Abstract
This is a literature review of the frequency of venous thromboembolism in hospitalized patients with cancer and of the available evidence supporting the use of thromboprophylaxis. Patients with cancer are at particularly high risk of venous thromboembolism and account for almost 20% of patients in the population. Hospitalization is an important risk factor in patients with cancer, with rates reported between 0.6% and 7.8%. The incidence has been increasing over the past decade. Three randomized controlled trials and meta-analyses indicate that prophylaxis with low molecular weight heparin, heparin, or fondaparinux significantly reduces the rate of venous thromboembolism in hospitalized medical patients who are at high risk. Patients with cancer were included in these studies, but prospective trials specifically focused on patients with cancer are not available. Evidence indicates that appropriate thromboprophylaxis is provided to a minority of hospitalized patients with cancer and that targeted educational efforts and computerized prompt systems can increase appropriate use. Guidelines developed by both oncology and thrombosis organizations support the use of thromboprophylaxis in hospitalized patients with cancer. In conclusion, most patients hospitalized with cancer are at high risk of venous thromboembolism, and thromboprophylaxis should be provided in the absence of active bleeding or a high bleeding risk.
MAGNITUDE OF THE PROBLEM
Venous thromboembolism occurs frequently in hospitalized medical patients and is a common but preventable cause of morbidity and mortality. In the general population, hospitalization for a medical illness increases the risk of venous thromboembolism by approximately eight-fold, and approximately 25% of all cases of venous thromboembolism are associated with hospitalization.1,2 Although hospitalized surgical patients are thought to be at greatest risk, 50% to 75% of venous thromboembolism in hospitalized patients actually occurs on the medical service.3–5 In prospective studies, venographic screening of high-risk hospitalized medical patients who are not receiving prophylaxis has shown rates of deep vein thrombosis of 10% to 15%, whereas ultrasonography has shown a rate of 5%.6–8 Pulmonary embolism occurred in 0.3% to 1.5% of these patients, and proximal deep vein thrombosis occurred in 2% to 4.9%. Approximately 70% of patients are asymptomatic.
The calf veins are most commonly affected, and the clinical significance of asymptomatic deep vein thrombosis that remains limited to the calf is controversial. However, studies of the natural history of venous thrombosis demonstrate that some such thrombi will extend proximally, increasing the risk of causing leg symptoms and often resulting in pulmonary embolism.9 Pulmonary embolism is associated with 5% to 10% of all deaths of hospitalized patients, but the diagnosis is often not suspected clinically.10–13
Patients with cancer are at particularly high risk of venous thromboembolism and account for almost 20% of all patients in the population.1,14,15 In population-based studies, cancer was associated with a 4.1-fold greater risk of thrombosis, and administration of chemotherapy increased the risk further to 6.5-fold.1,16 Several studies have reported on the frequency of venous thromboembolism specifically in hospitalized cancer patients. Levitan et al17 reported an analysis of Medicare claims data for patients older than age 65 hospitalized between 1988 and 1990 and found a rate of venous thromboembolism of 0.6%. This rate was significantly higher than the rate of 0.57% in a comparison group with nonmalignant disease (P = .001). Also, the probability of readmission or death within 183 days was between three- and four-fold higher among patients with cancer with venous thromboembolism compared with patients with nonmalignant disease. A more recent but smaller study that included both ambulatory and hospitalized patients found an incidence of venous thromboembolism of 7.8% over 26 months.18 In this study, the occurrence of thrombotic events did not adversely affect overall survival. A large study of patients with 19 selected malignancies who were hospitalized between 1979 and 1999 found a rate of deep vein thrombosis of 2% (827,000 of 40,787,000 patients) and a rate of pulmonary embolism of 1%.19 The incidence of venous thromboembolism was twice the rate observed in patients without cancer, and the incidence was observed to increase in the late 1980s, a trend that continued into the 1990s.
