Abstract
Background
Colorectal cancer (CRC) is a feared complication of chronic ulcerative colitis (UC). Annual endoscopic surveillance is recommended to detect early neoplasia. 5-aminosalicylates (5-ASAs) may prevent some UC-associated CRC. Therefore, in patients prescribed 5-ASAs for maintenance of remission, annual surveillance might be overly burdensome and inefficient. We aimed to determine the ideal frequency of surveillance in patients with UC maintained on 5-ASAs.
Methods
We performed systematic reviews of the literature, and created a Markov computer model simulating a cohort of 35 year-old men with chronic UC, followed until age 90. Twenty-two strategies were modeled: Natural History (no 5-ASA or surveillance), surveillance without 5-ASA at intervals of 1 to 10 years, 5-ASA plus surveillance every 1 to 10 years, and 5-ASA alone. The primary outcome was the ideal interval of surveillance in the setting of 5-ASA maintenance, assuming a third-party payer was willing to pay $100,000 for each quality-adjusted life-year (QALY) gained.
Results
In the Natural History strategy, the CRC incidence was 30%. Without 5-ASA, annual surveillance was the ideal strategy, preventing 89% of CRC and costing $69,100 per QALY gained compared to surveillance every 2 years. 5-ASA alone prevented 49% of CRC. In the setting of 5-ASA, surveillance every 3 years was ideal, preventing 87% of CRC. 5-ASA with surveillance every 2 years cost an additional 147,500 per QALY gained, and 5-ASA with annual surveillance cost nearly $1 million additional per QALY gained compared to every 2 years. In Monte Carlo simulations, surveillance every 2 years or less often was ideal in 95% of simulations.
Conclusions
If 5-ASA is efficacious chemoprevention for UC-associated CRC, endoscopic surveillance might be safely performed every 2 years or less often. Such practice could decrease burdens to patients and to endoscopic resources with a minimal decrease in quality-adjusted length of life, since 5-ASA with annual surveillance may cost nearly $1 million per additional QALY gained.
Keywords: Chemoprevention, Colonic Neoplasms, Decision Support Techniques, Surveillance, Ulcerative Colitis
INTRODUCTION
Colorectal cancer (CRC) is a feared complication of chronic ulcerative colitis (UC). The cumulative incidence of CRC after 30 years of UC is believed to be as high as 21.5%.1 Gastroenterology societies recommend regular colonoscopic surveillance with random biopsies every 1–2 years after 8–10 years of pancolitis.2–4 Colectomy is performed if high-grade dysplasia or cancer is identified and confirmed, and is often recommended for confirmed low-grade dysplasia.5 The effectiveness of this cancer prevention strategy is limited by sampling error, variation in histologic interpretation, and poor adherence. 5-ASA medications are used to treat chronic ulcerative colitis, and have been associated with decreased risk of the development of colorectal cancer.6–9 However, not all epidemiologic studies have found this association.10, 11 A meta-analysis of the existing studies found that 5-ASA use was associated with a net reduction of 49% in CRC incidence in chronic ulcerative colitis.12 It is believed that 5-ASA medications are plausible chemopreventive agents, as they have been shown to produce activation of caspases and to induce apoptosis in cancer cells in vitro.13–20 In fact, in vivo studies in humans with colon cancer have shown that mesalamine enemas can induce apoptosis in colon tumors.21 Mesalamine use also appears to decrease proliferation and increase the apoptosis rate in normal colonic mucosa.22 These results suggest that 5-ASA compounds could plausibly reduce the occurrence of dysplasia, or cause apoptosis and regression of pre-existing dysplasia.
Since many patients with UC are prescribed 5-ASA for maintenance of remission, they may simultaneously benefit from chemoprevention by 5-ASA for CRC. In that setting, annual colonoscopic surveillance may provide little incremental benefit in terms of detecting neoplasia compared to less frequent surveillance, and the risks of the procedure might outweigh those benefits. Furthermore, the cost of annual surveillance might not be justified by a very small incremental benefit. None of the published decision analyses regarding prevention of CRC in UC have accounted for the effect of chemoprevention.23–25 We hypothesized that among patients receiving 5-ASA, the most cost-effective frequency of colonoscopic surveillance would be less often than annually. We developed a Markov model and performed a cost-effectiveness analysis to answer this question, and to determine which variables significantly affect the ideal surveillance interval.
METHODS
Subjects
The hypothetical cohort consisted of 35 year-old men with a 10-year history of ulcerative pancolitis that is quiescent at the time of enrollment. The analysis followed the cohort until age 90 or death, whichever occurred first.
Model
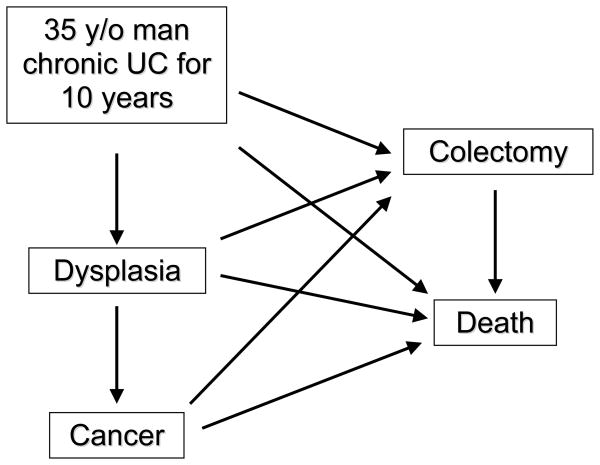
Decision analysis was modeled by creating a Markov process with TreeAge Pro 2007 software (TreeAge, Williamstown, MA). A Markov process is a mathematical simulation of hypothetical patients over time. Unlike decision trees, Markov processes are recursive, allowing movement back and forth between health states at the end of each one-year cycle. A simplified schematic of the model is shown in Figure 1. The actual model contains thousands of nodes, accounting for the natural history of patients with quiescent UC, the various strategies for CRC mortality prevention (5-ASA alone and surveillance in intervals of 1 to 10 years with or without 5-ASA). The model includes risks of iatrogenic complications, undetected dysplasia or cancer, and false positive results indicating neoplasia.
Figure 1. Simplified Schematic of Markov Model Health States and Transitions.
In any given one-year cycle, hypothetical patients start in one of the five health states (rectangles), and have specified rates of transitioning (arrows) to other health states. Each strategy has associated costs and an associated quality of life, measured by utility. Some of the transitions also have costs and effects on utility.
