CANCER AND THROMBOSIS
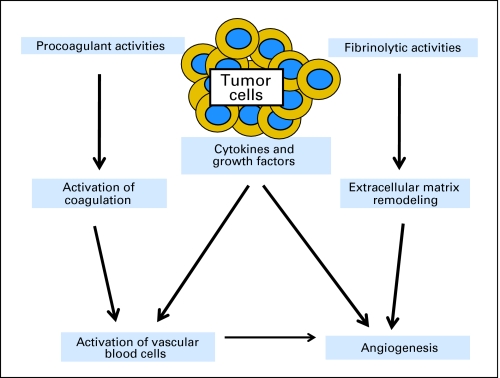
Long before reaching our current understanding of the complex relationship between malignancy and the elements of the coagulation system, it was recognized that patients with cancer were at increased risk for thrombosis. Armand Trousseau first described patients with thrombophlebitis as the presenting sign of a visceral malignancy, later known as Trousseau's syndrome, which he attributed to a special alteration of the blood.1 Increased risk of thrombosis is associated with alterations in normal blood flow, injury to the vascular endothelium, and alterations in the constitution of blood, referred to as Virchow's triad (Table 1).2 While the resulting hypercoagulable state rarely results in overt disseminated intravascular coagulation, virtually all patients with active malignancy demonstrate some degree of activation of the coagulation cascade.3 Today, it is recognized that thrombosis and cancer are linked by multiple pathophysiological mechanisms and that tumor biology and coagulation processes are integrally connected (Fig 1).4–9 The underlying biologic factors associated with the increased risk of thrombosis in patients with cancer include the activation of thrombin and fibrin formation both directly by the release of procoagulants by tumor cells and indirectly by the activation of endothelial cells, leukocytes, and platelets by cytokines and the production of a factor X-activating cysteine protease, mucinous glycoproteins, and circulating tissue factor–bearing microparticles.10 The molecular and genomic factors at the interface between malignant cell behavior and the hypercoagulable state in the patient with cancer are discussed in the articles by Boccaccio and Comoglio11 and by Kasthuri et al12 in this special issue of the Journal of Clinical Oncology.
Table 1.
Pathogenesis: Virchow's Triad
| Stasis | Bed rest and immobility, extrinsic compression of vessel by mass |
| Blood components | Tumors and macrophages produce procoagulants, inflammatory cytokines |
| Vessel damage | Direct tumor invasion, indwelling catheters, chemotherapy, erythropoietin, antiangiogenic agents |
Fig 1.
Schematic illustrating some of the interactions between tumor cells and the hemostatic system via procoagulant and fibrinolytic substances, various cytokines and growth factors, and their influence on hematopoietic elements and the vascular endothelium. Reproduced with permission.9
Venous thromboembolism (VTE) may indicate an occult cancer, may represent a complication of a known malignancy, or complicate hospitalization, surgery, or various systemic cancer treatments.13,14 VTE, including deep-vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and potentially life-threatening disorder representing the second leading cause of death in hospitalized patients with cancer, although often demonstrated only at autopsy.15–17 In addition, the occurrence of VTE may interrupt needed cancer treatment, even as the use of anticoagulants may result in serious bleeding complications. In addition to the human cost, the economic burden of VTE in patients with cancer is substantial with the average cost of hospitalization for DVT estimated at more than $20,000 and one fourth of patients with cancer with VTE requiring readmission as a result of bleeding or a recurrent VTE.18,19
Fortunately, there has been considerable progress not only in our basic understanding of the underlying pathophysiology, but also in the diagnosis of VTE and the options available for the treatment and prevention of thrombosis in patients with cancer. Current guidelines recommend primary prophylaxis in both surgical and medical hospitalized patients and for highly selected ambulatory patients with cancer.20 The development and validation of clinical risk models and the identification of new biomarkers for the selection of patients at increased risk for VYE represents an active area of research.21 Likewise, the potential impact of anticoagulation on cancer growth, invasion, metastases, and angiogenesis as well as overall survival continues to be actively investigated.22 It is essential that the results of ongoing research on underlying mechanisms as well as the optimal treatment and prevention of VTE in patients with cancer be rapidly integrated into clinical practice aided by frequently updated guidelines from major professional organizations.
RISK OF VTE IN PATIENTS WITH CANCER
It is estimated that approximately 500,000 Americans experience thromboembolism each year and at least 100,000 die from subsequent complications.23 Patients with cancer have nearly a six-fold greater risk of VTE compared with noncancer patients while patients with active cancer account for approximately 20% of all new VTE events.24 While the increased risk is most generally appreciated in patients with solid tumors, as discussed by Falanga and Marchetti25 in this issue, the risk extends to patients with the hematologic malignancies. Clinically, the risk of VTE in patients with cancer is most readily apparent among hospitalized individuals and those undergoing surgical or systemic medical treatment.26–29 The risk of thrombosis in ambulatory patients with cancer varies widely with the type of cancer, treatment, and comorbid conditions present.30–32 The risk of VTE appears to be particularly increased in ambulatory patients with cancer on active systemic therapy.33 As discussed by Shivakumar et al,34 many of these patients have indwelling central venous catheters, further increasing the risk of upper extremity VTE.
