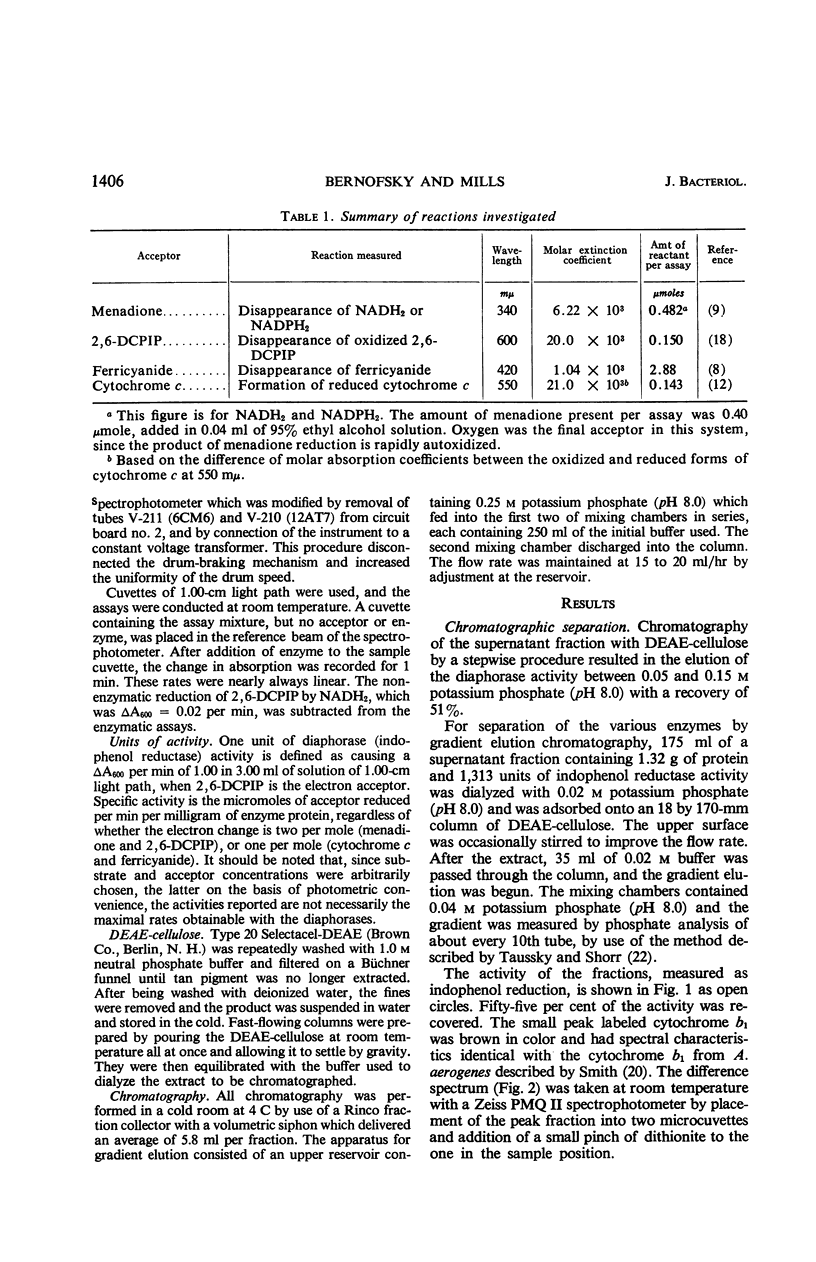
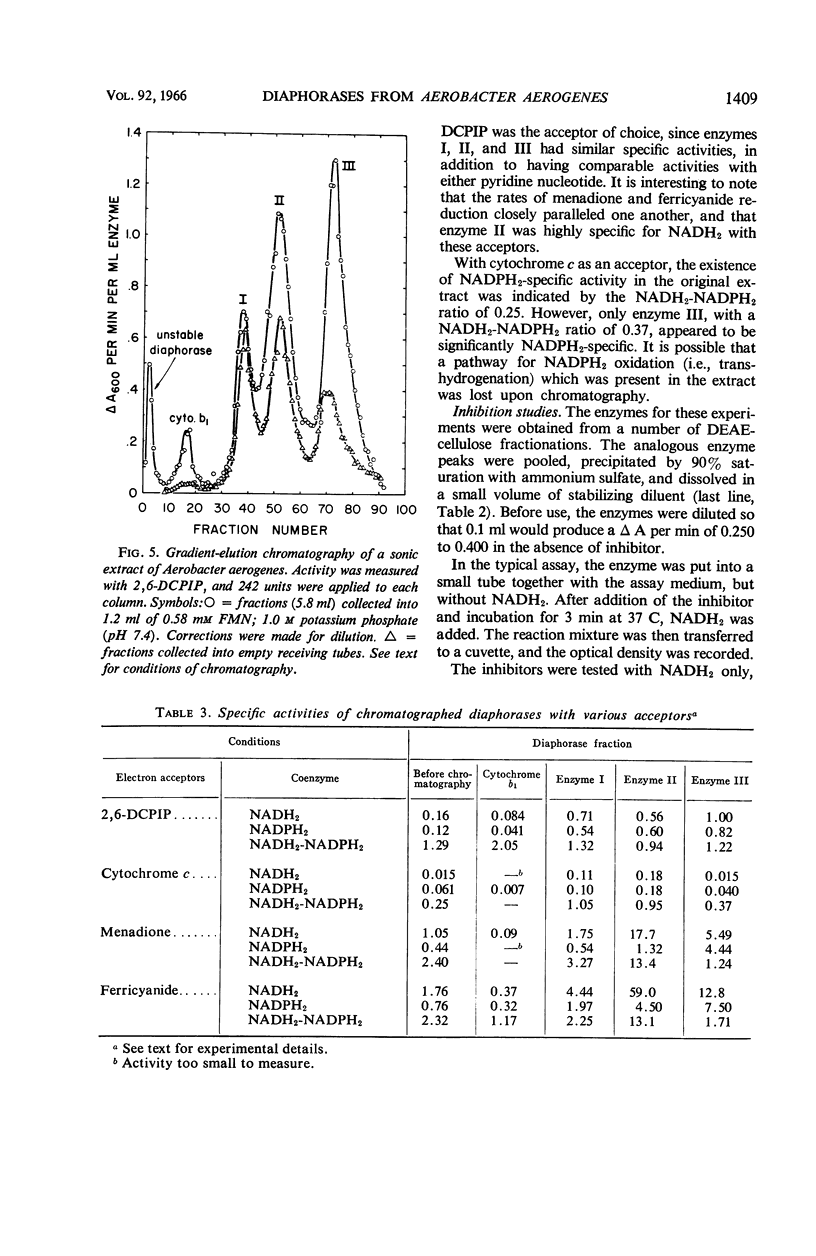
Abstract
Bernofsky, Carl (The University of Kansas, Kansas City), and Russell C. Mills. Diaphorases from Aerobacter aerogenes. J. Bacteriol. 92:1404–1414. 1966.—Five enzymes which catalyze the reduction of 2,6-dichlorophenol-indophenol by reduced nicotinamide adenine dinucleotide (NADH2) have been separated from sonic extracts of Aerobacter aerogenes B199 by diethylaminoethyl (DEAE) cellulose chromatography. Three major chromatographic fractions (enzymes I, II, and III) account for most of the activity in the extract. Of the two minor fractions, one is associated with cytochrome b1. The other is extremely labile, and was not studied further. The chromatographed diaphorases appear to have a specific requirement for flavin mononucleotide. They are also readily inactivated by dilution; however, this can be prevented by a combination of phosphate buffer, bovine serum albumin, and flavin mononucleotide. The different enzymes are clearly distinguishable by their activities with NADH2 and reduced nicotinamide adenine dinucleotide phosphate (NADPH2) in the presence of various electron acceptors (2,6-dichlorophenol-indophenol, ferricyanide, menadione, and cytochrome c), and by their responses to inhibitors (amobarbital, antimycin A, Atabrine, p-chloromercuribenzenesulfonate, dicumarol, and 2,4-dinitrophenol). With 2,6-dichlorophenol-indophenol as acceptor, enzymes I, II, and III have comparable activities with either NADH2 or NADPH2. With menadione and ferricyanide as acceptors, enzymes II and III exhibit very high, NADH2-specific activities. When cytochrome c is the acceptor, however, enzyme III shows greater activity with NADPH2 as the electron donor. Ferricyanide is the most active acceptor for the cytochrome b1-containing fraction. Coenzyme Q6 does not appear to serve as an acceptor. All the diaphorases, with the exception of that in the cytochrome b1-containing fraction, are inhibited by p-chloromercuribenzenesulfonate. Amobarbital is relatively ineffective