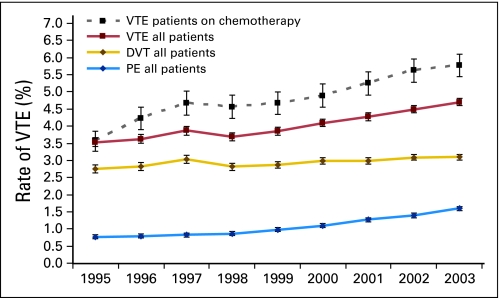
Khorana et al20 conducted a retrospective cohort study using a large discharge database of the University HealthSystem Consortium that included 66,106 adult neutropenic cancer patients with 88,074 hospitalizations between 1995 and 2002 at 115 medical centers in the United States. Neutropenia was selected as a marker for patients most likely to be receiving active chemotherapy. Thromboembolism occurred in 8% of patients, with 5.4% developing venous thromboembolism and 1.5% having arterial thrombosis. In-hospital mortality was significantly greater in patients with venous thrombosis (odds ratio [OR] = 2.01; 95% CI, 1.83 to 2.22) or arterial thrombosis (OR = 5.04; 95% CI, 4.38 to 5.79). Interestingly, from 1995 to 2002, there was a significant 36% increase in venous events and a 124% increase in arterial events (P = .0001). A second, larger retrospective cohort study was conducted by the same group using a database that included 1,824,316 hospitalizations between 1995 and 2003 at 133 US medical centers.21 A diagnosis of deep vein thrombosis and pulmonary embolism was made in 3.4% and 1.1% of patients, respectively, with an overall rate of venous thromboembolism of 4.1%. The rate of venous thromboembolism increased by 28% over the study period, with a near doubling of pulmonary embolism rates from 0.8% to 1.5% (P ≤ .0001). Among the patients receiving chemotherapy, the rate of venous thromboembolism increased by 47%, from 3.9% to 5.7% (P < .0001; Fig 1). The increasing rate of venous thromboembolism was not uniform but was disproportionately greater in patients receiving chemotherapy. In contrast, patients with cancer undergoing selected surgical procedures did not demonstrate a significant increase, suggesting that changes in awareness or diagnostic procedures did not account for the increase.
Fig 1.
Increase in the rate of venous thromboembolism (VTE) over time. Results are presented as annual rates of deep venous thrombosis (DVT), pulmonary embolism (PE) without DVT, and PE with DVT (VTE) between 1995 and 2003. Significant trends for increasing rates were observed for all three diagnoses (P < .0001). The rate of increase was found to be greater in the subgroup of patients who received chemotherapy. Error bars represent 95% CIs. Reprinted with permission.21
Overall, reported studies show a high but variable rate of venous thromboembolism among hospitalized patients with cancer (Table 1). More recent studies have found a higher rate, and evidence indicates that the frequency of venous thromboembolism among hospitalized patients with cancer is increasing. Important consequences in patients with cancer include increased mortality, high rates of recurrence and bleeding with anticoagulant therapy,22 prolonged hospitalization, and increased costs.23,24
Table 1.
Frequency of Venous Thrombosis in Hospitalized Patients With Cancer
| Study | No. of Hospitalizations or Patients | Events |
|
|---|---|---|---|
| No. | % | ||
| Levitan et al17* | 1,211,944 | 7,238 | 0.6 |
| Sallah et al18 | 1,041 | 81 | 7.8 |
| Stein et al19 | 40,787,000 | 837,000 | 2.0 |
| Khorana et al20 | 66,106 | 5,272 | 5.4 |
| Khorana et al21 | 1,015,598 | 41,666 | 4.1 |
Only includes patients age ≥ 65 years.
PROPHYLAXIS
Primary prophylaxis is the best strategy for reducing the burden of venous thromboembolism among hospitalized patients with cancer. For patients who present with signs or symptoms of deep vein thrombosis or pulmonary embolism, accurate diagnosis using proper laboratory and imaging studies is critical, and this should be followed by anticoagulant or other specific therapy as indicated.25 However, diagnosis and treatment of symptomatic disease is inadequate in the majority of hospitalized patients with cancer, in whom asymptomatic deep vein thrombosis can lead to sudden death from pulmonary embolism, which usually occurs rapidly before the diagnosis is suspected. A large number of clinical trials provide clear evidence that primary prophylaxis reduces both deep vein thrombosis and pulmonary embolism, and some studies have also shown that fatal pulmonary embolism is also prevented.26 Thromboprophylaxis is recognized as a highly effective strategy to improve patient outcomes in hospitals,26,27 and it also decreases costs.28,29 The practical steps involved in providing appropriate thromboprophylaxis include the following: awareness of the problem, assessment of risk, and choice of a prophylaxis modality. Prophylaxis can be provided using either mechanical or pharmacologic approaches.