The incidence of VTE among patients with cancer seems to have increased during the past couple of decades.30 This increase may be due, in part, to improved imaging studies and the extensive use of high-resolution computed tomography (CT) for cancer staging.28,35–37 Nevertheless, three fourths of patients with unsuspected PE found at the time of staging CT scans are symptomatic and are clinically significant.38 The risk of VTE is increased further among patients with cancer receiving systemic chemotherapy.24,33 The observed increase in VTE risk is also likely related to an overall increase in the acuteness of illness among hospitalized patients with cancer, the intensity of cancer treatment, and the availability of several new systemic cancer therapies with direct effects on the vascular endothelium.33,39–41 As discussed by Zangari et al42 in this issue, the newer antiangiogenic agents including bevacizumab have been associated with an increased risk of both arterial and venous thrombosis.43,44 In addition, supportive care strategies, such as the erythropoiesis-stimulating agents and both RBC and platelet transfusions, appear to further increase the risk of VTE in patients with cancer.45,46 In an effort to better identify ambulatory patients with cancer at risk, a predictive model for cancer and chemotherapy-associated thrombosis in patients undergoing outpatient chemotherapy has been developed and validated as discussed in the article by Khorana.47
ADVERSE CONSEQUENCES OF VTE IN PATIENTS WITH CANCER
VTE is associated with a variety of adverse medical consequences, including increased mortality.48–50 Overall, thromboembolism represents a leading cause of death in patients with cancer.15,49 Cancer diagnosed within 1 year of a VTE is often associated with an advanced stage and a poorer survival than among patients with newly diagnosed cancer without a preceding VTE event.51 Patients with cancer hospitalized with neutropenia and presumed infection with documented thromboembolism have a greater in-hospital mortality (P < .001).27 In a recent study of ambulatory patients with cancer receiving chemotherapy, of the 3.2% of patients who died over the first 3 to 4 cycles of treatment, nearly 10% died of thrombosis-related causes.16 Patients developing symptomatic VTE during chemotherapy have been found to have a greater risk of early mortality (hazard ratio, 4.90; P < .0001) than those without VTE.52 In another study of more than 100,000 patients with breast cancer, VTE was a significant predictor of decreased 2-year survival including patients with localized disease.50
On the basis of the increased risk of serious medical complications, it is essential that patients with cancer with symptoms or signs of thrombosis be promptly diagnosed and appropriately treated as discussed by Streiff53 in an article in this issue. In patients with cancer with established VTE, additional serious clinical consequences in patients with cancer with VTE include recurrent thrombosis as well as major bleeding complications associated with anticoagulation.54 The appropriate extended treatment and duration of anticoagulation in patients with cancer, particularly those with persistent active cancer, is discussed in this issue by Lee.55
PROPHYLAXIS OF VTE IN PATIENTS WITH CANCER
Given the considerable morbidity and mortality associated with VTE among patients with cancer, the often fatal course of PE and recent observations that asymptomatic VTE is common, prophylaxis is often recommended in high-risk patients with cancer.20,26 While hospitalized patients with cancer are at increased risk of VTE, no randomized controlled trial (RCT) of VTE prophylaxis specifically in hospitalized patients with cancer has been reported. Nevertheless, as discussed by Francis in this issue,56 three large RCTs of hospitalized acutely ill medical patients demonstrated that enoxaparin (Prophylaxis in Medical Patients with Enoxaparin trial [MEDENOX]), dalteparin (Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial [PREVENT]), and fondaparinux (Arixtra for Thromboembolism Prevention in a Medical Indications Study [ARTEMIS]) are effective in the prevention of VTE detected on the basis of screening with venography or ultrasound.57–59 As discussed by Kakkar et al,60,61 patients with cancer undergoing major surgical procedures are also at increased risk for VTE as well as a greater risk of bleeding complications. A review of VTE prophylaxis demonstrated a significant reduction in DVT with full-dose low molecular weight heparin (LMWH) in subgroup analysis in 26 studies of patients with cancer undergoing surgery.62 In a meta-analysis of RCTs of prolonged LMWH compared with standard postoperative prophylaxis in patients with cancer undergoing abdominal surgery, four RCTs comparing LMWH prophylaxis extended 4 to 5 weeks after surgery significantly reduced the risk of venographically detected DVT but not symptomatic VTE.63 An individual patient data meta-analysis of the two studies of the LMWH tinzaparin confirmed these findings.64
