental finding when a concomitant ASA was detected, with the probability of it being incidental estimated at 11% (4%, 31%) from age-inclusive studies, 9% (4%, 18%) in younger patients, and 26% (12%, 56%) in older patients (Table 2).
Figure 2. Probability that a PFO is incidental in patients with CS, based on case-control studies examining the prevalence of PFO in cases with CS versus controls with stroke of determined cause.
Individual studies are represented on the left with first author and year of publication, and prevalence of PFO (# PFO/total number of patients) in cases versus controls. Black boxes with sizes corresponding to each study’s weight in the analysis represent the point estimate of the probability that the PFO is incidental with 95% confidence intervals represented with the grey lines (P 95% 23 CI). The diamond in the last row represents the summary estimate of the probability. The dashed black line to the right of the panel represents a probability of 100% that the PFO was incidental (i.e. not related to the CS). Panel A: age-inclusive studies, Panel B: analyses in younger patients, and Panel C: analyses in older patients.
Table 2.
Probability a patent foramen ovale (PFO) with or without an atrial septal aneurysm (ASA) is incidental in patients with cryptogenic stroke, by age category, based on case-control studies of cases with cryptogenic stroke versus controls with stroke of determined cause (main analysis), or versus controls with no stroke (sensitivity analysis).
| Probability PFO is incidental | ||
|---|---|---|
| Main Analysis | Sensitivity Analysis | |
| PFO | ||
| Age-inclusive | 0.33 (0.28, 0.39) | 0.48 (0.39, 0.59) |
| Young | 0.20 (0.16, 0.25) | 0.20 (0.16, 0.25) |
| Old | 0.48 (0.34, 0.66) | 0.84 (0.60, 1.00) |
| PFO+ASA | ||
| Age-inclusive | 0.11 (0.04, 0.31) | --- |
| Young | 0.09 (0.04, 0.18) | 0.04 (0.01, 0.32) |
| Old | 0.26 (0.12, 0.56) | --- |
Sensitivity analyses
The above analyses were repeated using data from case-control studies examining the prevalence of PFO in CS cases versus non-stroke controls (10 age-inclusive studies, 6 studies in younger patients, and 3 studies in older patients).10, 11, 16, 18, 22, 23, 33–36 As per the primary analysis, there was a significant association between PFO and CS in age-inclusive studies (OR 2.1, [1.4, 3.1], I2 0.68, p = 0.001 for between-study heterogeneity, Figure 3A appendix) as well as in studies of younger patients (OR 5.0, [3.2, 7.6], I2 0.0, p = 0.44 for between-study heterogeneity, Figure 3B appendix), and a non-significant association in studies of older patients (OR 1.0, [0.5, 1.8], I2 0.06, p = 0.35 for between-study heterogeneity, Figure 3C appendix). The corresponding probabilities that a PFO is incidental in patients with CS were 48% (39%, 59%) for age-inclusive studies, 20% (16%, 25%) for studies of younger patients, and 84% (60%, 100%) for studies of older patients.
In the subgroup of studies that used referred controls, the summary OR for PFO in CS was 2.6 [1.7, 4.0] in age-inclusive studies, 6.5 [3.6, 11.7] in studies of younger patients, and 0.6 [0.2, 1.7] in studies of older patients.10, 11, 18, 27, 30, 33–37 The corresponding probabilities that a PFO was incidental were 39% [31%, 48%], 15% [11%, 20%], and 100% [76%, 100%], respectively. In contrast, in the subgroup of studies that used healthy controls, the summary OR for PFO in CS was 1.4 [0.9, 2.2] in age-inclusive studies, 3.7 [1.9, 7.2] in studies of younger patients, and 1.3 [0.6, 2.8] in studies of older patients.16, 22, 23, 36 The corresponding probabilities that a PFO was incidental were 71% [56%, 89%], 27% [19%, 37%], and 79% [53%, 100%], respectively.
Only one study reported the prevalence of a PFO with an ASA in CS cases versus controls without a stroke, and was limited to patients younger than 55 years (OR 23.9, [3.1, 185.4]).30 This yielded a corresponding probability that a PFO with ASA is incidental when detected in a CS patient younger than 55 years of only 4%, but with wide confidence limits (1%, 32%).
DISCUSSION
We used a Bayesian approach to estimate the probability that a PFO in a patient with CS is an incidental finding rather than pathogenic in 23 case-control studies and summarized these effects using meta-analytic techniques. Our analysis estimates that approximately one third of PFOs discovered in patients with CS are likely to be incidental and unrelated to the stroke (and somewhat higher when estimates are based on studies using non-stroke controls). This estimate is sensitive to patient age, and is higher in older patients. In addition, the probability that a PFO is incidental is much lower in any age when associated with an ASA.