The primary effect of mechanical methods of prophylaxis is to improve venous flow in the legs. Patients should be encouraged to ambulate when possible and be instructed in exercises such as foot extension that can improve venous flow. Pneumatic compression devices and graduated compression stockings reduce venous stasis and have been shown to be effective in reducing postoperative venous thromboembolism.30,31 Their effectiveness is supported by a Cochrane review showing that postoperative venous thrombosis is reduced by approximately 50%.31 Only limited data are available regarding the effectiveness of these approaches in hospitalized medical patients, and there is essentially no data specific for the cancer population. An additional limitation with studies of mechanical prophylaxis is lack of blinding, which could introduce bias into positive trial results. Also, compliance can be a problem because patients frequently remove the devices for comfort or ambulation. Despite these limitations, mechanical approaches do reduce risk of venous thrombosis and are a particularly good choice in patients who are bleeding or at high risk of bleeding.
Effective prophylaxis can be provided by administration of low doses of unfractionated heparin, low molecular weight heparins, or fondaparinux (Table 2). The most compelling data supporting the use of anticoagulant prophylaxis come from studies of surgical patients. A large international trial in patients after general surgery demonstrated that administration of low-dose unfractionated heparin reduced the incidence of pulmonary embolism and fatal pulmonary embolism.32 This seminal study was followed by numerous other trials in surgical patients, and a large meta-analysis found reduction in the rates of postoperative deep vein thrombosis and of total and fatal pulmonary embolism by 67%, 47%, and 64%, respectively.33
Table 2.
Medications and Doses for Prophylaxis of Venous Thromboembolism in Hospitalized Patients With Cancer
| Drug | Dose | Comment |
|---|---|---|
| Unfractionated heparin | 5,000 U subcutaneously every 8 hours | A dose of 5,000 U subcutaneously every 12 hours has also been used; expert opinion favors 8-hour dosing |
| Low molecular weight heparins | ||
| Enoxaparin | 40 mg subcutaneously once daily | More expensive than heparin; 20 mg daily not effective |
| Dalteparin | 5,000 U subcutaneously once daily | More expensive than heparin |
| Fondaparinux | 2.5 mg subcutaneously once daily | More expensive than heparin; not FDA approved for prophylaxis in medical patients |
NOTE. Anticoagulant prophylaxis should not be used if there is a risk of excessive bleeding, such as in patients with active or recent GI bleeding, hemorrhagic stroke, or hemostatic defects such as severe thrombocytopenia. Unfractionated heparin and low molecular weight heparins should not be used in patients with current or previous heparin-induced thrombocytopenia.
Abbreviation: FDA, US Food and Drug Administration.
RISK ASSESSMENT
The risk of venous thromboembolism is related to the presence of specific risk factors. Table 3 lists factors that increase risk of venous thromboembolism in general medical inpatients and also lists risks that have been identified especially in patients with cancer. The total risk increases if multiple risk factors are present, and review of these lists reveals that most hospitalized patients with cancer have multiple risk factors. For example, they are often older with immobility, and many have had recent surgery in addition to having active cancer and cancer therapy. Occasionally, young ambulatory patients with cancer are hospitalized who may need no specific prophylaxis. Unfortunately, no data are available from prospective clinical trials that have evaluated risk specifically for patients with cancer.
Table 3.
Risk Factors for Venous Thromboembolism in Hospitalized Patients
| Risk Factor |
|---|
| General medical patients |
| Active cancer |
| Cancer therapy |
| Congestive heart failure* |
| Acute myocardial infarction |
| Acute respiratory disease |
| Stroke |
| Rheumatic disease (eg, acute arthritis) |
| Inflammatory bowel disease |
| Previous venous thromboembolism |
| Older age |
| Recent surgery or trauma |
| Immobility or paresis |
| Obesity (BMI > 30 kg/m2) |
| Central venous catheterization |
| Inherited or acquired thrombophilic states |
| Varicose veins |
| Estrogen therapy |
| Erythropoiesis-stimulating agents |
| Patients with cancer |
| Age > 65 years |
| Black |
| Female |
| Site of cancer |
| Pancreas |
| Stomach |
| Other abdominal |
| Rectal |
| Ovary |
| Lung |
| Chemotherapy |
| Comorbidities |
| Arterial thrombosis |
| Pulmonary disease |
| Renal disease |
| Infection |
| Anemia |
| Transfusion |
Abbreviation: BMI, body mass index.
