Abstract
Background:
Attempts at cell-based dopamine replacement therapy in Parkinson disease (PD) have included surgical implantation of adrenal medullary, fetal mesencephalic, and cultured human mesencephalic tissue grafts. Trials involving putamenal implantation of human retinal pigment epithelial (RPE) cells in PD have also been performed. Neuropathologic findings in humans undergoing RPE cell implantation have not heretofore been reported. We describe the brain autopsy findings from a subject enrolled in a clinical trial of RPE cells in gelatin microcarriers for treatment of PD, and suggest factors which may have impacted cell survival.
Methods:
A 68-year-old man underwent bilateral surgical implantation of 325,000 RPE cells in gelatin microcarriers (Spheramine) but died 6 months after surgery. The left cerebral hemisphere was examined. Routine postmortem formalin fixation was performed and standard, as well as immunohistochemical methods used to highlight senile plaque and Lewy body pathologic changes, iron deposition, cellular inflammation, and reactive astrocytosis in implant regions. Manual cell counts were done of RPE cells.
Results:
Hematoxylin-eosin and α-synuclein immunostains confirmed the diagnosis of PD. Needle tracts with matrix material and RPE cells were observed in the context of local inflammatory and astrocytic reactive change. A total of 118 cells were counted (estimated 0.036% survival).
Conclusions:
Retinal pigment epithelial cells are seen in human brain 6 months postimplantation, but overall survival of implanted cells appeared poor.
GLOSSARY
- ABC
= avidin-biotin-peroxidase complex;
- DAB
= diaminobenzidine tetrahydrochloride;
- EGTA
= ethylene glycol-bis[β-aminoethyl ether]-N,N,N′N′-tetraacetic acid;
- GFAP
= glial fibrillary acidic protein;
- H-E
= hematoxylin-eosin;
- PD
= Parkinson disease;
- PEDF
= pigment epithelial derived factor;
- RPE
= retinal pigment epithelium.
Clinical trials using cell implantation for Parkinson disease (PD) have included fetal mesencephalic, autologous sympathetic ganglionic, and adrenal medullary graft implantation.1–7 These approaches have been hampered by limited cell survival or development of adverse motoric effects in subpopulations of subjects.5,8 More recent investigations have focused on grafting human retinal pigment epithelial (RPE) cells.9–13 In the human retina, RPE cells form a single layer adherent to the lamina vitrea of Bruch membrane. Four to 6 million cells measuring 12–18 μm in width and 10–14 μm in height make up the layer, which functions in recycling of vitamin A, absorption of scattered light, and transport of nutrients, and contributes to the blood–retina barrier.14 l-dopa is present intracellularly in RPE cells, as melanin synthesis begins with tyrosinase-mediated conversion of tyrosine to l-dopa.15
Possible RPE cell–mediated trophic support of basal ganglia neurons has been suggested by studies in rats showing that medium from RPE cell cultures promotes dopaminergic neuronal neuritic growth.16 Functional imaging studies in animals suggest enhancement of dopamine release.17 In humans, motor and quality of life measure improvements have been reported in open label studies up to 24 months after implantation.11,18,19 Histopathologic findings from studies on RPE cell implantation in humans have not been reported.
We describe the neuropathologic findings in a 68-year-old man enrolled in a blinded safety and tolerability study of stereotactic implantation of cultured human RPE cells on microcarriers.9 The subject underwent surgery 6 months prior to death.
METHODS
The subject was a 68-year-old man with an 18-year history of PD. He developed disabling motor fluctuations despite maximal medical management and enrolled in the Spheramine Safety and Efficacy Study (STEPS trial) investigating the safety, tolerability, and efficacy of bilateral putamenal implantation of cultured human RPE cells on microcarriers in PD. The procedure involved implantation of 325,000 RPE cells distributed over 5 tracts in each putamen.20 The subject did not note motoric benefit postimplantation and died 6 months after surgery after a fall leading to pulmonary empyema.