Natural History
The reference strategy was the Natural History of UC without any 5-ASA or surveillance. Patients could have a severe flare of disease requiring a total colectomy and ileal-pouch anal anastomosis (IPAA), develop dysplasia (although this would go undetected as they did not receive surveillance), and progress from dysplasia to cancer which would be detected only when it became symptomatic. The prevalence of cancer 10 years after diagnosis of UC was based on a published meta-analysis.1 The annual incidence of cancer thereafter was assumed to be constant over time, and was based on the same meta-analysis. We assumed that there was no possibility of regressing from true dysplasia, and that there was no possibility of developing cancer without first progressing through dysplasia; both assumptions bias the model in favor of surveillance. Localized or regional cancer was treated with total colectomy. Patients with regional cancer also received chemotherapy for 8 weeks. Patients with metastatic cancer did not undergo surgery, but received 4 cycles of chemotherapy.26 Patients could die from CRC, from a complication of surgery, from a colitis flare, or of causes unrelated to colitis. Stage-specific CRC mortality rates were obtained from the Surveillance Epidemiology and End Results registry.27 Age-specific mortality rates in patients with UC from causes other than CRC or directly related to colitis were derived from empiric data.28, 29
Surveillance Colonoscopy
We created 10 strategies of surveillance colonoscopy without 5-ASA, ranging from intervals of every 1 to every 10 years. In each of these strategies, random mucosal biopsies were obtained in 4 quadrants every 10 centimeters, and submitted to pathology in 4 separate specimen containers. If dysplasia was diagnosed, patients underwent total colectomy with IPAA. If cancer was detected, therapy was guided by stage as in the Natural History arm. The surveillance strategies incorporated false negative and false positive rates, as well as morbid and mortal complications of endoscopy. We assumed that cancers diagnosed by surveillance colonoscopy were asymptomatic and therefore less likely to be metastatic.30
5-ASA Alone
In this strategy, patients received Asacol 2.4 grams per day for maintenance of UC remission, but did not undergo any surveillance. A previously published meta-analysis was updated to include two more recently published articles assessing the effect of 5-ASA on the risk of UC-associated CRC.10, 12, 31 Using MIX 1.7 software,32, 33 the resulting random effects model provided a summary odds ratio of 0.57 (95% confidence interval 0.40, 0.81). The annual incidence risk ratio for 5-ASA was calibrated to this odds ratio after 10 years of follow-up in the model (20 years after UC diagnosis since the patients enter the model after a 10 year history of UC). We assumed that the protective effect of 5-ASA was equal for the rate of progression from no dysplasia to dysplasia and for the rate of progression from dysplasia to cancer. A sensitivity analysis was performed to determine whether the outcomes changed if the effect of 5-ASA was entirely in the first transition or the second transition.
5-ASA Plus Surveillance Colonoscopy
There were 10 combination strategies of 5-ASA with colonoscopic surveillance, ranging from every 1 to 10 years. These patients had a decreased rate of developing dysplasia and cancer (as in the 5-ASA strategy), underwent colectomy with IPAA for dysplasia when detected by surveillance, and benefited from surveillance by detecting cancers at earlier stages (as in the surveillance strategies).
Transition Rates
Transition rates between the health states were derived from the published literature (Table 1). MEDLINE was searched from 1966 through 2007 using the terms ulcerative colitis, aminosalicylic acids, mesalamine, mesalazine, 5-aminosalicyl$, 5-ASA, Asacol, Pentasa, Salofalk, Rowasa, Asamax, Canasa, SPD476, Llialda, Meavent, Mesasal, Claversal, Azulfidine, sulfasalazine, olsalazine, Dipentum, balsalazide, Colazide, Colazal, colectomy, ileal pouch anal anastomosis, colon cancer, prognosis, morbidity, mortality, natural history, clinical trials, follow-up studies and meta-analysis. Additional articles were identified by searching the bibliographies of these articles. Except as noted below, base case values were chosen from the means or medians of the published literature. Upper and lower limits were taken from 95% confidence intervals if available; otherwise, inclusive ranges were used.
Table 1.
Model Assumptions
| Variable | Base Case | One-Way Sensitivity Analysis Range | Monte Carlo Simulation Distribution (median; 95% CI) | References |
|---|---|---|---|---|
| Annual rate of ulcerative colitis flare requiring colectomy | 0.23% | 0.16%–0.49% | Beta (0.22%; 0.10%–0.42%) | 46,47 |
| Cancer | ||||
| Annual incidence | 0.84% | 0.71–1.5% | Beta (0.82%; 0.49%–1.3%) | 1 |
| Median time of progression from dysplasia to cancer | 3 years | 1–5 years | Lognormal (3.0; 1.7–5.2) | 48 |
| Median latency of cancer until symptomatic presentation | 2 years | 0.5–3 years | Lognormal (2.0; 1.2–3.3) | * |
| Annual risk ratio of cancer with 5 -ASA vs. no 5-ASA |
0.43 0.50 |
0.26–0.65 0.30–0.78 |
Lognormal (0.50; 0.30–0.81) | 10, 12, 31 |
| Proportion of cancers that are metastatic at presentation | 20.0% | 19.3%–20.7% | Beta (20.0%; 19.3%–20.7%) | 27 |
| Proportion of non-metastatic cancers that are local | 50.6% | 49.9%–51.4% | Beta (50.7%; 49.9%–51.4%) | 27 |
| Relative risk of metastatic cancer with surveillance vs. no surveillance | 0.31 | 0.11–1 | Lognormal (0.31; 0.10–0.93) | 30 |
| Colonoscopy test characteristics | ||||
| Sensitivity of surveillance for cancer | 95% | 80%–100% | Beta (97%; 81%–100%) | 49* |
| Sensitivity of surveillance for dysplasia | 90% | 55%–100% | Beta (94%; 58%–100%) | 49 |
| Specificity for neoplasia | 99.9% | 90–100% | Beta (99.99%; 99.2%–100%) | * |
| Complications | ||||
| Mortality from colectomy performed for flare | 9.5% | 3.0%–16.0% | Beta (9.1%; 4.0–16.9%) | 50 |
| Mortality from colectomy performed for surveillance | 1.9% | 0.5%–6.6% | Beta (1.5%; 0.1%–5.9%) | 50 |
| Morbid perioperative complication from colectomy. | 28.1% | 27.0%–29.3% | Beta (28.1%; 27.0%–29.3%) | 51 |
| Mortality from surveillance colonoscopy | 0.0060% | 0.0055–0.0065% | Beta (0.0056%; 0.0019%–0.012%) | 52 |
| Morbid complication from surveillance colonoscopy | 0.35% | 0.28%–0.42% | Beta (0.35%; 0.28%–0.43%) | 52,53 |
| Costs | ||||
| Annual 5 -ASA 2.4 gram/day | $1,476 | $738–$2,951 | Lognormal ($1,476; $738–$2,951) | 35 |
| Annual care of chronic ulcerative colitis | $2,687 | $1,344–$5,375 | Lognormal ($2,687; $1,344–$5,375) | 54,55 |
| Colonoscopy with surveillance biopsies | $1,054 | $527–$2,107 | Lognormal ($1,054; $527–$2,107) | CMS |
| Morbid complication of colonoscopy | $5,724 | $2,862–$11,448 | Lognormal ($5,724; $2,862–$11,447) | 24, 52, 53 |