Congestive heart failure is defined as New York Heart Association class III or IV disease.
However, retrospective studies of hospitalized patients with cancer have provided some insight into specific risk factors. Khorana et al20 conducted a retrospective cohort study of more than 66,000 adult neutropenic patients with cancer between 1995 and 2002. The variables that were significantly associated with venous thromboembolism using a multivariate logistical regression analysis included age ≥ 65 years; site of cancer, including brain, stomach, lung, pancreas, other abdominal, ovary, endometrium, and cervix; arterial thrombosis; the presence of comorbidities, including pulmonary infection and renal disease; and obesity. A subsequent larger retrospective cohort study of similar design included more than one million patients with cancer.21 The overall rate of venous thromboembolism was 4.1%, and groups with the highest rate included those with black race and patients receiving chemotherapy. Sites of cancer with the highest rates of venous thromboembolism included pancreas, kidney, ovary, lung, and stomach. Also, patients with myeloma, non-Hodgkin's lymphoma, and Hodgkin's disease had significantly higher rates. In multivariate analysis, risk factors associated with venous thrombosis included age ≥ 65 years, female sex, black race, use of chemotherapy, primary site of cancer, and presence of comorbidities. A recent study associated central venous catheters and RBC or platelet transfusions with increased risk of venous thromboembolism in hospitalized patients with cancer.34
CLINICAL TRIALS
Randomized clinical trials of prophylaxis for venous thromboembolism restricted specifically to patients with cancer are not available, and information regarding benefits and risks must be inferred from studies in surgical or general medical patients. For example, Dentali et al35 performed a meta-analysis of anticoagulant prophylaxis in hospitalized medical patients that included nine studies with 19,958 patients. Prophylaxis significantly reduced pulmonary embolism (relative risk [RR] = 0.43; 95% CI, 0.26 to 0.71), with an absolute risk reduction of 0.29% and a number needed to treat of 345 patients. Similarly, fatal pulmonary embolism was reduced (RR = 0.38; 95% CI, 0.21 to 0.69), with an absolute risk reduction of 0.25% and a number to treat of 400 patients. There was a nonsignificant reduction in symptomatic deep vein thrombosis (RR = 0.47; 95% CI, 0.22 to 1.0) and a nonsignificant increase in major bleeding (RR = 1.32; 95% CI, 0.73 to 3.27).
Three large, randomized, double-blind, placebo-controlled trials have demonstrated the benefits of anticoagulant prophylaxis in general medical patients (Table 4). The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study randomly assigned 1,102 hospitalized patients older than 40 years to receive enoxaparin 40 mg, enoxaparin 20 mg, or placebo subcutaneously once daily for 6 to 14 days.7 Eligible patients had congestive heart failure (New York Heart Association class III or IV), acute respiratory failure that did not require ventilator support, acute infection, or acute rheumatic disorder in association with an additional risk factor including cancer. The primary outcome was venous thromboembolism between days 1 and 14 that was either clinically symptomatic or detected by bilateral venography or ultrasound between days 6 and 14 in asymptomatic patients. The incidence of venous thromboembolism was significantly lower in the group that received 40 mg of enoxaparin (5.5%, 16 of 291 patients) than in the group that received placebo (14.9%, 43 of 288 patients; RR = 0.37; P < .001). There was no significant difference in the incidence of venous thromboembolism between the group that received 20 mg of enoxaparin (15.0%, 43 of 287 patients) and the placebo group. There was also no significant difference among the groups regarding bleeding complications.
Table 4.