Consent for autopsy including use of tissue for research purposes was obtained from next of kin. The brain was removed and a section from left midbrain was frozen. After fixation in 10% neutral buffered formalin, a mid-sagittal cut was made, and the right cerebral hemisphere with brainstem attached sent to the University of British Columbia (Dr. John O'Kusky, Vancouver, Canada) for processing under Dr. O'Kusky's research protocol. The left cerebral hemisphere was examined at UCLA. Coronal sections of the left hemisphere were taken at intervals of 1 cm beginning at the frontal pole and extending posteriorly to the beginning of the enlargement of the anterior horn of the lateral ventricle. At that point, 4-mm sections of the hemisphere were taken through the extent of the striatum (8 coronal slices). For each coronal level beginning at the anterior horn, 2 blocks were taken for histologic examination. An inferior block contained sampling of the periventricular white matter, corpus striatum, and thalamus (in most posterior levels). A block comprised of an area of overlying cortex and centrum semiovale at each level was also taken. Prefrontal, frontal, and cingulate cortices were therefore represented. Additional blocks were also sampled from cerebellum, hippocampus, occipital, and parietal cortices. The posterior (more caudal) face of each block was inked along its margins. After microscopic examination of hematoxylin-eosin (H-E)–stained sections, blocks containing implantation tracts were identified. An additional 100 serial 6-μm sections were taken from each of these blocks.
Staining.
Routine H-E, Perl's iron,21 and immunohistochemical stains were performed. Antibodies directed against CD 68 and glial fibrillary acidic protein (GFAP) highlighted monocyte/macrophage/microglial and astroglial cellular reactivity. One area of cortex and putamen were assessed for adaptive cellular immune response using antibodies targeting B and T cell markers CD4, CD8, and CD19. α-Synuclein immunohistochemistry identified Lewy body inclusion and Lewy neuritic elements, while amyloid β1–42 and tau immunostains enhanced visualization of parenchymal Alzheimer lesions (senile plaques and neurofibrillary tangles), and pigment epithelial derived factor (PEDF) immunostaining was used to identify RPE cells. Hippocampal and parietal cortices were examined with Bielschowsky silver stain to further highlight Alzheimer pathology and neuronal cytoarchitecture. Sections from the block containing a representative RPE cell profile on initial review were stained with anti RPE-65, EMMPRIN, and tyrosine hydroxylase antibodies (table e-1 on the Neurology® Web site at www.neurology.org). Immunostains used the avidin-biotin-peroxidase complex (ABC) (Vector, Burlingame, CA) and 3,3≪-diaminobenzidine tetrahydrochloride (DAB) chromogen for visualization. Appropriate positive and negative controls were used. For the majority of immunostains, microwave boiling 10 minutes in 0.01 M pH 6.0 sodium citrate buffer provided antigen retrieval prior to immunostaining. α-Synuclein immunostaining used this method of antigen retrieval. Antigen retrieval for CD4, CD8, and CD19 immunostains was done with microwave boiling 16 minutes in pH 9.0 10 mM Tris buffer with 0.5 mM ethylene glycol-bis[β-aminoethyl ether]-N,N,N′N′-tetraacetic acid (EGTA).
Cell counts.
Initial screening to locate tracts and cells was done on 8 6-μm sections through each block, revealing a single neuromelanin-containing profile. As cells were not encountered in uniform fashion, and to maximize sensitivity of cell detection, sectioning continued at 6 μm thickness. All sections were stained and manually examined. The protocol therefore was not amenable to nonbiased stereologic methods. Microscopic examination utilized a microscope with camera mount (Olympus BX-40, Tokyo, Japan) and reticule with engraved grid. Sections were screened under 20× objective lens and cell profiles confirmed under 40× objective lens. The presence and location of needle tracts, presence of foreign material consistent with microcarrier matrix, and presence and number of neuromelanin-containing profiles were noted on H-E sections. Neuromelanin-containing cellular profiles were counted. Counts took into account the typical dimensions encountered in single cells. Where cells in close approximation were encountered, neuromelanin-containing profiles with contiguous border extending beyond 1 grid square (25 μm) under 40× objective were counted as 2 cells. Neuromelanin-containing profiles were seen maximally on 3 consecutive serial sections due to their A-P (rostral-caudal) dimension, and where this occurred, were not counted as distinct profiles. Thirty sections were submitted for interrater reliability assessment. Spearman correlation coefficient determination and Bland-Altman analysis of counts were done using GraphPad Prism 5 software (GraphPad Software Inc., LA Jolla, CA).
RESULTS
Gross examination.
The calvarium contained symmetric bilateral plates and screws in the superior frontal parasagittal region consistent with prior surgery. The prefixation brain weight was 1,200 g. The cortical gyral/sulcal appearance and base of brain were remarkable for a mild degree of cortical atrophy over the frontal convexities. Over the superior frontal gyri were 2 symmetric irregularities in the cortical mantle, each measuring 3 mm, consistent with instrumentation entry points. Gross pathologic examination suggested a mildly to moderately atrophic brain without significant topographic accentuation.