The optimal method for the treatment of VTE in patients with cancer as well as the prevention of recurrent VTE (secondary prophylaxis) continues to be investigated.65 The impact of different anticoagulants on cancer-specific mortality, including post hoc analyses of cancer subgroups, has been studied in a number of RCTs. A previous meta-analysis comparing LMWH with unfractionated heparin (UFH) before initiating warfarin demonstrated a reduction in VTE recurrences and major bleeding complications favoring LMWH.66 Another meta-analysis of RCTs in patients with VTE reported a significantly lower 3-month mortality for the subgroup of patients with cancer treated with LMWH compared with those receiving UFH.67 No significant difference in cancer mortality was found in a meta-analysis of eight RCTs comparing LMWH with vitamin K antagonists (VKAs) overall or limited to patients with cancer.68 The impact of LMWH versus VKAs on recurrence of VTE specifically in patients with cancer has been addressed in four RCTs.69–72 In the largest of these studies, extended treatment for 6 months with dalteparin was found to be superior to maintenance therapy with oral warfarin in preventing recurrent VTE (P = .002) with no significant difference in the rates of bleeding.71
As discussed by Rana and Levine73 in this issue of the Journal, there have been few studies of primary VTE prophylaxis in ambulatory patients with cancer. In a study of 311 women with metastatic breast cancer receiving chemotherapy, the author and his colleagues demonstrated a significant reduction in the risk of VTE in those randomly assigned to receive low-dose warfarin compared with placebo (P = .03) with no significant increase in bleeding.74 Of the six reported RCTs of LMWH in ambulatory patients with cancer, only two have been published and none have demonstrated a significant reduction in VTE.75–79 The results of a placebo-controlled, double-blind, multicenter, clinical outcome–based trial (PROTECHT) presented at the 2008 Annual Meeting of the American Society of Hematology, demonstrated a significant reduction in the composite outcome of arterial and venous thrombosis.75 While a recent meta-analysis confirms that the use of bevacizumab is associated with a significantly increased risk of VTE in patients with cancer, an increased risk of bleeding has also been observed in such patients and no RCTs of VTE prophylaxis have been reported.43
Despite evidence of benefit and favorable risk-benefit ratio for VTE prophylaxis in seriously ill medical patients, in the perioperative setting and for extended secondary prophylaxis after an initial VTE event, evidence from population surveys indicate that VTE prophylaxis is underutilized or inappropriately administered in both medical and surgical cancer patients.80–84 A prospective United States DVT registry found that 28% of patients with active cancer received VTE prophylaxis compared with 35% in patients without cancer (P < .0001).84 Prophylaxis rates among some 2 million US medical patient hospital discharges with other indications for thromboprophylaxis were significantly lower among patients with cancer (range, 18% to 25%) than observed in patients with acute myocardial infarction (range, 71% to 74%), heart failure (range, 29% to 38%), severe lung disease (range, 24% to 32%), and ischemic stroke (range, 27% to 32%).81 A recent hospital audit of VTE prophylaxis demonstrated that patients with cancer were less likely to receive VTE prophylaxis than noncancer acutely ill medical patients (odds ratio, 0.40; P = .0007).82 The Fundamental Research in Oncology and Thrombosis survey of oncologists found lower rates of VTE prophylaxis among medical oncologists (< 5%) than among surgeons (> 50%).83 Finally, although some VTE prophylaxis was reported in approximately half of medical patient discharges from US hospitals, only one fourth received prophylaxis according to current guidelines.80
ANTICOAGULATION AS CANCER TREATMENT TO IMPROVE SURVIVAL
The apparent impact of VTE on early mortality in patients with cancer naturally raises the question whether anticoagulation might improve long-term survival in this population.85,86 At the same time, the heparins may influence malignant cell growth by inhibiting heparin-binding growth factors that stimulate malignant cell growth, tumor cell heparinases that influence tumor cell invasion and metastasis, and cell surface selectin–mediated tumor cell metastasis.87 Likewise, studies have demonstrated that LMWHs may inhibit angiogenesis, block thrombin-induced platelet aggregation, inhibit platelet interaction with vascular endothelium, and stimulate platelet production.88 The impact of anticoagulation on overall survival in patients with cancer has been the focus of a number of prospective clinical trials as reviewed by Kuderer et al89 in this issue of the Journal.68,90–95 In a recent meta-analysis of 11 randomized controlled trials on the use of anticoagulants in patients with cancer by Kuderer and her colleagues, a significant decrease in mortality was observed in patients treated with anticoagulants versus no anticoagulants.22 The relative risk for overall mortality was 0.88 across trials of LMWH (95% CI, 0.79 to 0.98; P = .015) and 0.94 for warfarin studies (95% CI, 0.85 to 1.04; P = .239). An absolute decrease in 1-year mortality in studies of LMWH was estimated at 8.0% (95% CI, 1.6 to 14.3). Major bleeding complications were greater in patients randomly assigned to anticoagulation but only reached statistical significance in warfarin studies, which were associated with an absolute increase in major bleeding compared with controls of 11.5% (P < .001).