Given the extensive literature associating PFO with CS, the observation that a substantial proportion of discovered PFOs in patients with CS may be incidental might seem surprising. However, the literature to date has focused only on estimating the strength of the association between a PFO and CS, presenting estimates in the form of an OR, the clinical significance of which can be difficult to interpret. The Bayesian approach adopted in the present analysis goes beyond an estimate of association to address the clinically relevant question of whether the discovered PFO is likely to be etiologically related to the stroke.
The heterogeneity between the studies included in the present analysis is not surprising both because study and diagnostic methods vary, and because numerous factors can potentially affect the degree of association between PFO and CS, and thus the likelihood that a discovered PFO in the setting of CS is incidental. For example, PFO has been found to be more likely to be associated with CS in patients who were younger, did not have hypertension (HTN), hyperlipidemia, diabetes or tobacco use.4, 38
In addition to age and conventional stroke risk factors, morphologic features of a PFO may influence the association between PFO and CS and hence the probability that the PFO is incidental.14, 23, 24, 39 In the present analysis, the presence of a PFO with a concomitant ASA yielded a lower probability of it being an incidental finding. Additionally, multiple studies comparing PFO characteristics of patients with CS versus strokes of known cause have found that larger PFO, greater right-to-left shunt and higher septal wall motion mobility, are more frequent in patients with CS.14, 23, 24, 39 Thus, the likelihood that PFO is pathogenic when found in CS patients is sensitive to multiple patient characteristics. For example, even in patients younger than 55, incidental PFOs would be more likely if patients are near the upper margin of the age category and/or have conventional ischemic stroke risk factors (HTN, high cholesterol, DM, smoking) and/or lower risk morphologic/physiologic PFO features on TEE. Conversely, PFOs may more likely be pathogenic even in patients in the older age range in those who do not have other stroke risk factors but do have high-risk features on TEE.
While patient factors associated with PFOs increase the confidence that paradoxical embolism is the likely mechanism for an individual patient’s stroke, these factors may not be the same as those that predict risk of recurrent paradoxical embolism. Additionally, procedural complications of PFO closure include stroke, e.g. from thrombus formation on the device, and so the risk may increase with the intervention.40 Given the significant number of incidental PFOs that are likely to be present and the relatively low risk of stroke recurrence while on medical therapy in patients with CS and PFO, careful assessment of the risks and benefits of treatment options is essential.3
For interventions where the risks and benefits are finely balanced, it has been demonstrated that multivariate predictive modeling can be useful to identify subgroups in clinical trials likely to benefit or not from the tested therapy.41–43 Using our Bayesian framework, baseline characteristics and PFO morphologic features that predict the presence of a PFO in patients with CS can be used to predict the probability that a discovered PFO is likely to be incidental. Predictive modeling can also be performed separately to estimate the probability of stroke recurrence in patients with PFO and CS.4 For trials testing the efficacy of endovascular closure (RESPECT, PC-Trial), stratifying results by the joint probability that the CS was related to the PFO and the probability the stroke will recur offers the potential for a refined and novel approach to patient selection.44, 45
The present Bayesian approach used to derive the probability of an incidental PFO was informed by estimates obtained from case-control studies of PFO in CS, and relied on two basic assumptions. Therefore, proper interpretation of these findings should take into account the inherent limitations of case-control studies including potential selection bias, presence of unmeasured confounders, and possible differential intensity in the investigation of a PFO in CS cases versus controls. Furthermore, the findings are based on two assumptions, namely that the prevalence of PFO would be similar in patients with CS compared to controls if not for those strokes attributable to PFO, and that CS in patients without a detected PFO is not caused by an undetected PFO. While the first assumption is intuitive, the second may not necessarily be true; though presumably PFOs that go undetected are less likely to be of clinical significance. Despite these caveats, the present analysis offers a novel contribution to our intuitive interpretation of case-control studies, by extending such interpretation from association to a measure of attributable risk.
In conclusion, in patients with otherwise CS, about a third of discovered PFOs are likely to be incidental, and hence endovascular closure is not likely to reduce their recurrent stroke risk. This probability is sensitive to patient characteristics such as age, and morphologic features of a PFO such as the presence of a concurrent ASA.
Supplementary Material
Acknowledgments
This study was partially supported by R01 NS062153. Dr. Alsheikh-Ali is a recipient of a Pfizer/Tufts Medical Center faculty development award. We thank Dr Thomas Trikalinos for insightful discussions regarding statistical analysis, and Jennifer Donovan for help with manuscript preparation.