Microscopic findings.
Normal cortical neuronal laminar architecture was seen throughout the cortical mantle. Senile plaques were inconspicuous, and neurofibrillary tangles were absent in the hippocampus and neocortex. Cerebral infarcts were not seen.
Significant depigmentation and neuron loss in the substantia nigra was noted with characteristic Lewy bodies in locus ceruleus. Lewy body pathology was less prominent in substantia nigra due to the severe degree of neuron loss. Lewy body–containing neurons were sparse in the frontal cortex and cingulum, with rare hippocampal intraneuronal inclusions (figure 1). The findings were consistent with those of advanced PD.
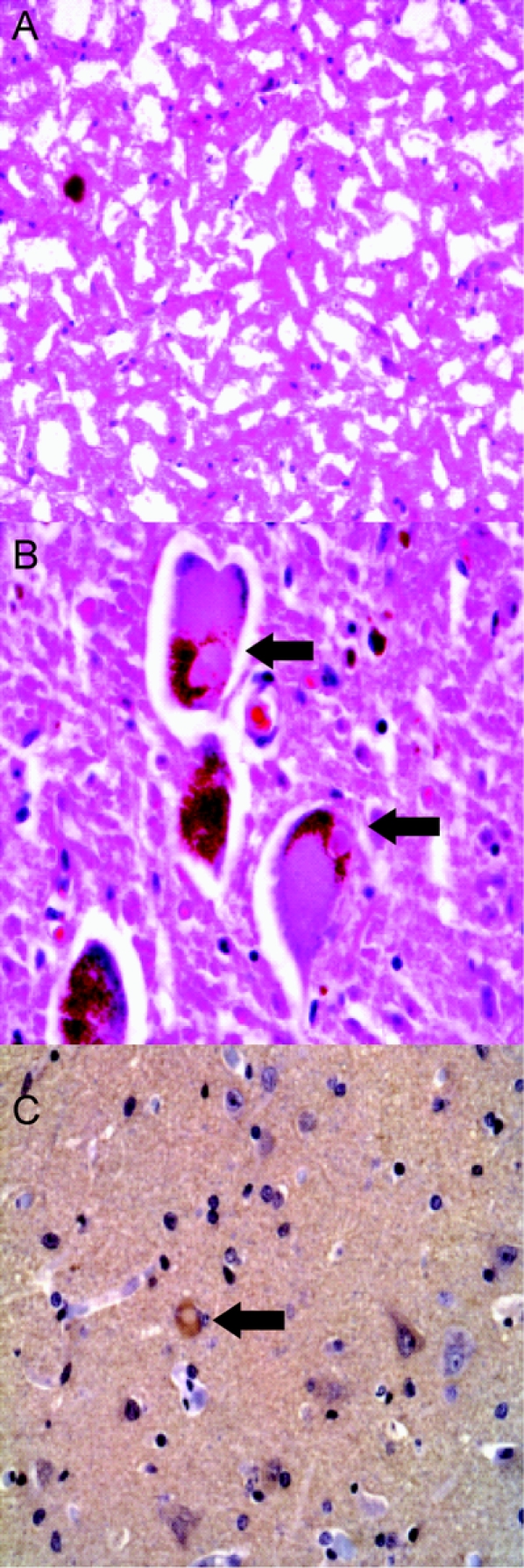
Figure 1 Parkinson disease neuropathology
Hematoxylin-eosin (H-E) stained frozen section of substantia nigra (SN) (A) and H-E stained paraffin section of locus ceruleus (LC) (B) showing severe pigmented neuron loss in SN and Lewy bodies in residual LC neurons (arrows). α-Synuclein immunostaining highlights rare immunoreactive neuron in frontal cortex (C).