A meta-analysis of five randomized controlled trials of LMWH in patients with limited disease small-cell lung cancer reported a survival benefit with LMWH.96 At the same time, studies of the impact of anticoagulants on survival in patients with metastatic disease have produced inconsistent results. In an RCT of patients with small-cell lung cancer, median overall survival of 8 months and 13 months were observed with chemotherapy alone versus chemotherapy with the addition of dalteparin, respectively (P = .01), with similar improvements in survival observed in patients with both limited and extensive disease stages.97 Likewise, in a study of patients with advanced cancer, median survivals of 6.6 and 8.0 months were observed in patients receiving placebo versus LMWH (P = .021).98 Sideras et al,79 however, observed no significant difference in survival between placebo and LMWH therapy in patients with cancer with advanced malignancy. Further studies are needed to better define the potential clinical value of anticoagulants as an adjunct to other cancer therapies.
GUIDELINES FOR THE TREATMENT AND PREVENTION OF VTE
Despite the evidence for increased risk of VTE among patients with cancer and the benefit of prophylactic anticoagulation in specific high-risk settings, surveys of oncologists have demonstrated low rates of compliance with thromboprophylaxis guidelines.20,83 The American College of Chest Physicians has recently updated general guidelines for VTE prevention including a limited discussion of patients with cancer.24 VTE prophylaxis is recommended for both surgical patients with cancer as well as hospitalized patients considered acutely ill. The National Comprehensive Cancer Network provides consensus guidelines on the diagnosis and initial evaluation of VTE in patients with cancer, available therapies for prophylaxis and treatment of VTE and the risks and contraindications of anticoagulation.99 Several international organizations have also developed guidelines for patients with cancer at risk for VTE.100–102 In 2007, the American Society of Clinical Oncology (ASCO) published evidence-based guidelines on the prevention and treatment of VTE in patients with cancer.20
The major questions put to the ASCO VTE Prophylaxis Guidelines Panel are summarized in Table 2. Recommendations provided by the ASCO Guidelines include: (1) hospitalized patients with cancer should be considered for VTE prophylaxis in the absence of bleeding or other contraindications to anticoagulation; (2) routine thromboprophylaxis is not recommended in ambulatory patients with cancer, although those with multiple myeloma receiving thalidomide or lenalidomide with chemotherapy or dexamethasone are at high risk and warrant prophylaxis; (3) all patients undergoing major surgical intervention for malignant disease should be considered for pharmacologic thromboprophylaxis initiated either preoperatively, or as early as possible in the postoperative period. Extended prophylaxis up to 4 weeks and combined pharmacologic and mechanical prophylaxis may be considered in high-risk patients; (4) LMWH represents the preferred approach for the initial 5 to 10 days of anticoagulant therapy of patients with cancer with established VTE and should be continued for up to 6 months for secondary prophylaxis and indefinitely in patients with active malignancy; and (5) while anticoagulant treatment in patients with cancer without VTE to improve survival is not recommended, patients should be encouraged to participate in ongoing clinical trials evaluating this issue. The consistency of recommendations across multiple international guidelines along with a call for further research into the optimal management of cancer patients at risk for VTE are highlighted in this special issue on cancer and thrombosis.103
Table 2.
American Society of Clinical Oncology VTE Prophylaxis and Treatment Guidelines Clinical Questions19
|
Abbreviation: VTE, venous thromboembolism.