Trials of Anticoagulant Prophylaxis for Venous Thromboembolism in Hospitalized Medical Patients
| Study | Total No. of Patients | Patients With Cancer |
Placebo Events |
Treatment Events |
Relative Risk | 95% CI | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No./Total No. | % | No./Total No. | % | |||||
| MEDENOX | 579* | 72 | 12.4 | 43/288 | 14.9 | 16/291 | 5.5 | 0.37 | 0.22 to 0.63 | < .001 |
| PREVENT | 3,706 | 190 | 5.1 | 73/1,473 | 4.96 | 42/1,518 | 2.77 | 0.55 | 0.38 to 0.8 | .0015 |
| ARTEMIS | 849† | 131 | 15.4 | 34/323 | 10.5 | 18/321 | 5.6 | 0.47 | 0.08 to 0.69 | .029 |
Abbreviations: MEDENOX, Prophylaxis in Medical Patients with Enoxaparin; PREVENT, Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial; ARTEMIS, Arixtra for Thromboembolism Prevention in a Medical Indications Study.
MEDENOX included a 20-mg enoxaparin arm of 287 patients with event rates equivalent to placebo. No. includes only patients receiving enoxaparin 40 mg or placebo.
Total patients assessable for safety analysis; only 644 patients were assessable for the primary end point.
The results were similar in the Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT).8 This study included 3,706 patients older than age 40 who were admitted to the hospital with an acute medical condition requiring hospitalization for more than 4 days. Patients had acute congestive heart failure, acute respiratory failure, infection and acute rheumatologic disease, or inflammatory bowel disease. Most patients had one or more additional risk factors for venous thromboembolism including cancer. Eligible patients were randomly assigned to receive once-daily subcutaneous injections of either dalteparin 5,000 U or placebo for 14 days. The primary end point was venous thromboembolism, which included symptomatic deep vein thrombosis or pulmonary embolism and asymptomatic proximal deep vein thrombosis detected by compression ultrasound at day 21. The incidence of venous thromboembolism was reduced from 4.96% (73 of 1,473 patients) in the placebo group to 2.77% (42 of 1,518 patients) in the dalteparin group, an absolute risk reduction of 2.19% or a relative risk reduction of 45% (95% CI, 38% to 80%; P = .0015). There were no significant differences in mortality up to 90 days or in major bleeding complications between the two groups.
The Arixtra for Thromboembolism Prevention in a Medical Indication Study (ARTEMIS) had a similar design and involved 849 hospitalized patients older than age 60 who were admitted to hospital for congestive heart failure, acute respiratory illness in the presence of chronic lung disease, or acute infectious or inflammatory disease and who were expected to remain for at least 4 days.6 They were randomly allocated to receive either fondaparinux 2.5 mg or placebo subcutaneously once daily for 6 to 14 days. The primary outcome was symptomatic venous thromboembolism or asymptomatic deep vein thrombosis detected by routine bilateral venography up to day 15. Venous thromboembolism was detected in 5.6% of patients (18 of 321 patients) treated with fondaparinux and 10.5% of patients (34 of 323 patients) administered placebo, a relative risk reduction of 46.7% (95% CI, 7.7% to 69.3%). At 1 month, the mortality was 3.3% in patients in the fondaparinux group compared with 6.0% in the placebo group (P = .06). Major bleeding was low and not significantly different in the two groups.
Taken together, the results of the meta-analysis and three prospective trials clearly demonstrate that primary prophylaxis using heparin, low molecular weight heparins, or fondaparinux reduces venous thromboembolism in hospitalized medical patients. Limited information is available comparing different anticoagulant regimens for prophylaxis. However, a meta-analysis that included nine trials (4,669 patients) compared low molecular weight heparins with unfractionated heparin for prophylaxis in medical patients, excluding those with acute myocardial infarction or stroke.36 There was no significant difference between low molecular weight heparins and unfractionated heparin concerning the incidence of deep vein thrombosis (RR = 0.083; 95% CI, 0.56 to 1.2), symptomatic pulmonary embolism (RR = 0.74; 95% CI, 0.29 to 1.88), or mortality. However, low molecular weight heparins reduced the risk of major hemorrhagic complications compared with unfractionated heparin (RR = 0.48; 95% CI, 0.23 to 1.00; P = .049). There are no direct comparisons of fondaparinux with heparin or low molecular weight heparins for prophylaxis, and there are no comparisons of anticoagulant regimens specifically in patients with cancer.