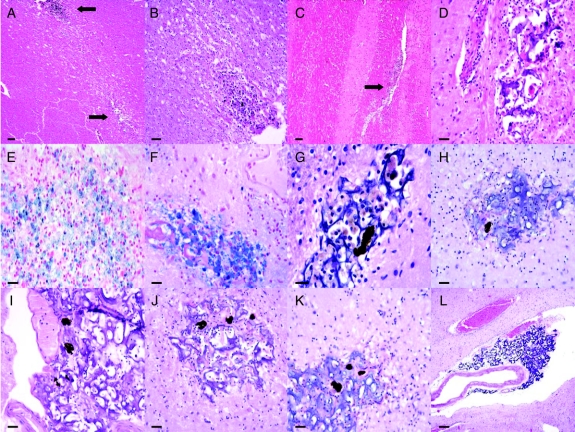
In the region of the periventricular white matter, internal capsule, and putamen, needle tracts were identified based on the presence of characteristic tissue changes. These consisted of foci of irregular cystic cavitation with varying degrees of surrounding glial reactivity. Size and orientation varied from punctate to linear (in internal capsule). The internal capsular region between the anterior horn of the lateral ventricle and putamen contained 2 to 3 of these lesions, depending on the coronal level examined, with the remainder typically within the lateral putamen (figure e-1). Frequent infiltration by CD68-positive macrophages and other inflammatory cells was noted, at times filling these spaces. Frequently, macrophages were seen within matrix crevices (figures 2G and 3A). No vascular-centered inflammation or vascular infiltration was observed. Perl's iron stain confirmed the presence of iron within macrophages and in the parenchyma surrounding certain lesion sites. A subset of lesions contained a basophilic substance with intrinsic porosity consistent with matrix support material employed in RPE cell implantation in animals.23,30 The cross-sectional profile of the substance typically evolved and regressed along its linear (A-P axis) extent, consistent with irregularly semi-cylindrical or spherical aggregations. Deposition was therefore nonuniform along the A-P axis. All additional sections contained needle tract lesions, and the majority of foci contained matrix material. In the majority of sections, no cells—excluding inflammatory cells and rare glia—were seen within the matrix. At tract sites near the medial putamen/lateral internal capsule border, and more posteriorly in the inferior putamen, 2 tracts appeared to fuse. Representative tract lesions, RPE cells, and associated tissue changes are shown in figure 2.
Figure 2 Representative tract lesions and associated findings
(A) Low power view of 2 tract sites, with upper lesion containing cluster of hemosiderin-containing cells shown in higher power in (B), and stained with Perl's iron in (E). (C) Linear tract lesion in internal capsule (arrow). (D) Tract focus containing matrix material without retinal pigment epithelial (RPE) cells. (F) Perl's iron stain of another tract site with matrix material and dense aggregate of likely hemosiderin-containing macrophages. (G–K) Hematoxylin-eosin stain of tract sites showing basophilic matrix material with representative RPE cell neuromelanin profiles. (L) Matrix material within Virchow-Robin space and abutting small artery in inferior putamen. Scale bar: A, C 200 μm; B 50 μm; D–F 30 μm; G 20 μm; H–K 30 μm; L 150 μm.
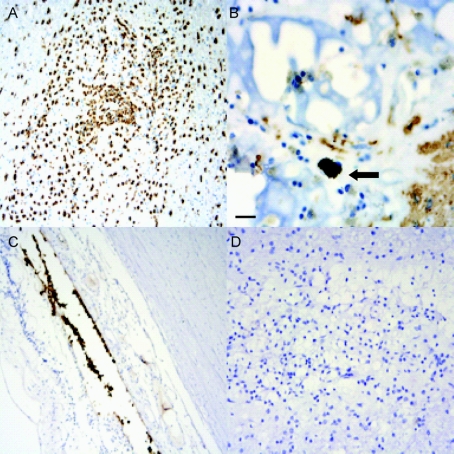
Figure 3 Representative immunostaining results
(A) CD68 immunostain of tract lesion demonstrating inflammatory cell infiltration. (B) Glial fibrillary acidic protein immunostain shows rare immunoreactive astrocytes encroaching on matrix, with retinal pigment epithelial (RPE) cell within matrix lattice (arrow). (C) RPE 65 immunostain of normal human retina highlights RPE cells. (D) RPE 65 immunostain of matrix-containing tract site showing absence of immunoreactivity. Scale bar: 20 μm.