| Colectomy with IPAA | $22,129 | $11,065–$44,258 | Lognormal ($22,129; $11,065–$44,257) | CMS |
| Annual care of IPAA | $173 | $87–$346 | Lognormal ($173; $87–$346) | 55–58 CMS |
| Diagnosis and staging of cancer | $1,512 | $756–$3,024 | Lognormal ($1,512; $756–$3,024) | CMS |
| Chemotherapy for regional cancer | $12,613 | $6,307–$25,226 | Lognormal ($12,613; $6,307–$25,226) | 26 |
| Chemotherapy for metastatic cancer | $50,452 | $25,226–$100,904 | Lognormal ($50,452; $25,227–$100,903) | 26 |
| Annual post-surgical care of cancer (excludes chemotherapy) | $8,813 | $4,406–$17,626 | Lognormal ($8,813; $4,407–$17,626) | 59 |
| Annual care of unresectable cancer (excludes chemotherapy) | $41,991 | $20,996–$83,983 | Lognormal ($41,991; $20,996–$83,981) | 60 |
| Utilities | ||||
| Chronic ulcerative colitis (no 5-ASA) | 0.97 | 0.86–0.99 | Beta (0.98; 0.88–1.00) | see methods |
| Chronic ulcerative colitis (on 5-ASA) | 0.98 | 0.89–1.00 | Beta (0.99; 0.90–1.00) | see methods |
| IPAA | 0.93 | 0.75–1.00 | Beta (0.95; 0.76–1.00) | 61 |
| Local cancer | 0.74 | 0.69–0.78 | Beta (0.74; 0.69–0.78) | 62 |
| Regional cancer | 0.63 | 0.44–0.77 | Beta (0.63; 0.56–0.70) | 62 |
| Metastatic cancer | 0.24 | 0.16–0.36 | Beta (0.24; 0.16–0.32) | 62 |
| Toll for complication of colectomy | 8.3% | 2.1%–18.8% | Beta (7.6%; 2.0%–18.4%) | * |
| Toll for complication colonoscopy | 3.1% | 1.0%–12.5% | Beta (2.3%; 0.1%–10.9%) | * |
| Discount rate | 3% | 0%–5% | Not varied | 34 |
Author consensus
CI–confidence interval
CMS–Centers for Medicare and Medicaid Services
IPAA–colectomy with ileal-pouch anal anastomosis
Costs and Utilities
The perspective was that of a third-party payer; therefore, modeled costs included direct healthcare costs, but not indirect healthcare costs (such as lost productivity costs for patients and their families), or direct non-healthcare costs (such as patient transportation costs) (Table 1).34 The wholesale cost of Asacol 2.4 gram daily was estimated by the average price offered by 8 online discount pharmacies.35 The base case cost of chemotherapy was based on 5-flurouracil, leucovorin and oxaliplatin given as the FOLFOX regimen; the upper limit added the cost of bevacizumab.26 Discrete procedural and hospitalization costs were based on median national reimbursements from the Centers for Medicare and Medicaid Services for year 2007. Listed costs for endoscopies include the cost of histopathologic processing and interpretation. The cost of diagnosing and staging symptomatic cancer included the cost of colonoscopy with biopsy, computed tomogram and laboratory tests. The costs of chronic care of UC, and complications of colonoscopy were derived from published estimates, adjusting for 3% annual inflation to the year 2007. If 95% confidence limits were not available, the upper and lower limits were set at ½ and twice the base case estimate of cost, respectively.
Utilities are ratios reflecting patient preferences for particular health states, ranging from 0 (death) to 1 (perfect health). Utilities were based on published empiric data for CRC and IPAA (Table 1). The utilities of UC on and off 5-ASA were estimated as weighted averages of the utility of UC in remission and an outpatient UC flare, assuming the decrement of quality of life from a UC flare lasted 10 weeks, and accounting for the annual likelihood of a symptomatic flare on and off 5-ASA. The utility of an outpatient UC flare was derived as previously described.36 The utility for UC in remission was determined empirically using the time trade-off method from n=46 patients at the University of Michigan (median utility = 0.99, interquartile range = 0.91, 1.00). The Institutional Review Board of the University of Michigan approved that portion of the study. Subjects were deemed in remission if their Simple Clinical Colitis Index was less than 3.37 All costs and utilities were discounted at an annual rate of 3%, with sensitivity analysis from 0% to 5%.
Outcomes
The time horizon of the model was age 90 or death (whichever was earliest), and the outcomes were judged from the perspective of a third-party payer. The incremental cost-effectiveness ratio (ICER) is defined as the difference in cost in dollars, divided by the difference in effectiveness in quality-adjusted life-years (QALYs) between competing strategies. The primary outcome was the ideal strategy in a patient already taking 5-ASA; we defined the ideal strategy as the one providing the most QALYs at a cost of no more than $100,000 per each additional QALY compared with the next most effective strategy (willingness to pay = $100,000/QALY).38 Additional outcomes measured for each strategy included the lifetime risk of CRC, life expectancy, and the proportion undergoing colectomy for cancer, dysplasia, false positive neoplasia, and flare.
Sensitivity Analyses
One-way sensitivity analyses were performed over the specified ranges for each variable (Table 1), comparing all strategies simultaneously to determine which strategy was ideal. The relative effects of 5-ASA on the progression to dysplasia, and from dysplasia to cancer, were explored by attributing the incidence risk ratio of cancer entirely to the first transition or the second.
Probabilistic Monte Carlo simulation was performed to simultaneously randomly vary each variable in the model for strategies with 5-ASA. The type of distribution used for each variable, their median and 95% confidence interval, are displayed in Table 1. Beta distributions were used for probabilities and utilities. Lognormal distributions were used for relative risks and costs. The covariance structure was assumed to be symmetrical. The model was run with 1,000 independent simulations, and the proportion of simulations in which each strategy was ideal at any particular willingness to pay was calculated. Since the strategies being compared are different in frequency of surveillance rather than categorical differences, the data is presented as cumulative acceptability curves stacking more frequent intervals on less frequent intervals. The Monte Carlo simulations were also repeated with 1,000 simulations with the efficacy of 5-ASA held constant at its most and least efficacious.
RESULTS
Natural History
Over their entire lifetimes, 30% of patients not receiving 5-ASA or surveillance colonoscopy developed colorectal cancer (Figure 2). In 23% of patients, the cancer was found at an early enough stage that they underwent colectomy, and 7% had distant metastatic CRC. An additional 7% underwent colectomy for a severe flare of UC (Figure 3). The average life expectancy was 71.4 years (Figure 4). Patients experienced 20.07 QALYs in their remaining lives at a discounted lifetime cost of $71,000 (Figure 5).
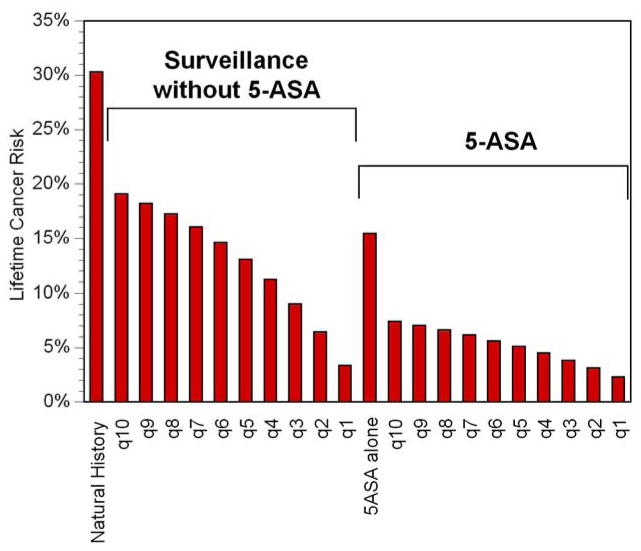
Figure 2. Proportion of Patients Developing Cancer.