It should be noted that all of the studies cited include a heterogeneous population of medical patients in whom those with cancer represent a minority. Thus, in the PREVENT, MEDENOX, and ARTEMIS studies, patients with cancer represented 5.1%, 12.4%, and 15.4% of the total study groups, respectively. No studies are available in which prophylaxis has been evaluated in a population limited to patients with cancer. However, a subgroup analysis of the MEDENOX study showed that patients with cancer had an elevated risk of venous thromboembolism37 and that the relative risk reduction for venous thromboembolism in patients with cancer treated with enoxaparin 40 mg/d was 0.50 (95% CI, 0.14 to 1.72), which was similar to the benefits observed in the whole group and in other predefined risk factor subgroups. However, patients with cancer have an increased risk for bleeding, resulting from factors such as thrombocytopenia and the performance of invasive procedures that occurs more commonly than in other medical patients. Thus, care should be exercised in administering anticoagulant prophylaxis in patients with a high bleeding risk, such as those with severe thrombocytopenia or recent GI bleeding.
Despite the limitations of the available data, it is clear that hospitalized patients with cancer are at high risk of venous thromboembolism and that this contributes significantly to morbidity and mortality. Evidence is strong that mechanical or pharmacologic prophylaxis reduces venous thromboembolism in hospitalized surgical and general medicine patients and in patients with cancer by inference. Overall, the bleeding risk seems small, and the benefit-to-risk ratio strongly favors providing prophylaxis.
GUIDELINES
Several groups have provided guidelines for the use of thromboprophylaxis in cancer patients. The American Society of Clinical Oncology performed a comprehensive systematic review of the literature on the prevention of venous thromboembolism in patients with cancer and recommended that hospitalized patients with cancer should be considered candidates for venous thromboembolism prophylaxis with anticoagulants in the absence of bleeding or other contraindications to anticoagulation.24 The American College of Chest Physicians issued guidelines in 2008 and recommended that routine prophylaxis be provided for patients with cancer who are bedridden with acute medical illness as for other high-risk medical patients (grade 1A).26 The National Comprehensive Cancer Network recommends that hospitalized patients with a diagnosis of cancer or a clinical suspicion of cancer who have no contraindication to anticoagulation receive prophylactic pharmacologic prophylaxis with or without sequential compression devices. Those with a contraindication to anticoagulation should receive mechanical prophylaxis with either sequential compression devices or graduated compression stockings.38
UTILIZATION OF PROPHYLAXIS
Evidence indicates that prophylaxis for venous thromboembolism is underused in hospitalized patients.3–5,39–41 A community-wide study of 16 hospitals in Massachusetts indicated that prophylaxis was provided for only 32% of patients at high risk.3 A prospective study of patients in the intensive care unit showed that only 33% received prophylaxis, which was administered after an average delay of 2 days.41 Data from a registry showed that only 42% of 2,726 patients with symptomatic deep vein thrombosis that developed during hospitalization had received prophylaxis,5 and in a survey of 106 oncologists, approximately 80% indicated that they did not routinely provide prophylaxis for inpatients undergoing active treatment for cancer.42 A chart audit of 29 Canadian hospitals examined 4,124 medical admissions and found that thromboprophylaxis was indicated in 90% of patients but was administered to only 23% of these patients and only 16% received appropriate thromboprophylaxis.43 In this study, patients with cancer had a significantly reduced likelihood of receiving prophylaxis (OR = 0.40; 95% CI, 0.24 to 0.68).
Analysis of a prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis from 183 hospitals in the United States found that 1,096 patients (20%) had active cancer.44 Patients with cancer received prophylaxis for venous thromboembolism less often before development of deep vein thrombosis compared with patients with no cancer (28.2% v 34.6%, respectively; P < .0001). The Frontline Survey collected information regarding perceived risks and patterns of practice with regard to venous thromboembolism in cancer patients from 3,891 respondents primarily in Europe and North America.45 More than 50% of surgeons reported that they provided thromboprophylaxis routinely, whereas medical oncologists reported using prophylaxis in less than 5% of patients. Much of the data were collected before recent guidelines were publicized. However, it seems clear that thromboprophylaxis is underused in cancer inpatients and that strategies to improve prophylaxis are indicated.