CONCLUSIONS AND FUTURE DIRECTIONS
The US Surgeon General has recently issued a Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism 200823 to alert both health care professionals and the public about the need for better education and greater research into the many remaining questions and controversies around VTE, including the clear relationship with cancer. Patients with cancer, especially those hospitalized and those undergoing major surgery or systemic treatment, are at significantly increased risk for VTE. The role of primary VTE prophylaxis in high-risk patients as well as secondary prevention of recurrent VTE remains a continuing clinical challenge for the practicing oncologist. Likewise, the possible adjunctive role of anticoagulants for improving survival for patients with cancer remains an intriguing opportunity that will require additional controlled clinical trials. Studies are also needed to better define the benefits and risks associated with anticoagulation in high-risk patients with cancer, such as the elderly or those with CNS malignancies as well as patients in the ambulatory setting receiving cancer chemotherapy. In addition, as novel systemic therapies are introduced, the VTE risk must be evaluated and preventive measures considered, if appropriate. While a recent meta-analysis confirms that the use of bevacizumab is associated with a significantly increased risk of VTE in patients with cancer, the increase in bleeding risk accompanying such treatment indicates the need for well-designed randomized controlled trials of VTE prophylaxis to clearly demonstrate benefit and risk before routine anticoagulation is recommended in such patients.43 As discussed by Levine in this issue,104 the need for more efficacious, safe, and convenient anticoagulants has sparked the development of a number of new agents including the direct thrombin inhibitors, which have and are being studied in patients with cancer. While recently developed and validated clinical risk models for VTE among ambulatory patients with cancer are promising, new biomarkers for VTE and PE are awaited to further improve selection of high-risk patients for effective and safe prophylactic strategies. Evidence-based guidelines from ASCO and other professional organizations can provide clinicians with a balanced discussion of the benefits and risks associated with the use of anticoagulants in the specific treatment of patients with cancer. Further efforts are needed, however, to improve the implementation and utilization of available guidelines in order to bring clinical practice into compliance with current recommendations.105 Through the optimal application of current preventive strategies along with increased investment into basic and clinical research, significant reductions in the morbidity and mortality associated with thromboembolic complications in patients with cancer should be realized.
Acknowledgment
Gary H. Lyman is supported by Grant No. 1R01HL095109-01 from the National Heart, Lung and Blood Institute; Alok A. Khorana is supported by Grant No. 5K23CA120587-03 from the National Cancer Institute, Grant No. 1R01HL095109-01 from the National Heart, Lung and Blood Institute, and the V Foundation.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Alok A. Khorana, sanofi-aventis (C), Eisai (C) Stock Ownership: None Honoraria: Alok A. Khorana, sanofi-aventis, Eisai Research Funding: Alok A. Khorana, sanofi-aventis, Eisai, Bristol-Myers Squibb Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Gary H. Lyman, Alok A. Khorana
Administrative support: Gary H. Lyman
Provision of study materials or patients: Gary H. Lyman
Collection and assembly of data: Gary H. Lyman
Data analysis and interpretation: Gary H. Lyman
Manuscript writing: Gary H. Lyman, Alok A. Khorana
Final approval of manuscript: Gary H. Lyman, Alok A. Khorana
REFERENCES
- 1.Khorana AA. Malignancy, thrombosis and Trousseau: The case for an eponym. J Thromb Haemost. 2003;1:2463–2465. doi: 10.1111/j.1538-7836.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 2.Brotman DJ, Deitcher SR, Lip GY, Matzdorff AC. Virchow's triad revisited. South Med J. 2004;97:213–214. doi: 10.1097/01.SMJ.0000105663.01648.25. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Bettigole RE, Robson E, et al. Fibrinogen kinetics in patients with neoplastic disease. Cancer Mar. 1978;41:1113–1122. doi: 10.1002/1097-0142(197803)41:3<1113::aid-cncr2820410346>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Bluff JE, Brown NJ, Reed MW, et al. Tissue factor, angiogenesis and tumour progression. Breast Cancer Res. 2008;10:204. doi: 10.1186/bcr1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter PC. The pathogenesis of venous thromboembolism in cancer: Emerging links with tumour biology. Hematol Oncol. 2006;24:126–133. doi: 10.1002/hon.785. [DOI] [PubMed] [Google Scholar]
- 6.Bobek V, Kovarik J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother. 2004;58:213–219. doi: 10.1016/j.biopha.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Hejna M, Raderer M, Zielinski CC. Inhibition of metastases by anticoagulants. J Natl Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]
- 8.ten Cate H, Falanga A. The pathophysiology of cancer and thrombosis: Summary and conclusions. Pathophysiol Haemost Thromb. 2008;36:212–214. doi: 10.1159/000175159. [DOI] [PubMed] [Google Scholar]