and inhibits only the indophenol reductase activity of enzyme I. The menadione reductase activity of enzymes I, and II, and the diaphorases in the cytochrome b1-containing fraction are strongly inhibited by antimycin A, 2,4-dinitrophenol, dicumarol, and Atabrine. However, the menadione reductase activity of enzyme III is affected only by the last three of these inhibitors. The diaphorases in sonic-treated extracts do not appear to be associated with a particulate fraction.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bragg P. D. Quinone-independent reduction of cytochrome b-1 by reduced nicotinamide adenine dinucleotide in a purified soluble system from Escherichia coli. J Bacteriol. 1965 Nov;90(5):1498–1499. doi: 10.1128/jb.90.5.1498-1499.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIN M. I., WOOD N. P. The Streptococcus faecalis oxidases for reduced diphosphopyridine nucleotide. V. A flavin mononucleotike-containing diaphorase. J Biol Chem. 1960 Jun;235:1809–1814. [PubMed] [Google Scholar]
- ERNSTER L., DANIELSON L., LJUNGGREN M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962 Apr 9;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- FUJITA T., ITAGAKI E., SATO R. Purification and properties of cytochrome bl from Escherichia coli. J Biochem. 1963 Apr;53:282–290. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V. The identity of diaphorase and lipoyl dehydrogenase. Biochim Biophys Acta. 1960 Jan 15;37:314–322. doi: 10.1016/0006-3002(60)90239-0. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. A., MILLS R. C. Oxidation of reduced pyridine nucleotides in Pasteurella tularensis. II. Separation of diaphorases and transhydrogenases. Biochim Biophys Acta. 1961 Mar 18;48:85–92. doi: 10.1016/0006-3002(61)90518-2. [DOI] [PubMed] [Google Scholar]
- Repaske R., Lizotte C. L. The electron transport system of Hydrogenomonas eutropha. II. Reduced nicotinamide adenine dinucleotide-menadione reductase. J Biol Chem. 1965 Dec;240(12):4774–4779. [PubMed] [Google Scholar]