Evidence-based educational programs that provide hospital-specific data demonstrating the problem of venous thromboembolism can be successful in increasing use of prophylaxis by clinicians, as shown in a study that evaluated the use of prophylaxis in 15 community hospitals before and after a targeted continuing medical educational program was conducted.46 In large randomized trials, computer prompts that remind physicians to consider the appropriate use of prophylaxis have been shown to increase its use.47,48 In a trial that assessed the effects of a computerized reminder system on the use of heparin and outcomes among 2,501 inpatients identified by the computer to be at high risk, the intervention group received prophylactic treatment at a higher rate than the control group (34% v 15%, respectively; P < .001).48 Patients judged to be at high risk were those admitted with cancer, previous venous thromboembolism, hypercoagulability, major surgery, advanced age, obesity, on bed rest, or using postmenopausal hormone therapy or oral contraceptives. The intervention group that received computerized prompts also had a lower incidence of symptomatic confirmed deep vein thrombosis or pulmonary embolism at 90 days compared with the control group (4.9% v 8.2%, respectively; P < .001).
AREAS OF UNCERTAINTY
Although the evidence is clear that administration of prophylaxis can reduce venous thromboembolism in hospitalized surgical and medical patients, areas of uncertainty remain, and additional information is needed. For example, recommendations for hospitalized cancer patients are inferential and derived from studies of medical inpatients with a variety of high-risk conditions, with cancer representing a minority. Therefore, data from clinical trials of prophylaxis focused on inpatients with cancer would be of considerable interest, particularly because patients with cancer often have increased risks of bleeding. Additional information regarding the value of mechanical prophylaxis in patients with cancer is also needed. Furthermore, no data are available regarding the comparative efficacy of fondaparinux with low molecular weight heparins or unfractionated heparin for prophylaxis, and additional comparisons with more anticoagulants in late-phase clinical development will also be needed.
Finally, greater awareness of oncologists is needed to increase the use of prophylaxis for venous thromboembolism in hospitalized patients with cancer. Evidence from chart reviews and surveys clearly shows that only a minority of hospitalized patients with cancer are receiving appropriate thromboprophylaxis. The use of continuing medical education programs and especially computer-based prompts should be strongly considered to increase compliance with current guidelines.
Footnotes
Supported in part by Grant No. RO1HL095109-01 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Author's disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Charles W. Francis, Boehringer Ingelheim (C) Stock Ownership: None Honoraria: Charles W. Francis, Eisai Research Funding: Charles W. Francis, Eisai Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 2.Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy: III. Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. BMJ. 1994;308:235–246. [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson FA, Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: The Worcester DVT Study. Arch Intern Med. 1991;151:933–938. [PubMed] [Google Scholar]
- 4.Goldhaber SZ, Dunn K, MacDougall RC. New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118:1680–1684. doi: 10.1378/chest.118.6.1680. [DOI] [PubMed] [Google Scholar]
- 5.Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol. 2004;93:259–262. doi: 10.1016/j.amjcard.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 6.Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: Randomised placebo controlled trial. BMJ. 2006;332:325–329. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samama MM, Cohen AT, Darmon J-Y, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 8.Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 9.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 10.Baglin TP, White K, Charles A, et al. Fatal pulmonary embolism in hospitalised medical patients. J Clin Pathol. 1997;50:609–610. doi: 10.1136/jcp.50.7.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindblad B, Eriksson A, Bergqvist D. Autopsy-verified pulmonary embolism in a surgical department: Analysis of the period from 1951 to 1988. Br J Surg. 1991;78:849–852. doi: 10.1002/bjs.1800780725. [DOI] [PubMed] [Google Scholar]
- 12.Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: Are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82:203–205. doi: 10.1177/014107688908200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108:978–981. doi: 10.1378/chest.108.4.978. [DOI] [PubMed] [Google Scholar]