- 9.Falanga A. The incidence and risk of venous thromboembolism associated with cancer and nonsurgical cancer treatment. Cancer Invest. 2009;27:105–115. doi: 10.1080/07357900802563028. [DOI] [PubMed] [Google Scholar]
- 10.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 11.Boccaccio C, Comoglio P. Genetic link between cancer and thrombosis. J Clin Oncol. 2009;27:4827–4833. doi: 10.1200/JCO.2009.22.7199. [DOI] [PubMed] [Google Scholar]
- 12.Kasthuri RS, Mackman N, Taubman MB. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–4838. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varki A. Trousseau's syndrome: Multiple definitions and multiple mechanisms. Blood. 2007;110:1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prandoni P, Lensing AW, Buller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med. 1992;327:1128–1133. doi: 10.1056/NEJM199210153271604. [DOI] [PubMed] [Google Scholar]
- 15.Ambrus JL, Ambrus CM, Mink IB, et al. Causes of death in cancer patients. J Med. 1975;6:61–64. [PubMed] [Google Scholar]
- 16.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 17.Shen VS, Pollak EW. Fatal pulmonary embolism in cancer patients: Is heparin prophylaxis justified? South Med J. 1980;73:841–843. doi: 10.1097/00007611-198007000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Bullano MF, Willey V, Hauch O, et al. Longitudinal evaluation of health plan cost per venous thromboembolism or bleed event in patients with a prior venous thromboembolism event during hospitalization. J Manag Care Pharm. 2005;11:663–673. doi: 10.18553/jmcp.2005.11.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653–1661. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: Recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 21.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuderer NM, Khorana AA, Lyman GH, et al. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: Impact on survival and bleeding complications. Cancer. 2007;110:1149–1161. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 23.General US. Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism 2008. http://www.surgeongeneral.gov/topics/deepvein/ [PubMed]
- 24.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (ed 8) Chest. 2008;133(suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 25.Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27:4848–4857. doi: 10.1200/JCO.2009.22.8197. [DOI] [PubMed] [Google Scholar]
- 26.Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356:1438–1444. doi: 10.1056/NEJMcp067264. [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 28.Stein PD, Beemath A, Meyers FA, et al. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med Jan. 2006;119:60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 29.Dentali F, Douketis JD, Gianni M, et al. Meta-analysis: Anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 30.Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 31.Khorana AA. The NCCN Clinical Practice Guidelines on Venous Thromboembolic Disease: Strategies for improving VTE prophylaxis in hospitalized cancer patients. Oncologist. 2007;12:1361–1370. doi: 10.1634/theoncologist.12-11-1361. [DOI] [PubMed] [Google Scholar]
- 32.Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285–291. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–2829. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 34.Shivakumar SP, Anderson DR, Couban S. Catheter-associated thrombosis in patients with malignancy. J Clin Oncol. 2009;27:4858–4864. doi: 10.1200/JCO.2009.22.6126. [DOI] [PubMed] [Google Scholar]
- 35.Gosselin MV, Rubin GD, Leung AN, et al. Unsuspected pulmonary embolism: Prospective detection on routine helical CT scans. Radiology. 1998;208:209–215. doi: 10.1148/radiology.208.1.9646815. [DOI] [PubMed] [Google Scholar]
- 36.Storto ML, Di Credico A, Guido F, et al. Incidental detection of pulmonary emboli on routine MDCT of the chest. Am J Roentgenol. 2005;184:264–267. doi: 10.2214/ajr.184.1.01840264. [DOI] [PubMed] [Google Scholar]
- 37.Sebastian AJ, Paddon AJ. Clinically unsuspected pulmonary embolism–an important secondary finding in oncology CT. Clin Radiol. 2006;61:81–85. doi: 10.1016/j.crad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.O'Connell CL, Boswell WD, Duddalwar V, et al. Unsuspected pulmonary emboli in cancer patients: Clinical correlates and relevance. J Clin Oncol. 2006;24:4928–4932. doi: 10.1200/JCO.2006.06.5870. [DOI] [PubMed] [Google Scholar]
- 39.Cavo M, Zamagni E, Cellini C, et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood. 2002;100:2272–2273. doi: 10.1182/blood-2002-06-1674. [DOI] [PubMed] [Google Scholar]
- 40.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 41.Kuenen BC, Levi M, Meijers JC, et al. Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol. 2003;21:2192–2198. doi: 10.1200/JCO.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 42.Zangari M. Thrombotic events in patients with cancer receiving anti-angiogenesis agents. J Clin Oncol. 2009;27:4865–4873. doi: 10.1200/JCO.2009.22.3875. [DOI] [PubMed] [Google Scholar]