References
- 1.Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, Wolf PA. Infarcts of undetermined cause: The NINCDS stroke data bank. Ann Neurol. 1989;25:382–390. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 2.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: A meta-analysis of case-control studies. Neurology. 2000;55:1172–1179. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 3.Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005;112:1063–1072. doi: 10.1161/CIRCULATIONAHA.104.524371. [DOI] [PubMed] [Google Scholar]
- 4.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–1746. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 5.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 6.Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, Whisnant JP, Wiebers DO, Covalt JL, Petterson TM, Christianson TJ, Agmon Y. Patent foramen ovale: Innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47:440–445. doi: 10.1016/j.jacc.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 9.Cramer H. Mathematical methods of statistics. Princeton Univeristy Press; 1999. [Google Scholar]
- 10.Hausmann D, Mugge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol. 1992;70:668–672. doi: 10.1016/0002-9149(92)90210-p. [DOI] [PubMed] [Google Scholar]
- 11.de Belder MA, Tourikis L, Leech G, Camm AJ. Risk of patent foramen ovale for thromboembolic events in all age groups. Am J Cardiol. 1992;69:1316–1320. doi: 10.1016/0002-9149(92)91228-v. [DOI] [PubMed] [Google Scholar]
- 12.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–465. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]
- 13.Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke. 1993;24:1020–1024. doi: 10.1161/01.str.24.7.1020. [DOI] [PubMed] [Google Scholar]
- 14.Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Li Mandri G, Mohr JP. Characteristics of patent foramen ovale associated with cryptogenic stroke. A biplane transesophageal echocardiographic study. Stroke. 1994;25:582–586. doi: 10.1161/01.str.25.3.582. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Comess KA, DeRook FA, Bracci P, Atwood JE, Bolger A, Hotson J. Transesophageal echocardiographic findings in stroke subtypes. Stroke. 1994;25:23–28. doi: 10.1161/01.str.25.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Jones EF, Calafiore P, Donnan GA, Tonkin AM. Evidence that patent foramen ovale is not a risk factor for cerebral ischemia in the elderly. Am J Cardiol. 1994;74:596–599. doi: 10.1016/0002-9149(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 17.Klotzsch C, Janssen G, Berlit P. Transesophageal echocardiography and contrast-tcd in the detection of a patent foramen ovale: Experiences with 111 patients. Neurology. 1994;44:1603–1606. doi: 10.1212/wnl.44.9.1603. [DOI] [PubMed] [Google Scholar]
- 18.Zahn R, Lehmkuhl S, Lotter R, Zander M, Senges J. Cardiac sources of cerebral ischemic events with special regard to a patent foramen ovale. Herz Kreislauf. 1995;27:279–284. [Google Scholar]
- 19.Schminke U, Ries S, Daffertshofer M, Staedt U, Hennerici M. Patent foramen ovale: A potential source of cerebral embolism? Cerebrovasc Dis. 1995;5:133–138. [Google Scholar]
- 20.Yeung M, Khan KA, Shuaib A. Transcranial doppler ultrasonography in the detection of venous to arterial shunting in acute stroke and transient ischaemic attacks. J Neurol Neurosurg Psychiatry. 1996;61:445–449. doi: 10.1136/jnnp.61.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petty GW, Khandheria BK, Chu CP, Sicks JD, Whisnant JP. Patent foramen ovale in patients with cerebral infarction. A transesophageal echocardiographic study. Arch Neurol. 1997;54:819–822. doi: 10.1001/archneur.1997.00550190013008. [DOI] [PubMed] [Google Scholar]
- 22.Roijer A, Lindgren A, Algotsson L, Norrving B, Olsson B, Eskilsson J. Cardiac changes in stroke patients and controls evaluated with transoesophageal echocardiography. Scand Cardiovasc J. 1997;31:329–337. doi: 10.3109/14017439709075949. [DOI] [PubMed] [Google Scholar]
- 23.Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Davalos A. The need to quantify right-to-left shunt in acute ischemic stroke: A case-control study. Stroke. 1998;29:1322–1328. doi: 10.1161/01.str.29.7.1322. [DOI] [PubMed] [Google Scholar]
- 24.Steiner MM, Di Tullio MR, Rundek T, Gan R, Chen X, Liguori C, Brainin M, Homma S, Sacco RL. Patent foramen ovale size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;29:944–948. doi: 10.1161/01.str.29.5.944. [DOI] [PubMed] [Google Scholar]