Each column represents a different strategy. The height of the column represents the lifetime cumulative risk of colorectal cancer. Among patients not taking 5-ASA, more frequent surveillance leads to substantial decreases in risk of cancer, but among those taking 5-ASA, there is much less incremental improvement in cancer risk from more frequent surveillance.
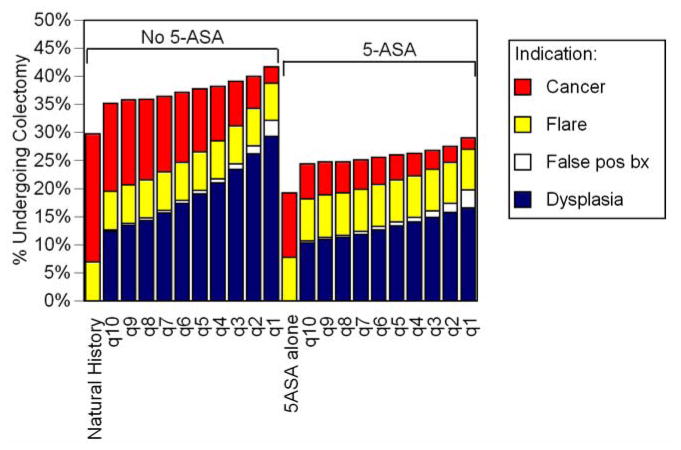
Figure 3. Proportion of Patients Undergoing Colectomy.
The x-axis displays each strategy. The total height of each column displays the cumulative lifetime chance of undergoing colectomy for any cause. Each colored section of a column represents the lifetime chance of undergoing colectomy for a specific indication indicated by the legend. Patients receiving 5-ASA were less likely to undergo colectomy, due to fewer cancers and dysplasias. More frequent surveillance shifts the indication for colectomy from cancer to dysplasia, and slightly increases the chance of undergoing colectomy for a false positive finding.
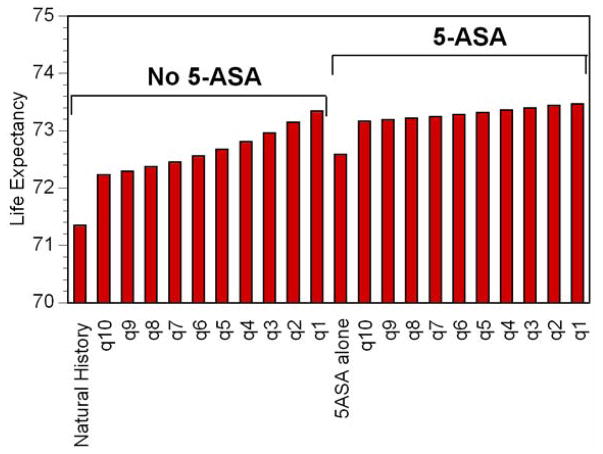
Figure 4. Life Expectancy.
The x-axis displays each strategy. The height of each column represents the average lifespan for a 35 year-old man with 10 prior years of ulcerative colitis for each strategy. In the setting of no 5-ASA, progressively shorter intervals between surveillance colonoscopy substantially improves life expectancy. Among patients already taking 5-ASA, shorter surveillance intervals also improves life expectancy, but with smaller incremental improvements.
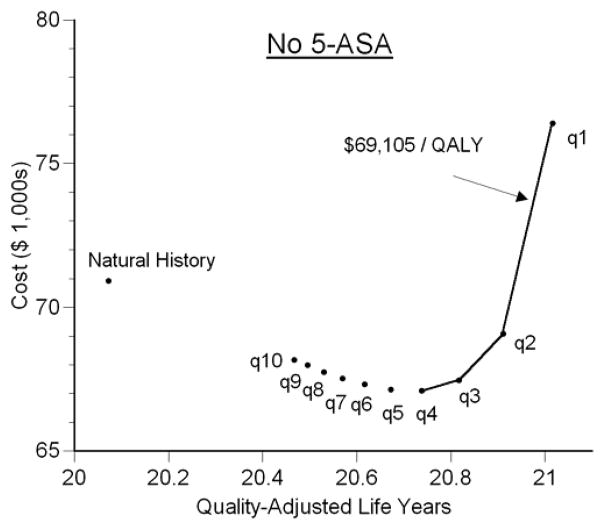
Figure 5. Cost-QALY Plot for Patients without 5-ASA.
The x-axis displays average remaining quality-adjusted life-years (QALYs) for each of 11 strategies without 5-ASA. The y-axis displays average lifetime costs accrued after age 35. Up until surveillance every 3 years, progressively shorter surveillance intervals dominate the longer intervals meaning that they provide more QALYs at lower cost. Beginning with surveillance every 3 years, the incremental cost-effectiveness ratio (ICER) is depicted by the slope of the line connecting two strategies (the extra cost per QALY gained). Annual surveillance is ideal since it provides the most QALYs at cost that is less than the threshold of $100,000 per QALY.
Surveillance Colonoscopy Alone
In the absence of 5-ASA, performing surveillance every 10 years decreased the lifetime risk of CRC to 19% (Figure 2). Shorter intervals between surveillance were associated with progressively lower risks of CRC, such that annual surveillance led to a lifetime risk of CRC of 3%. Surveillance led to a higher incidence of colectomy, largely due to the detection of dysplasia, but also a small incidence attributable to false positive findings of neoplasia (Figure 3). Twenty-nine percent of those undergoing annual surveillance eventually underwent colectomy due to dysplasia. Shorter intervals between surveillance led to substantial incremental improvements in life expectancy (Figure 4). Despite the decrement in quality of life related to colectomy resulting from surveillance, this improvement in life expectancy led to substantial incremental benefits in QALYs (Figure 5). Annual surveillance cost $69,100 per each additional QALY compared to surveillance every other year, an incremental cost-effectiveness ratio that falls below the $100,000/QALY willingness-to-pay threshold.
5-ASA Alone
5-ASA without any surveillance prevented 49% of expected cancers (Figure 2), a proportion equivalent to that prevented by surveillance every 6–7 years without 5-ASA use. Life expectancy with 5-ASA alone was similar to that with surveillance every 5–6 years in the absence of 5-ASA (Figure 4). The lifetime risk of colectomy for any indication was reduced to 19%, the lowest of any strategy (Figure 3).