- 43.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 44.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 45.Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: Updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98:708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 46.Khorana AA, Francis CW, Blumberg N, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–4847. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: Incidence and effect on survival. J Clin Oncol. 2006;24:1112–1118. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 49.Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 50.Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 52.Kuderer NM, Francis CW, Culakova E, et al. E. Venous thromboembolism represents a major risk factor for early all-cause mortality in patients receiving cancer chemotherapy. J Clin Oncol. 2008;26:506s. abstr 9521. [Google Scholar]
- 53.Streiff MB. Diagnosis and initial treatment of venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27:4889–4894. doi: 10.1200/JCO.2009.23.5788. [DOI] [PubMed] [Google Scholar]
- 54.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 55.Lee AY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27:4895–4901. doi: 10.1200/JCO.2009.22.3958. [DOI] [PubMed] [Google Scholar]
- 56.Francis CW. Prevention of venous thromboembolism in hospitalized patients with cancer. J Clin Oncol. 2009;27:4874–4880. doi: 10.1200/JCO.2009.22.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: Randomised placebo controlled trial. BMJ. 2006;332:325–329. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 59.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients: Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 60.Kakkar AK, Haas S, Wolf H, et al. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: The MC-4 cancer substudy. Thromb Haemost. 2005;94:867–871. doi: 10.1160/TH04-03-0189. [DOI] [PubMed] [Google Scholar]
- 61.Kakkar A. Prevention of venous thromboembolism in the cancer surgical patient. J Clin Oncol. 2009;27:4881–4884. doi: 10.1200/JCO.2009.23.2009. [DOI] [PubMed] [Google Scholar]
- 62.Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol. 2007;14:929–936. doi: 10.1245/s10434-006-9183-9. [DOI] [PubMed] [Google Scholar]
- 63.Rasmussen E, Wille-Jorgensen P, Jorgensen LN. Extended out-of-hospital low-molecular-weight heparin prophylaxis against venous thromboembolism in patients after cancer operations: A meta-analysis. ISTH. 2005 abstract. [Google Scholar]
- 64.Jorgensen LN, Lausen I, Rasmussen MS, et al. Prolonged thromboprophylaxis with low molecular weight heparin (tinzaparin) following major general surgery primarily for cancer: An individual patient data meta-analysis. J Thromb Haemost. 2005;(suppl 1) abstr P1870. [Google Scholar]
- 65.Buller HR, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):401S–428S. doi: 10.1378/chest.126.3_suppl.401S. [DOI] [PubMed] [Google Scholar]
- 66.Siragusa S, Cosmi B, Piovella F, et al. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: Results of a meta-analysis. Am J Med. 1996;100:269–277. doi: 10.1016/S0002-9343(97)89484-3. [DOI] [PubMed] [Google Scholar]
- 67.Hettiarachchi RJ, Smorenburg SM, Ginsberg J, et al. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemost. 1999;82:947–952. [PubMed] [Google Scholar]
- 68.Conti S, Guercini F, Iorio A. Low-molecular-weight heparin and cancer survival: Review of the literature and pooled analysis of 1,726 patients treated for at least three months. Pathophysiol Haemost Thromb. 2003;33:197–201. doi: 10.1159/000081508. [DOI] [PubMed] [Google Scholar]
- 69.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch Intern Med. 2002;162:1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 70.Deitcher SR, Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic events (VTE) in patients with active malignancy: A randomized study of enoxaparin sodium alone vs. initial enoxaparin sodium followed by warfarin for a 180-day period. J Thromb Haemost. 2003;(suppl 1):1. doi: 10.1177/1076029606293692. abstr OC194. [DOI] [PubMed] [Google Scholar]
- 71.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 72.Hull RD, Pineo GF, Brant RF, et al. Self-managed long-term low-molecular-weight heparin therapy: The balance of benefits and harms. Am J Med. 2007;120:72–82. doi: 10.1016/j.amjmed.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 73.Rana P, Levine M. Prevention of thrombosis in ambulatory patients with cancer. J Clin Oncol. 2009;27:4885–4888. doi: 10.1200/JCO.2009.23.5481. [DOI] [PubMed] [Google Scholar]
- 74.Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343:886–889. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 75.Agnelli G, Gussoni G, Bianchini C, et al. A randomized double-blind placebo-controlled study on nadroparin for prophylaxis of thromboembolic events in cancer patients receiving chemotherapy: The PROTECHT Study. Blood. 2008;112:6. abstr 984. [Google Scholar]
- 76.Haas S, Kakkar A, Kemkes-Matthes B, et al. Prevention of venous thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer: Results of the TOPIC studies. J Thromb Haemost. 2005 abstr 0R059. [Google Scholar]
- 77.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Perry JR, Rogers L, Laperriere N, et al. PRODIGE: A phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin (LMWH) in patients with newly diagnosed malignant glioma. J Clin Oncol. 2007;25(2011) doi: 10.1111/j.1538-7836.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 79.Sideras K, Schaefer PL, Okuno SH, et al. Low-molecular-weight heparin in patients with advanced cancer: A phase 3 clinical trial. Mayo Clin Proc. 2006;81:758–767. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]