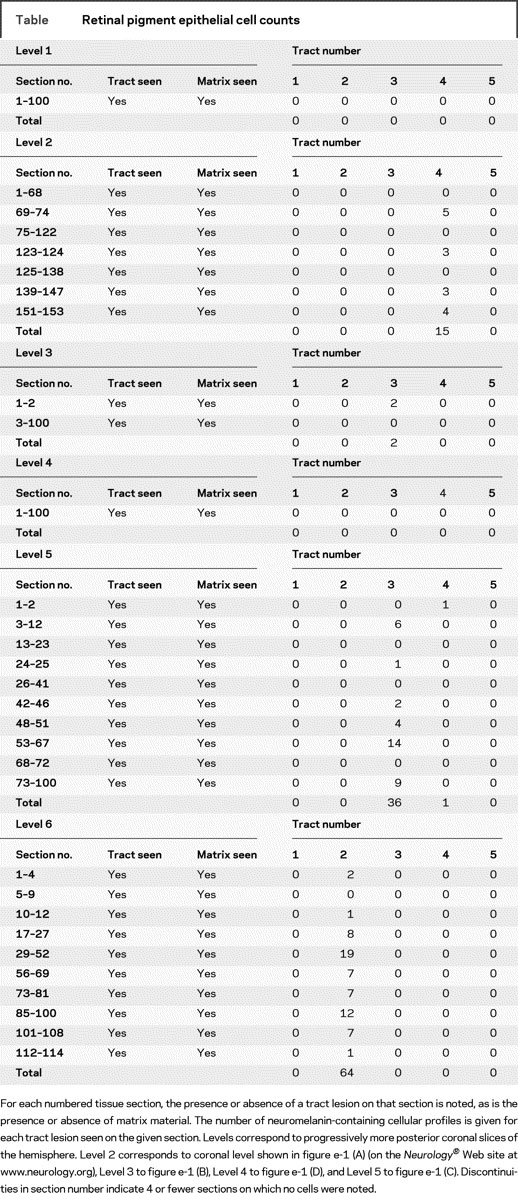
Intermittently, within the matrix substance, cellular profiles were encountered whose size, cytoarchitectural features, and tinctorial characteristics differed from those of neuronal or glial elements. They contained dense clusters of darkly pigmented coarse granules typically obscuring the nucleus and other cytoplasmic features. The overall appearance was consistent with that of neuromelanin-containing cells. Their size in the A-P axis averaged 18 μm, and length in coronal plane was typically 25 μm, though cell contours were irregular. At times, larger pigmented profiles suggestive of cellular clusters were noted. In the posterior putamen, the matrix material was present in the Virchow-Robin space surrounding a single blood vessel (figure 2L). The internal capsule lesions rarely contained matrix material. Tyrosine hydroxylase, PEDF, and RPE-65 immunostains did not highlight cellular or neuritic structures. Though areas containing RPE cells tended to have less inflammation within matrix, there did not appear to be a qualitative difference in inflammatory cell presence between tract regions devoid of RPE cells and those with relatively more RPE cells. The limited staining done using B- and T-cell markers suggested that the inflammatory response in those areas examined involved primarily macrophage presence with mild CD8-positive T-cell presence, in the absence of appreciable CD19 and CD4 immunoreactivity. Characteristic immunostaining results are shown in figure 3. Sections on which the stains were performed did not capture neuromelanin-containing cell profiles. Cell counts are summarized in the table. A total of 118 RPE cells were observed from the 5 tract sites. Spearman correlation coefficient determination and Bland-Altman analysis of interrater reliability counts yielded significant correlation (table e-2).
Table Retinal pigment epithelial cell counts
DISCUSSION
The gross and microscopic findings were consistent with those of a subject with advanced PD undergoing surgical implantation of RPE cells within the putamen. Lesions consistent with needle tracts were identified, having their most inferocaudal extent within the inferolateral putamen near the coronal level of the amygdala. Most foci contained microcarrier matrix support material. Within needle tract lesions, and often within matrix material, a variable macrophage-predominant inflammatory cellular presence was noted, at times filling the tract focus. Relatively few RPE cells were observed.