Surveillance Colonoscopy in the Setting of 5-ASA
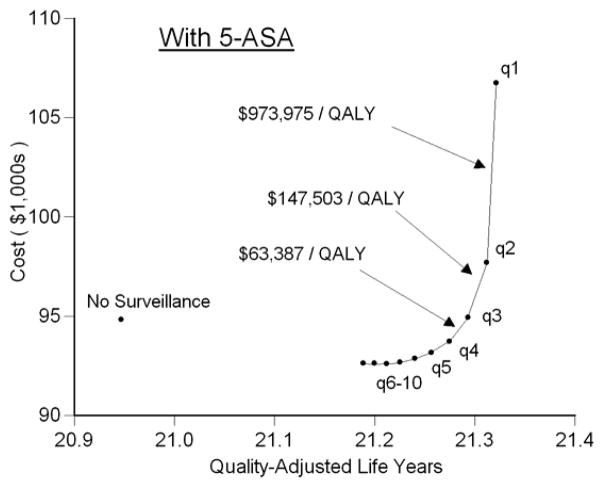
In the setting of a patient taking 5-ASA for maintenance of remission, performing surveillance colonoscopy every 10 years reduced the risk of cancer to 7%, a risk similar to that of a patient not treated with 5-ASA who undergoes surveillance every other year (Figure 2). Surveillance performed at progressively shorter intervals resulted in incremental reductions in risk of cancer of less than 1%. Surveillance every 3 years while taking 5-ASA led to a slightly greater risk of cancer as annual surveillance in the absence of 5-ASA (3.9% vs. 3.4%). Regardless of the surveillance interval, all patients taking 5-ASA and undergoing surveillance had a lower risk of colectomy for any indication compared to the natural history arm (Figure 3). 5-ASA plus surveillance every 4–5 years led to a similar life expectancy as annual surveillance without 5-ASA (Figure 4). Surveillance every 3 years cost $63,400 per additional QALY compared to surveillance every 4 years (Figure 6). Because of the small incremental improvements in life expectancy, more frequent surveillance in the setting of 5-ASA may be construed as too expensive. Surveillance every other year cost $147,500 per QALY gained compared to every 3 years, and annual surveillance cost nearly $1 million per QALY gained compared to surveillance every other year.
Figure 6. Cost-QALY Plot for Patients Taking 5-ASA.
This plot is similar to Figure 5, but displays the 11 strategies for patients taking 5-ASA for maintenance of remission. Surveillance every 10 years dominates no surveillance, but progressively shorter intervals incur additional costs. Annual surveillance is extremely expensive (nearly $1 million per QALY gained). Surveillance every 3 years is ideal in the setting of a patient taking 5-ASA, since the cost of surveillance every 2 years is greater than that the threshold of $100,000 per QALY. Note that the label for strategies of surveillance every 6 to 10 years are condensed due to space limitations; in this range (as in the range of shorter intervals), progressively shorter intervals provide more QALYs.
One-Way Sensitivity Analyses
In order to assess the robustness of the model results, we varied each of the variables across their ranges listed in Table 1, determining the ideal strategy in the setting of 5-ASA. Only alterations in the variables listed in Table 2 resulted in an ideal surveillance interval that differed from the base case of every 3 years. For instance, if the incidence of cancer were at its upper limit, then in the setting of a patient taking 5-ASA, surveillance every 2 years was ideal. Surveillance every 2 years was also ideal if the efficacy of 5-ASA for preventing colorectal cancer were at its lower limit. In no scenario was annual surveillance ideal in the setting of 5-ASA. In contrast, if the quality of life with ileo-anal anastomosis were at its lower limit, then surveillance every 7 years was ideal. Similarly, if the specificity of colonoscopy for neoplasia were at its lower limit (the false positive rate was at its upper limit) then surveillance every 9 years was ideal.
Table 2.
One-Way Sensitivity Analyses
| Ideal at Low Value | Variable | Ideal at High Value |
|---|---|---|
| 5-ASA + q2 years | Cost of colonoscopy | 5-ASA + q4years |
| 5-ASA + q2 years | Time for progression of dysplasia to cancer | 5-ASA + q4 years |
| 5-ASA + q2 years | Discount rate | 5-ASA + q3 years |
| 5-ASA + q3 years | Incidence of cancer | 5-ASA + q2 years |
| 5-ASA + q5 years | Risk ratio of cancer with 5-ASA | 5-ASA + q2 years |
| 5-ASA + q7years | Quality of life with IPAA | 5-ASA + q2 years |
| 5-ASA + q9 years | Specificity of colonoscopy for neoplasia | 5-ASA + q3 years |
One-way sensitivity analyses were performed on each variable across their ranges listed in Table 1, evaluating for changes in the ideal strategy in the setting of a patient receiving 5-ASA for maintenance therapy. Only those analyses that resulted in changes from the base case result of 5-ASA with surveillance every 3 years are presented. For instance, if the cost of colonoscopy were at its lowest value, then surveillance every 2 years was ideal, and if the cost were at its highest value, then surveillance every 4 years was ideal. In no case was annual surveillance ideal.
The range identified in the annual incidence of cancer, and used in Table 1 and Table 2 was obtained from a published meta-analysis.1 However, if the lower limit of the incidence of cancer were reduced so that the lifetime risk of cancer in the natural history were 10% (which calibrated to annual risk of 0.24% in the model), then in the setting of 5-ASA, surveillance every 7 years would be ideal.
We assessed the robustness of the model to variations in the assumption of the mechanism of action of 5-ASA. In the base case, we had assumed that the effect of 5-ASA was equally weighted on the progression from no dysplasia to dysplasia and from dysplasia to cancer. If the effect was entirely on the progression from dysplasia to cancer, then surveillance colonoscopy would needlessly direct some proportion of patients with dysplasia to colectomy who would not have needed colectomy to prevent cancer. We found that in such a scenario, surveillance every 4 years was ideal. Further varying the efficacy of 5-ASA resulted in ideal surveillance intervals of 2–7 years. If the effect of 5-ASA were entirely on the progression from no dysplasia to dysplasia with no effect on the progression to cancer, then the ideal surveillance interval was every 2 years. In that case, varying the efficacy of 5-ASA resulted in ideal intervals of 2–3 years.
Monte Carlo Simulations
The robustness of the models with and without 5-ASA were further tested by 1,000 independent Monte Carlo simulations, which simultaneously randomly varies each variable in the model. At a willingness to pay of $100,000 for each additional QALY gained, in the absence of 5-ASA, annual surveillance was ideal in 67% of simulations, and surveillance every 2 years was ideal in 29% of simulations. At a willingness to pay of $50,000 per QALY, the corresponding proportions were 31% and 57% for annual and biennial surveillance, respectively. At a willingness to pay of $250,000 per QALY, annual surveillance was ideal in 97% of simulations.