- 80.Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: Success or failure? J Thromb Haemost. 2007;5:1610–1616. doi: 10.1111/j.1538-7836.2007.02650.x. [DOI] [PubMed] [Google Scholar]
- 81.Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm. 2006;63(suppl 6):S23–S29. doi: 10.2146/ajhp060390. [DOI] [PubMed] [Google Scholar]
- 82.Kahn SR, Panju A, Geerts W, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145–155. doi: 10.1016/j.thromres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 83.Kakkar AK, Levine M, Pinedo HM, et al. Venous thrombosis in cancer patients: Insights from the FRONTLINE survey. Oncologist. 2003;8:381–388. doi: 10.1634/theoncologist.8-4-381. [DOI] [PubMed] [Google Scholar]
- 84.Seddighzadeh A, Shetty R, Goldhaber SZ. Venous thromboembolism in patients with active cancer. Thromb Haemost. 2007;98:656–661. [PubMed] [Google Scholar]
- 85.Gerotziafas GT, Papageorgiou C, Hatmi M, et al. Clinical studies with anticoagulants to improve survival in cancer patients. Pathophysiol Haemost Thromb. 2008;36:204–211. doi: 10.1159/000175158. [DOI] [PubMed] [Google Scholar]
- 86.Vestjens JH, Sassen S, Prins MH. Blood coagulation and cancer: Thrombosis and survival, clinical relevance and impact: An introduction. Pathophysiol Haemost Thromb. 2008;36:113–121. doi: 10.1159/000175149. [DOI] [PubMed] [Google Scholar]
- 87.Castelli R, Porro F, Tarsia P. The heparins and cancer: Review of clinical trials and biological properties. Vasc Med. 2004;9:205–213. doi: 10.1191/1358863x04vm566ra. [DOI] [PubMed] [Google Scholar]
- 88.Cosgrove RH, Zacharski LR, Racine E, et al. Improved cancer mortality with low-molecular-weight heparin treatment: A review of the evidence. Semin Thromb Hemost. 2002;28:79–87. doi: 10.1055/s-2002-20566. [DOI] [PubMed] [Google Scholar]
- 89.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902–4911. doi: 10.1200/JCO.2009.22.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zacharski LR, Henderson WG, Rickles FR, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate: Final report of VA Cooperative study #75. Cancer. 1984;53:2046–2052. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 91.Chahinian AP, Propert KJ, Ware JH, et al. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B. J Clin Oncol. 1989;7:993–1002. doi: 10.1200/JCO.1989.7.8.993. [DOI] [PubMed] [Google Scholar]
- 92.Maurer LH, Herndon JE, Jr, Hollis DR, et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: A Cancer and Leukemia Group B study. J Clin Oncol. 1997;15:3378–3387. doi: 10.1200/JCO.1997.15.11.3378. [DOI] [PubMed] [Google Scholar]
- 93.Lebeau B, Chastang C, Brechot JM, et al. Subcutaneous heparin treatment increases survival in small cell lung cancer: “Petites Cellules” Group. Cancer. 1994;74:38–45. doi: 10.1002/1097-0142(19940701)74:1<38::aid-cncr2820740108>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 94.Lazo-Langner A, Goss GD, Spaans JN, et al. The effect of low-molecular-weight heparin on cancer survival: A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5:729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]
- 95.Tagalakis V, Blostein M, Robinson-Cohen C, et al. The effect of anticoagulants on cancer risk and survival: Systematic review. Cancer Treat Rev. 2007;33:358–368. doi: 10.1016/j.ctrv.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Akl EA, van Doormaal FF, Barba M, et al. Parenteral anticoagulation may prolong the survival of patients with limited small cell lung cancer: A Cochrane systematic review. J Exp Clin Cancer Res. 2008;27:4. doi: 10.1186/1756-9966-27-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266–1271. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 98.Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 99.Wagman LD, Baird MF, Bennett CL, et al. Venous thromboembolic disease: NCCN Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2008;6:716–753. doi: 10.6004/jnccn.2008.0055. [DOI] [PubMed] [Google Scholar]
- 100.Debourdeau P, Farge-Bancel D, Bosquet L, et al. 2008 Standards, options: Recommendations for venous thromboembolic events (VTE) treatment and central venous catheter thrombosis (CVCT) management in cancer patients. Bull Cancer. 2008;95:750–761. doi: 10.1684/bdc.2008.0678. [DOI] [PubMed] [Google Scholar]
- 101.Di Minno G, Tufano A. Venous thromboembolism in oncologic patients: Guidelines for prevention and therapy and areas of uncertainty. Tumori. 2006;92(suppl):1–15. [PubMed] [Google Scholar]
- 102.Mandala M, Falanga A, Roila F. Management of venous thromboembolism in cancer patients: ESMO clinical recommendations. Ann Oncol. 2008;19(suppl 2):ii126–ii127. doi: 10.1093/annonc/mdn110. [DOI] [PubMed] [Google Scholar]
- 103.Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: A consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27:4919–4926. doi: 10.1200/JCO.2009.22.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Levine M. New antithrombotic drugs: Potential for use in oncology. J Clin Oncol. 2009;27:4912–4918. doi: 10.1200/JCO.2009.24.7346. [DOI] [PubMed] [Google Scholar]
- 105.Somerfield MR, Einhaus K, Hagerty KL, et al. American Society of Clinical Oncology clinical practice guidelines: Opportunities and challenges. J Clin Oncol. 2008;26:4022–4026. doi: 10.1200/JCO.2008.17.7139. [DOI] [PubMed] [Google Scholar]