- 25.Kanda N, Yasaka M, Otsubo R, Nagatsuka K, Minematsu K, Yamaguchi T. [right-to-left shunt and atrial septal aneurysm in stroke patients: A contrast transesophageal echocardiographic study] Rinsho Shinkeigaku. 1998;38:213–218. [PubMed] [Google Scholar]
- 26.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–2268. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 27.Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 28.Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, Glasgow GL. Patent foramen ovale in young stroke patients. Lancet. 1988;2:11–12. doi: 10.1016/s0140-6736(88)92944-3. [DOI] [PubMed] [Google Scholar]
- 29.Jeanrenaud X, Bogousslavsky J, Payot M, Regli F, Kappenberger L. [patent foramen ovale and cerebral infarct in young patients] Schweiz Med Wochenschr. 1990;120:823–829. [PubMed] [Google Scholar]
- 30.Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, Chedru F, Guerin F, Bousser MG, de Recondo J. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–1873. doi: 10.1161/01.str.24.12.1865. [DOI] [PubMed] [Google Scholar]
- 31.Ranoux D, Cohen A, Cabanes L, Amarenco P, Bousser MG, Mas JL. Patent foramen ovale: Is stroke due to paradoxical embolism? Stroke. 1993;24:31–34. doi: 10.1161/01.str.24.1.31. [DOI] [PubMed] [Google Scholar]
- 32.Job FP, Ringelstein EB, Grafen Y, Flachskampf FA, Doherty C, Stockmanns A, Hanrath P. Comparison of transcranial contrast doppler sonography and transesophageal contrast echocardiography for the detection of patent foramen ovale in young stroke patients. Am J Cardiol. 1994;74:381–384. doi: 10.1016/0002-9149(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 33.Van Camp G, Schulze D, Cosyns B, Vandenbossche JL. Relation between patent foramen ovale and unexplained stroke. Am J Cardiol. 1993;71:596–598. doi: 10.1016/0002-9149(93)90518-h. [DOI] [PubMed] [Google Scholar]
- 34.Labovitz AJ, Camp A, Castello R, Martin TJ, Ofili EO, Rickmeyer N, Vaughn M, Gomez CR. Usefulness of transesophageal echocardiography in unexplained cerebral ischemia. Am J Cardiol. 1993;72:1448–1452. doi: 10.1016/0002-9149(93)90195-i. [DOI] [PubMed] [Google Scholar]
- 35.Mesa D, Franco M, Suarez de Lezo J, Munoz J, Rus C, Delgado M, Ruiz M, Pan M, Romo E, Valles F, Vinals M, Bescansa E. Prevalence of patent foramen ovale in young patients with cerebral ischemic accident of unknown origin. Rev Esp Cardiol. 2003;56:662–668. doi: 10.1016/s0300-8932(03)76936-x. [DOI] [PubMed] [Google Scholar]
- 36.Petty GW, Khandheria BK, Meissner I, Whisnant JP, Rocca WA, Christianson TJ, Sicks JD, O'Fallon WM, McClelland RL, Wiebers DO. Population-based study of the relationship between patent foramen ovale and cerebrovascular ischemic events. Mayo Clin Proc. 2006;81:602–608. doi: 10.4065/81.5.602. [DOI] [PubMed] [Google Scholar]
- 37.Vella MA, Sulke AN, Rodrigues CA, McNabb WR, Lewis RR. Patent foramina ovale in elderly stroke patients. Postgrad Med J. 1991;67:745–746. doi: 10.1136/pgmj.67.790.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: Patent foramen ovale in cryptogenic stroke study. Circulation. 2002;105:2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 39.De Castro S, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, Beccia M, Colonnese C, Fedele F, Fieschi C, Pandian NG. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–2413. doi: 10.1161/01.str.31.10.2407. [DOI] [PubMed] [Google Scholar]
- 40.Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. doi: 10.1016/j.jacc.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: The need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 43.Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. Treating individuals 3: From subgroups to individuals: General principles and the example of carotid endarterectomy. Lancet. 2005;365:256–265. doi: 10.1016/S0140-6736(05)17746-0. [DOI] [PubMed] [Google Scholar]
- 44.Randomized evaluation of recurrent stroke comparing pfo closure to established current standard of care treatment. Clinicaltrials.Gov id = NCT00465270. Information at http://www.Strokecenter.Org/trials/trialdetail.Aspx?Tid=482.
- 45.Pc-trial: Patent foramen ovale and cryptogeni embolism. Information at http://clinicaltrials.Gov/ct2/show/nct00166257.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.