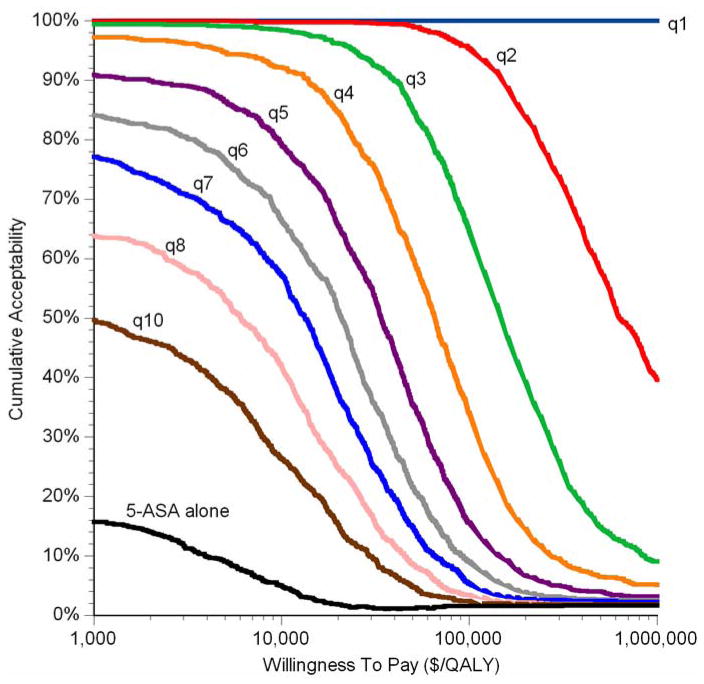
In the setting of a patient taking 5-ASA, a surveillance interval every 2 years or less often was ideal in 95% of simulations, at a willingness to pay of $100,000 per QALY gained (Figure 7). An interval of every 3 years or less often was ideal in 65% of simulations. At a willingness to pay of $50,000/QALY, the corresponding proportions were 99% and 85%, respectively. At a willingness to pay of $250,000/QALY, the corresponding proportions were 78% and 32% respectively. If the efficacy of 5-ASA were held constant at the upper limit of relative risk (0.78), then surveillance every 2 years or less often was ideal in 79% of simulations at a willingness to pay of $100,000 per QALY (data not shown in figures). If the efficacy of 5-ASA were held constant at the lower limit of relative risk (0.30), then at a willingness to pay of $100,000 per QALY, surveillance every 3 years or less often was ideal in 94% of simulations, and surveillance every 4 years or less often was ideal in 80% of simulations (data not shown in figures).
Figure 7. Cumulative Acceptability Curves in Patients Taking 5-ASA.
Each area between lines represents the proportion of 1,000 Monte Carlo simulations in which that strategy is ideal at a particular willingness to pay. Since the strategies are different from each other in degree (frequency of surveillance), rather than category, the curves are stacked to present the cumulative acceptability. Any point on a curve represents the proportion of simulations in which surveillance at that frequency or less often is ideal at that willingness to pay. Note that surveillance every 9 years was never ideal in more than 0.5% of simulations at any willingness to pay, and as omitted from the graph. Also note the x-axis is on a logarithmic scale.
DISCUSSION
We systematically reviewed the literature and performed a cost-effectiveness analysis of surveillance intervals to decrease mortality from CRC in the setting of chronic UC. In the setting of a patient taking 5-ASA medication to maintain remission of symptoms, we found that the chemopreventive properties of the medication allows for less intensive surveillance. In the base case scenario, surveillance every 3 years appeared ideal, as more frequent surveillance may be prohibitively expensive compared to its tiny incremental benefits. Annual surveillance cost $1 million for each additional QALY compared to surveillance every other year, an order of magnitude greater than any accepted threshold of willingness to pay.24 One-way sensitivity analyses indicated multiple scenarios at the extremes where surveillance every 2 years was ideal, but no scenario in which annual surveillance would be considered cost-effective. Probabilistic Monte Carlo simulation demonstrated that at a willingness to pay of $100,000 per QALY, surveillance every 2 years or less often was ideal in 95% of simulations. In the absence of 5-ASA, there is considerable uncertainty regarding the ideal surveillance interval; the currently accepted practice of annual surveillance was found to be ideal in only 67% of simulations.
This study was limited by the available empiric data, particularly regarding the natural history of UC developing into CRC. We could not identify a large study of incidence of CRC in a cohort of patients receiving neither surveillance nor 5-ASA. Likewise, the effect of 5-ASA on carcinogenesis is inferred from epidemiologic studies and basic science studies, but is not proven from prospective randomized controlled studies in humans. The observed epidemiologic relationship may be due to unmeasured confounders such as patient adherence and health-seeking behaviors. Furthermore, the locus of effect of 5-ASA on carcinogenesis (whether solely on the development of dysplasia, solely on the progression from dysplasia to cancer, or both), is not known. As a result, published guidelines do not recommend 5-ASA for chemoprevention, and the practice is controversial.3, 39 However, one-way sensitivity analyses across plausible ranges failed to identify annual surveillance as the ideal strategy, and in Monte Carlo simulations, annual surveillance was the ideal strategy in only 5% of simulations at a willingness to pay of $100,000 per QALY. Strengths of the study include the systematic review for input variables, the use of results from meta-analyses for key inputs, the structured comparison of multiple potential strategies for multiple outcomes, and the robustness of the results to the sensitivity analyses. Given the potential limitations of the study, we prefer to conservatively interpret the results, erring on the side of fewer cancers (and of too frequent costly surveillance). Therefore, until better empiric data are available, we would suggest surveillance every 2 years in patients taking 5-ASA, but less frequent surveillance may actually be ideal.
5-ASAs are not the only potential chemopreventive medications for UC-associated CRC. Folate, ursodiol, non-steroidal anti-inflammatory drugs, and statins may also be effective.40–45 The results of this model could be applied to these other pharmaceuticals by applying their particular cost, efficacy, and adverse effect profile. A key conclusion from this study is that efficacious chemoprevention, particularly if efficacious for the step from dysplasia to cancer, could decrease the burden of surveillance for patients and the healthcare system.
Previous decision analyses have examined surveillance for CRC associated with UC. Provenzale and others performed a cost-effectiveness analysis comparing surveillance at various intervals and doing nothing.24 They concluded that surveillance every 3 to 4 years was the optimal strategy, and that more frequent surveillance extended life expectancy but at prohibitively high incremental cost. Our analysis also found that more frequent surveillance extended life expectancy, but we included the costs of chronic care of UC to bias the model in favor of surveillance and away from chemoprevention. Because we included this additional real cost, we found that in the absence of 5-ASA, annual surveillance is the ideal strategy. Delcò and Sonnenberg compared biannual surveillance to doing nothing in a cost-benefit analysis, and concluded that surveillance could be preferred if the cumulative incidence of CRC was greater than 27%.23 That result is consistent with our analysis. Our study is different from both of these previous decision analyses in that it incorporates a novel strategy of chemoprevention.
In summary, we found that chemoprevention of colorectal cancer is a promising strategy for the management of patients with chronic ulcerative colitis. Until better empiric data on the efficacy of 5-ASA and natural history of UC is available, this analysis may help guide patients and their physicians in choosing an appropriate surveillance interval. Since 5-ASA compounds are so safe, and any randomized controlled trial of surveillance interval would require a very large sample size and long follow-up, unfortunately additional empiric data are unlikely to be available any time soon. In the setting of a patient already taking 5-ASA for maintenance of remission, the current study suggests that surveillance colonoscopy might be deferred to at least every other year, particularly in patients who find annual surveillance too burdensome. Annual surveillance likely provides very little incremental benefit at very substantial incremental cost.
What is current knowledge
The risk of colorectal cancer is increased in patients with chronic ulcerative colitis.
Frequent surveillance with colonoscopy is recommended to detect early neoplasia.
5-Aminosalicylates (5-ASA) are associated with a decreased risk of colorectal cancer.
What is new here
In a UC patient taking 5-ASA, surveillance every 3 years is ideally cost-effective.
In some scenarios, surveillance every 2 years is ideal.
Annual surveillance provided marginal benefits, at a cost of nearly $1 million per quality-adjusted life-year gained.