- 14.Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch Intern Med. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 15.White RH, Wun WT. The burden of cancer-associated venous thromboembolism and its impact on cancer survival. In: Khorana A, Francis C, editors. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare; 2007. pp. 115–129. [Google Scholar]
- 16.Silverstein MD, Heit J, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 17.Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: Risk analysis using Medicare claims data. Medicine. 1999;78:285–291. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb Haemost. 2002;87:575–579. [PubMed] [Google Scholar]
- 19.Stein PD, Beemath A, Meyers FA, et al. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 20.Khorana A, Francis CW, Culakova E, et al. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 21.Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 22.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 23.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653–1661. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 24.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: Recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 25.Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 2008;133(suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 26.Geerts WH, Bergqvist D, Pineo GG, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 27.Shojania KG, Duncan B, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. http://www.ahrq.gov/clinic/ptsafety/ [PMC free article] [PubMed]
- 28.Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health. 2003;6:59–74. doi: 10.1046/j.1524-4733.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 29.Avorn J, Winkelmayer W. Comparing the costs, risks, and benefits of competing strategies for the primary prevention of venous thromboembolism. Circulation. 2004;110(suppl):IV25–IV32. doi: 10.1161/01.CIR.0000150642.10916.ea. [DOI] [PubMed] [Google Scholar]
- 30.Agu O, Hamilton G, Baker D. Graduated compression stockings in the prevention of venous thromboembolism. Br J Surg. 1999;86:992–1004. doi: 10.1046/j.1365-2168.1999.01195.x. [DOI] [PubMed] [Google Scholar]
- 31.Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2000;3:CD001484. doi: 10.1002/14651858.CD001484. [DOI] [PubMed] [Google Scholar]
- 32.Prevention of fatal postoperative pulmonary embolism by low doses of heparin: An international multicenter trial. Lancet. 1975;2:45–51. [PubMed] [Google Scholar]
- 33.Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin: Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173. doi: 10.1056/NEJM198805053181805. [DOI] [PubMed] [Google Scholar]
- 34.Khorana A, Francis CW, Blumberg N, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dentali F, Doukexis J, Gianni M, et al. Meta-analysis: Anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 36.Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: A meta-analysis of randomised clinical trials. Thromb Haemost. 2000;83:14–19. [PubMed] [Google Scholar]
- 37.Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness. Arch Intern Med. 2004;164:963–968. doi: 10.1001/archinte.164.9.963. [DOI] [PubMed] [Google Scholar]
- 38.National Comprehensive Cancer Network. www.nccn.org.
- 39.Ageno W, Squizzato A, Ambrosini F, et al. Thrombosis prophylaxis in medical patients: A retrospective review of clinical practice patterns. Haematologica. 2002;87:746–750. [PubMed] [Google Scholar]
- 40.Anderson FA, Jr, Wheeler H, Goldberg RJ, et al. Physician practices in the prevention of venous thromboembolism. Ann Intern Med. 1991;115:591–595. doi: 10.7326/0003-4819-591. [DOI] [PubMed] [Google Scholar]
- 41.Keane MG, Ingenito EP, Goldhaber SZ. Utilization of venous thromboembolism prophylaxis in the medical intensive care unit. Chest. 1994;106:13–14. doi: 10.1378/chest.106.1.13. [DOI] [PubMed] [Google Scholar]
- 42.Kirwan CC, Nath E, Byrne GJ, et al. Prophylaxis for venous thromboembolism during treatment for cancer: Questionnaire survey. BMJ. 2003;327:597–598. doi: 10.1136/bmj.327.7415.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahn SR, Panju A, Geerts W, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145–155. doi: 10.1016/j.thromres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Seddighzadeh A, Shetty R, Goldhaber S. Venous thromboembolism in patients with active cancer. Thromb Haemost. 2007;98:656–661. [PubMed] [Google Scholar]
- 45.Kakkar AK, Levine M, Pinedo HM, et al. Venous thrombosis in cancer patients: Insights from the FRONTLINE survey. Oncologist. 2003;8:381–388. doi: 10.1634/theoncologist.8-4-381. [DOI] [PubMed] [Google Scholar]
- 46.Anderson FA, Jr, Wheeler H, Goldberg RJ, et al. Changing clinical practice: Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism. Arch Intern Med. 1994;154:669–677. doi: 10.1001/archinte.154.6.669. [DOI] [PubMed] [Google Scholar]
- 47.Dexter PR, Perkins S, Overhage JM, et al. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345:965–970. doi: 10.1056/NEJMsa010181. [DOI] [PubMed] [Google Scholar]
- 48.Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]