In addition to depigmentation and neuronal dropout in the substantia nigra, and Lewy body presence in the locus ceruleus, sparse Lewy body presence in frontal neocortex and cingulum, with very rare hippocampal involvement, was seen in the hemisphere examined. Cortical Lewy bodies are seen in longstanding PD, as well as in a significant subset of the asymptomatic elderly.22,23 Though the precise histopathologic relationship between PD, PD dementia, and dementia with cortical Lewy bodies has yet to be definitively determined,22,24–26 the subject's longstanding history of levodopa-responsive parkinsonism with characteristic motor fluctuations and primarily motoric rather than cognitive symptoms antedating decline would not be consistent with the clinical diagnosis of diffuse Lewy body disease.27
Generally, matrix material was encountered as expected, in the putamen. Matrix material was also seen rarely within tracts in the internal capsule, suggesting rostral translocation of matrix within a tract, or deposition at those sites. The presence of matrix did not reliably predict the presence of RPE cells. The degree, if any, of immunogenicity imparted by matrix material or RPE cells is unknown from the current examination, though cellular reactivity was clearly observed. Studies in humans and animals have reported motoric benefits from RPE cell therapy without immunosuppression.18,28–30 An inflammatory response up to 5 months post RPE cell implantation has been reported in certain animal studies but in the context of preserved cell survival,29 though other studies suggest absence of notable host response as assessed by immunostaining for activated microglial markers.28 Pathologic studies after fetal mesencephalic grafting have generally documented little to no inflammation associated with grafts, but in subjects who received immunosuppression5,31; nonetheless, these studies did find some degree of graft-associated macrophage presence, and rarely, extensive macrophage presence with significant tract-to-tract variability within the same subject.32 Macrophage presence associated with adrenal medullary autografts has likewise been seen in a protocol employing different surgical technique.33 Provocation of cellular reactivity by surgical procedure–related factors can also be considered. Focal inflammation at the site of ablation has been described 3 weeks post pallidotomy for PD.34 This likely represents, however, a qualitatively different coagulative necrotic process associated with thermal injury. No conclusions can be drawn overall regarding the relative contribution of innate and adaptive cellular immune response given the limited sampling. The degree of cellular inflammation associated with tract foci may have had ramifications on cell survival, and the current findings suggest that more investigation may be warranted with respect to inflammation, immunosuppression, and RPE cell implants in humans.
In keeping with descriptions of RPE cells in animal models, the cobblestone and monolayer appearances seen in vivo and in culture were replaced with an irregular spherical or curvilinear cell structure which has been speculated to contribute to cell survival.29 The cells contained typical-appearing neuromelanin granules. The carrier matrix itself has been reported to impart trophic support to the RPE cells.16 In congruence with this possibility, and with findings in animal studies, RPE cells were nearly exclusively observed within matrix. The nature of the implantation therapy, and the current results, suggest at least 3 possible explanations for the relatively low number of cells encountered. First, the inflammatory response noted may have impacted cell survival. Secondly, potential changes in matrix function may have altered the cellular trophic environment. Finally, as suggested by recent reports showing Lewy bodies in surviving cells in fetal nigral graft recipients, host–graft interactions related to background neurodegenerative pathophysiology may have influenced cell longevity.35–37 This is a novel concept with even more uncertain applicability to non-neuronal cell types. No Lewy bodies were seen in RPE cells in this subject.
Poor cell implant survival may have contributed to the lack of postoperative benefit in this subject, and potentially to lack of overall study efficacy,38 though it should be emphasized that these results are from a single cerebral hemisphere, and that variable cell survival is well-established in cell implantation trials. Past experience in fetal transplants has shown up to sixfold variability in cell survival linked to such variables as number of graft donors and target region of implant (e.g., striatum vs midbrain).5,31 Within the same subject, and the same hemisphere, near 100-fold differences in cell survival have been seen between tracts, while interhemispheric differences ranging from absence of survival to survival of 100,000 cells have been described.31,32 In addition, there were instances in which RPE cell borders were indistinct, making the cell counts likely an underestimate.
A limitation of the current study lies in its quantitation of RPE cells by morphologic rather than immunohistochemical or enzymatic detection. To our knowledge, however, there are no published descriptions of RPE cells postimplantation in human tissue; likewise, the antigenic profile of implanted RPE cells in humans (i.e., on postmortem examination) has not been described. The neuromelanin-containing profiles counted, however, were distinct from glial or neuronal cells, and were encountered only within matrix material. EMMPRIN, RPE-65, and tyrosine hydroxylase have been used to characterize RPE cells in xenotransplantation in animal models.28,29 Host sprouting of tyrosine hydroxylase immunoreactive cell processes or terminals was not seen, suggesting that the mechanisms underlying behavioral improvements may have involved humoral factors. Tyrosine hydroxylase immunoreactivity has been variable in these studies, indicating that tyrosine hydroxylase staining may not reliably assess the functional state of RPE cells. The same reports suggested that the antigenic phenotype of RPE cells may be partially state dependent, varying between in vivo ocular and culture conditions, and that the reliability of these antibodies as markers of RPE cells in humans is unclear. RPE cell production of PEDF has been shown to differ according to state of differentiation using a calcium switch protocol.16 The utility of the antibodies and their antigenic targets in characterizing RPE cells in human tissue cannot be assessed.