- SANADI D. R., LITTLEFIELD J. W. Studies on alpha-ketoglutaric oxidase. III. Role of coenzyme A and diphosphopyridine nucleotide. J Biol Chem. 1953 Mar;201(1):103–115. [PubMed] [Google Scholar]
- SAVAGE N. Preparation and properties of highly purified diaphorase. Biochem J. 1957 Sep;67(1):146–155. doi: 10.1042/bj0670146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARLS R. L., SANADI D. R. A comparative study of dihydrolipoyl dehydrogenase and diaphorase. J Biol Chem. 1961 Feb;236:580–583. [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes. Bacteriol Rev. 1954 Jun;18(2):106–130. doi: 10.1128/br.18.2.106-130.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub F. B. Isolation and properties of a flavoprotein from heart muscle tissue. Biochem J. 1939 May;33(5):787–792. doi: 10.1042/bj0330787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- VERNON L. P. Bacterial cytochromes. I. Cytochrome composition of Micrococcus denitrificans and Pseudomonas denitrificans. J Biol Chem. 1956 Oct;222(2):1035–1044. [PubMed] [Google Scholar]
- WATARI H., KEARNEY E. B., SINGER T. P., BASINSKI D., HAUBER J., LUSTY C. J. A comparison of reduced nicotinamide adenine dinucleotide-cytochrome c reductases from heart mitochondria and their formation from the respiratory chain-linked dehydrogenase. J Biol Chem. 1962 May;237:1731–1734. [PubMed] [Google Scholar]
- WOISLAIT W. D. Role of vitamin K in electron transport. Fed Proc. 1961 Dec;20:1005–1011. [PubMed] [Google Scholar]
- WOSILAIT W. D., NASON A. Pyridine nucleotide-menadione reductase from Escherichia coli. J Biol Chem. 1954 Jun;208(2):785–798. [PubMed] [Google Scholar]
- Walker G. A., Kilgour G. L. Pyridine nucleotide oxidizing enzymes of Lactobacillus casei. I. Diaphorase. Arch Biochem Biophys. 1965 Sep;111(3):529–533. doi: 10.1016/0003-9861(65)90231-6. [DOI] [PubMed] [Google Scholar]
- Walker G. A., Kilgour G. L. Pyridine nucleotide oxidizing enzymes of Lactobacillus casei. II. Oxidase and peroxidase. Arch Biochem Biophys. 1965 Sep;111(3):534–539. doi: 10.1016/0003-9861(65)90232-8. [DOI] [PubMed] [Google Scholar]