Acknowledgments
JHR is the Damon Runyon-Gordon Family Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI-36-07), and was supported by NIH K23DK079291. PDRH was supported by the NIH Mentored Research Award, 1K08DK080172, and the Crohn’s and Colitis Foundation Senior Research Award. JJ was supported by NIH Oncology Research Training Grant 2T32 CA009357. FV was supported by the Crohn’s and Colitis Career Development Award and the NIH/NCRR/OD UCSF-CTSI Award, KL2 RR024130. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
PDRH has received research support from Shire, and PDRH, UL, and FV have received research support from Proctor and Gamble. JHR created the model, performed the analyses, drafted the manuscript, and is the guarantor of the paper. AW, JJ, and PDRH performed the systematic review, contributed to the development of the model, and edited the manuscript. FV and UL contributed to the development of the model and the systematic review, and edited the manuscript.
The authors greatly appreciate the advice provided by Paul Taheri, M.D. and Michael Chernew, Ph.D.
Abbreviations
- ASA
aminosalicylic acid
- CRC
colorectal cancer
- ICER
incremental cost-effectiveness ratio
- IPAA
ileal-pouch anal anastomosis
- QALY
quality-adjusted life year
- UC
ulcerative colitis
References
- 1.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C. Colorectal cancer screening and surveillance: Clinical guidelines and rationale - Update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 3.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. American Journal of Gastroenterology. 2004;99:1371–85. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous The role of colonoscopy in the management of patients with inflammatory bowel disease. American Society for Gastrointestinal Endoscopy Gastrointestinal Endoscopy. 1998;48:689–90. doi: 10.1016/s0016-5107(98)70062-8. [DOI] [PubMed] [Google Scholar]
- 5.Steven HI, Noam H. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 6.van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–8. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Alimentary Pharmacology & Therapeutics. 2000;14:145–53. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 8.Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO. Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology. 1994;107:117–20. doi: 10.1016/0016-5085(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg BU, Broome U, Persson B. Proximal colorectal dysplasia or cancer in ulcerative colitis. The impact of primary sclerosing cholangitis and sulfasalazine: results from a 20-year surveillance study. Diseases of the Colon & Rectum. 2001;44:77–85. doi: 10.1007/BF02234825. [DOI] [PubMed] [Google Scholar]
- 10.Terdiman JP, Steinbuch M, Blumentals WA, Ullman TA, Rubin DT. 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflammatory Bowel Diseases. 2007;13:367–71. doi: 10.1002/ibd.20074. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein CN, Blanchard JF, Metge C, Yogendran M. Does the use of 5-aminosalicylates in inflammatory bowel disease prevent the development of colorectal cancer? American Journal of Gastroenterology. 2003;98:2784–8. doi: 10.1111/j.1572-0241.2003.08718.x. [DOI] [PubMed] [Google Scholar]
- 12.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–53. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 13.Stolfi C, Fina D, Caruso R, Caprioli F, Sarra M, Fantini MC, Rizzo A, Pallone F, Monteleone G. Cyclooxygenase-2-dependent and -independent inhibition of proliferation of colon cancer cells by 5-aminosalicylic acid. Biochemical Pharmacology. 2008;75:668–76. doi: 10.1016/j.bcp.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Fang HM, Mei Q, Xu JM, Ma WJ. 5-aminosalicylic acid in combination with nimesulide inhibits proliferation of colon carcinoma cells in vitro. World Journal of Gastroenterology. 2007;13:2872–7. doi: 10.3748/wjg.v13.i20.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu EC, Chai J, Ahluwalia A, Tarnawski AS. Mesalazine downregulates c-Myc in human colon cancer cells. A key to its chemopreventive action? Alimentary Pharmacology & Therapeutics. 2007;25:1443–9. doi: 10.1111/j.1365-2036.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 16.Luciani MG, Campregher C, Fortune JM, Kunkel TA, Gasche C. 5-ASA affects cell cycle progression in colorectal cells by reversibly activating a replication checkpoint. Gastroenterology. 2007;132:221–35. doi: 10.1053/j.gastro.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fina D, Franchi L, Caruso R, Peluso I, Naccari GC, Bellinvia S, Testi R, Pallone F, Monteleone G. 5-aminosalicylic acid enhances anchorage-independent colorectal cancer cell death. European Journal of Cancer. 2006;42:2609–16. doi: 10.1016/j.ejca.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Monteleone G, Franchi L, Fina D, Caruso R, Vavassori P, Monteleone I, Calabrese E, Naccari GC, Bellinvia S, Testi R, Pallone F. Silencing of SH-PTP2 defines a crucial role in the inactivation of epidermal growth factor receptor by 5-aminosalicylic acid in colon cancer cells. Cell Death & Differentiation. 2006;13:202–11. doi: 10.1038/sj.cdd.4401733. [DOI] [PubMed] [Google Scholar]
- 19.Brown WA, Farmer KC, Skinner SA, Malcontenti-Wilson C, Misajon A, O’Brien PE. 5-aminosalicyclic acid and olsalazine inhibit tumor growth in a rodent model of colorectal cancer. Digestive Diseases & Sciences. 2000;45:1578–84. doi: 10.1023/a:1005517112039. [DOI] [PubMed] [Google Scholar]
- 20.Reinacher-Schick A, Schoeneck A, Graeven U, Schwarte-Waldhoff I, Schmiegel W. Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis. 2003;24:443–51. doi: 10.1093/carcin/24.3.443. [DOI] [PubMed] [Google Scholar]
- 21.Bus PJ, Nagtegaal ID, Verspaget HW, Lamers CB, Geldof H, Van Krieken JH, Griffioen G. Mesalazine-induced apoptosis of colorectal cancer: on the verge of a new chemopreventive era? Alimentary Pharmacology & Therapeutics. 1999;13:1397–402. doi: 10.1046/j.1365-2036.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 22.Reinacher-Schick A, Seidensticker F, Petrasch S, Reiser M, Philippou S, Theegarten D, Freitag G, Schmiegel W. Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy. 2000;32:245–54. doi: 10.1055/s-2000-135. [DOI] [PubMed] [Google Scholar]
- 23.Delco F, Sonnenberg A. A decision analysis of surveillance for colorectal cancer in ulcerative colitis. Gut. 2000;46:500–6. doi: 10.1136/gut.46.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzale D, Wong JB, Onken JE, Lipscomb J. Performing a cost-effectiveness analysis: surveillance of patients with ulcerative colitis. Am J Gastroenterol. 1998;93:872–80. doi: 10.1111/j.1572-0241.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 25.Provenzale D, Kowdley KV, Arora S, Wong JB. Prophylactic colectomy or surveillance for chronic ulcerative colitis? A decision analysis. Gastroenterology. 1995;109:1188–96. doi: 10.1016/0016-5085(95)90578-2. [DOI] [PubMed] [Google Scholar]