Past implantation trial autopsy results highlight the importance of autopsy in assessing cell implantation strategies, and in understanding the biology of the graft objects. Incorporation of autopsy into research protocols in such trials should become routine. Research encompassing preimplantation engineering of cell implants to provide known in vivo imaging–based and post mortem immunohistopathologic stigmata of cell implants facilitates postmortem characterization of such studies.39,40
ACKNOWLEDGMENT
The authors thank Justine Pomakian and Spencer Tung for assistance with immunohistochemistry and interrater reliability determinations. Computer support was provided by Richard May.
DISCLOSURE
Dr. Farag receives research support from the NIH [P01 AG12435 (Research Associate)] and from the VA Southwest Parkinson's Disease Research Education and Clinical Center. Dr. Vinters receives research support from the NIH [P01 AG12435 (Core leader or Core PI) and P50 AG 16570 (Core leader or Core PI)] and from the UCLA Geffen School of Medicine (Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine). Dr. Bronstein has received research support from Bayer Schering Pharma (Site PI at UCLA) and from the VA Southwest Parkinson's Disease Research Education and Clinical Center.
Supplementary Material
Address correspondence and reprint requests to Dr. Emad Farag, Movement Disorders Program, UCLA Department of Neurology, 300 UCLA Medical Plaza Ste. B200, Los Angeles, CA 90095 efarag@mednet.ucla.edu.
Supplemental data at www.neurology.org
Editorial, page 1086.
e-Pub ahead of print on September 2, 2009, at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received March 20, 2009. Accepted in final form July 27, 2009.
REFERENCES
- 1.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 2001;344:710–719. [DOI] [PubMed] [Google Scholar]
- 2.Olanow CW, Koller W, Goetz CG, et al. Autologous transplantation of adrenal medulla in Parkinson's disease: 18-month results. Arch Neurol 1990;47:1286–1289. [DOI] [PubMed] [Google Scholar]
- 3.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci 1996;19:102–109. [DOI] [PubMed] [Google Scholar]
- 4.Hauser RA, Freeman TB, Snow BJ, et al. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 1999;56:179–187. [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003;54: 403–414. [DOI] [PubMed] [Google Scholar]
- 6.Itakura T, Uematsu Y, Nakao N, et al. Transplantation of autologous sympathetic ganglion into the brain with Parkinson's disease: long-term follow-up of 35 cases. Stereotact Funct Neurosurg 1997;69:112–115. [DOI] [PubMed] [Google Scholar]
- 7.Laguna Goya R, Tyers P, Barker RA. The search for a curative cell therapy in Parkinson's disease. J Neurol Sci 2008;265:32–42. [DOI] [PubMed] [Google Scholar]
- 8.Kompoliti K, Chu Y, Shannon KM, Kordower JH. Neuropathological study 16 years after autologous adrenal medullary transplantation in a Parkinson's disease patient. Mov Disord 2007;22:1630–1633. [DOI] [PubMed] [Google Scholar]
- 9.Stover NP, Watts RL. Spheramine for treatment of Parkinson's disease. Neurotherapeutics 2008;5:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosset D, Grosset K. Spheramine Titan/Schering. Curr Opin Invest Drugs 2005;6:722–728. [PubMed] [Google Scholar]
- 11.Bakay RA, Raiser CD, Stover NP, et al. Implantation of Spheramine in advanced Parkinson's disease (PD). Front Biosci 2004;9:592–602. [DOI] [PubMed] [Google Scholar]
- 12.Watts RL, Raiser CD, Stover NP, et al. Stereotaxic intrastriatal implantation of human retinal pigment epithelial (hRPE) cells attached to gelatin microcarriers: a potential new cell therapy for Parkinson's disease. J Neural Transm Suppl 2003:215–227. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian T. Cell transplantation for the treatment of Parkinson's disease. Semin Neurol 2001;21:103–115. [DOI] [PubMed] [Google Scholar]
- 14.Marmor MF, Wolfensberger TJ, eds. The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. [Google Scholar]
- 15.Ming M, Le WD. Retinal pigment epithelial cells: biological property and application in Parkinson's disease. Chin Med J (Engl) 2007;120:416–420. [PubMed] [Google Scholar]
- 16.McKay BS, Goodman B, Falk T, Sherman SJ. Retinal pigment epithelial cell transplantation could provide trophic support in Parkinson's disease: results from an in vitro model system. Exp Neurol 2006;201:234–243. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Zhang J, Guo Z, et al. In-vivo PET imaging of implanted human retinal pigment epithelium cells in a Parkinson's disease rat model. Nucl Med Commun 2008;29:455–461. [DOI] [PubMed] [Google Scholar]
- 18.Stover NP, Bakay RA, Subramanian T, et al. Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease. Arch Neurol 2005;62:1833–1837. [DOI] [PubMed] [Google Scholar]
- 19.Stover NP, Watts RL, Freeman A, et al. Spheramine improves health-related quality of life in patients with moderate to advanced Parkinson's disease. International Congress of Parkinson's Disease and Movement Disorders, Vol. 23/Supplement 1, 2008. Chicago; 2008:131.
- 20.Bayer, Titan Pharmaceuticals. STEPS Trial: Spheramine Safety and Efficacy Study. In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine. Available at: http://clinicaltrials.gov/ct2/show/NCT00206687.
- 21.Luna LG, ed. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. Third ed. New York: McGraw-Hill; 1968. [Google Scholar]
- 22.Jellinger KA. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm 2004;111:1219–1235. [DOI] [PubMed] [Google Scholar]
- 23.Aarsland D, Perry R, Brown A, et al. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol 2005;58:773–776. [DOI] [PubMed] [Google Scholar]
- 24.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology 2006;67:1605–1611. [DOI] [PubMed] [Google Scholar]
- 25.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 2008;116:1–16. [DOI] [PubMed] [Google Scholar]
- 26.McKeith I. Dementia with Lewy bodies and Parkinson's disease with dementia: where two worlds collide. Pract Neurol 2007;7:374–382. [DOI] [PubMed] [Google Scholar]
- 27.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 2006;9:417–423. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian T, Marchionini D, Potter EM, Cornfeldt ML. Striatal xenotransplantation of human retinal pigment epithelial cells attached to microcarriers in hemiparkinsonian rats ameliorates behavioral deficits without provoking a host immune response. Cell Transplant 2002;11:207–214. [PubMed] [Google Scholar]
- 29.Flores J, Cepeda IL, Cornfeldt ML, et al. Characterization and survival of long-term implants of human retinal pigment epithelial cells attached to gelatin microcarriers in a model of Parkinson disease. J Neuropathol Exp Neurol 2007;66:585–596. [DOI] [PubMed] [Google Scholar]
- 30.Cepeda IL, Flores J, Cornfeldt ML, et al. Human retinal pigment epithelial cell implants ameliorate motor deficits in two rat models of Parkinson disease. J Neuropathol Exp Neurol 2007;66:576–584. [DOI] [PubMed] [Google Scholar]
- 31.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain 2005;128:1498–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kordower JH, Rosenstein JM, Collier TJ, et al. Functional fetal nigral grafts in a patient with Parkinson's disease: chemoanatomic, ultrastructural, and metabolic studies. J Comp Neurol 1996;370:203–230. [DOI] [PubMed] [Google Scholar]
- 33.Kordower JH, Cochran E, Penn RD, Goetz CG. Putative chromaffin cell survival and enhanced host-derived TH-fiber innervation following a functional adrenal medulla autograft for Parkinson's disease. Ann Neurol 1991;29:405–412. [DOI] [PubMed] [Google Scholar]
- 34.Bhidayasiri R, Miyata H, Bronstein JM, et al. Postmortem analysis of pallidotomy in Parkinson's disease. J Neurol 2005;252:100–102. [DOI] [PubMed] [Google Scholar]
- 35.Mendez I, Vinuela A, Astradsson A, et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med 2008;14:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kordower JH, Chu Y, Hauser RA, et al. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med 2008;14:504–506. [DOI] [PubMed] [Google Scholar]
- 37.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 2008;14:501–503. [DOI] [PubMed] [Google Scholar]
- 38.Bayer. Stockholders' Newsletter Second Quarter 2008 Management Report. Available at: http://www.stockholders-newsletter-q2-08.bayer.com.
- 39.Acton PD, Zhou R. Imaging reporter genes for cell tracking with PET and SPECT. Q J Nucl Med Mol Imaging 2005;49:349–360. [PubMed] [Google Scholar]
- 40.Ko IK, Song HT, Cho EJ, et al. In vivo MR imaging of tissue-engineered human mesenchymal stem cells transplanted to mouse: a preliminary study. Ann Biomed Eng 2007;35:101–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.