- 26.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004;351:317–9. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 27.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, Mariotto A, Feuer E, Edwards B, editors. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2002. [Accessed: November 2, 2007]. based on November 2004 SEER data submission, posted to the SEER web site 2005. [Google Scholar]
- 28.Card T, Hubbard R, Logan RF. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 2003;125:1583–90. doi: 10.1053/j.gastro.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Survival and cause-specific mortality in ulcerative colitis: follow-up of a population-based cohort in Copenhagen County. Gastroenterology. 2003;125:1576–82. doi: 10.1053/j.gastro.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 30.Choi PM, Nugent FW, Schoetz DJ, Jr, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–24. doi: 10.1016/0016-5085(93)90715-o. [DOI] [PubMed] [Google Scholar]
- 31.Ullman T, Croog V, Harpaz N, Hossain S, Kornbluth A, Bodian C, Itzkowitz S. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clinical Gastroenterology & Hepatology. 2008;6:1225–30. doi: 10.1016/j.cgh.2008.05.020. quiz 1177. [DOI] [PubMed] [Google Scholar]
- 32.Bax L, Yu L, Ikeda N, Tsuruta H, Moons K. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Medical Research Methodology. 2006:6. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bax L, Yu L, Ikeda N, Tsuruta N, Moons K. MIX: Comprehensive free software for meta-analysis of causal research data - version 1.7. 2008 doi: 10.1186/1471-2288-6-50. http://www.mix-for-meta-analysis.info. [DOI] [PMC free article] [PubMed]
- 34.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 35.Gossner L, May A, Stolte M, Seitz G, Hahn EG, Ell C. KTP laser destruction of dysplasia and early cancer in columnar-lined Barrett’s esophagus. Gastrointestinal Endoscopy. 1999;49:8–12. doi: 10.1016/s0016-5107(99)70438-4. [DOI] [PubMed] [Google Scholar]
- 36.Yen E, Kane S, Ladabaum U. Cost-effectiveness of 5-aminosalicylic acid therapy for maintenance of remission in ulcerative colitis. American Journal of Gastroenterology. 2008 Sep 4; doi: 10.1111/j.1572-0241.2008.02130.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Higgins PDR, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54:782–8. doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Archives of Internal Medicine. 2003;163:1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel BM, Ho W, Esrailian E, Targan S, Higgins PD, Siegel CA, Dubinsky M, Melmed GY. Controversies in ulcerative colitis: a survey comparing decision making of experts versus community gastroenterologists. Clinical Gastroenterology & Hepatology. 7:168–74. doi: 10.1016/j.cgh.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology. 1997;112:29–32. doi: 10.1016/s0016-5085(97)70215-4. [DOI] [PubMed] [Google Scholar]
- 41.Pardi DS, Loftus EV, Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–93. doi: 10.1053/gast.2003.50156. [see comment] [DOI] [PubMed] [Google Scholar]
- 42.Bansal P, Sonnenberg A. Risk factors of colorectal cancer in inflammatory bowel disease. American Journal of Gastroenterology. 1996;91:44–8. [PubMed] [Google Scholar]
- 43.Velayos FS, Loftus EV, Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941–9. doi: 10.1053/j.gastro.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 44.Poynter JN, Gruber SB, Higgins PDR, Almog R, Bonner JD, Rennert H, Low M, Greenson JK, Rennert G. Statins and the Risk of Colorectal Cancer. New England Journal of Medicine. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 45.Chan EP, Lichtenstein GR. Chemoprevention: risk reduction with medical therapy of inflammatory bowel disease. Gastroenterology Clinics of North America. 2006;35:675–712. doi: 10.1016/j.gtc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 47.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clinical Gastroenterology & Hepatology. 2004;2:1088–95. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 48.Lashner BA, Hanauer SB, Silverstein MD. Optimal timing of colonoscopy to screen for cancer in ulcerative colitis. Ann Intern Med. 1988;108:274–8. doi: 10.7326/0003-4819-108-2-274. [DOI] [PubMed] [Google Scholar]
- 49.Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611–20. doi: 10.1016/0016-5085(92)91185-7. [DOI] [PubMed] [Google Scholar]
- 50.Frykholm G, Pahlman L, Enblad P, Krog M, Ejerblad S. Early outcome after emergency and elective surgery for ulcerative colitis. Acta Chir Scand. 1989;155:601–5. [PubMed] [Google Scholar]
- 51.Longo WE, Virgo KS, Johnson FE, Oprian CA, Vernava AM, Wade TP, Phelan MA, Henderson WG, Daley J, Khuri SF. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. doi: 10.1007/BF02237249. [DOI] [PubMed] [Google Scholar]
- 52.Dominitz JA, Eisen GM, Baron TH, Goldstein JL, Hirota WK, Jacobson BC, Johanson JF, Leighton JA, Mallery JS, Raddawi HM, Vargo JJ, 2nd, Waring JP, Fanelli RD, Wheeler-Harbough J, Faigel DO. Complications of colonoscopy. Gastrointest Endosc. 2003;57:441–5. doi: 10.1016/s0016-5107(03)80005-6. [DOI] [PubMed] [Google Scholar]
- 53.Garbay JR, Suc B, Rotman N, Fourtanier G, Escat J. Multicentre study of surgical complications of colonoscopy. Br J Surg. 1996;83:42–4. doi: 10.1002/bjs.1800830112. [DOI] [PubMed] [Google Scholar]
- 54.Murray L. 2004 Red Book. 108. Montvale, NJ: Thomson PDR; 2004. [Google Scholar]
- 55.Hay AR, Hay JW. Inflammatory bowel disease: medical cost algorithms. J Clin Gastroenterol. 1992;14:318–27. doi: 10.1097/00004836-199206000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Ikeuchi H, Nakano H, Uchino M, Nakamura M, Yanagi H, Noda M, Yamamura T. Incidence and therapeutic outcome of pouchitis for ulcerative colitis in Japanese patients. Digestive Surgery. 2004;21:197–201. doi: 10.1159/000079372. [DOI] [PubMed] [Google Scholar]
- 57.Madiba TE, Bartolo DC. Pouchitis following restorative proctocolectomy for ulcerative colitis: incidence and therapeutic outcome. Journal of the Royal College of Surgeons of Edinburgh. 2001;46:334–7. [PubMed] [Google Scholar]
- 58.Shen B, Shermock KM, Fazio VW, Achkar JP, Brzezinski A, Bevins CL, Bambrick ML, Remzi FH, Lashner BA. A cost-effectiveness analysis of diagnostic strategies for symptomatic patients with ileal pouch-anal anastomosis. American Journal of Gastroenterology. 2003;98:2460–7. doi: 10.1111/j.1572-0241.2003.07710.x. [DOI] [PubMed] [Google Scholar]
- 59.Berkowitz N, Gupta S, Silberman G. Medical resource utilization and cost in patients following colectomy. Managed Care and Cancer. 2000;2:14–21. [Google Scholar]
- 60.Pyenson B, Connor S, Fitch K, Kinzbrunner B. Medicare cost in matched hospice and non-hospice cohorts. J Pain Symptom Manage. 2004;28:200–10. doi: 10.1016/j.jpainsymman.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Muir AJ, Edwards LJ, Sanders LL, Bollinger RR, Koruda MJ, Bachwich DR, Provenzale D. A prospective evaluation of health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol. 2001;96:1480–5. doi: 10.1111/j.1572-0241.2001.03801.x. [DOI] [PubMed] [Google Scholar]
- 62.Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94:1650–7. doi: 10.1111/j.1572-0241.1999.01157.x. [DOI] [PubMed] [Google Scholar]