Abstract
Receptor-activated Ca2+ influx is mediated largely by store-operated channels (SOCs). TRPC channels mediate a significant portion of the receptor-activated Ca2+ influx. However, whether any of the TRPC channels function as a SOC remains controversial. Our understanding of the regulation of TRPC channels and their function as SOCs is being reshaped with the discovery of the role of STIM1 in the regulation of Ca2+ influx channels. The findings that STIM1 is an ER resident Ca2+ binding protein that regulates SOCs allow an expanded and molecular definition of SOCs. SOCs can be considered as channels that are regulated by STIM1 and require the clustering of STIM1 in response to depletion of the ER Ca2+ stores and its translocation towards the plasma membrane. TRPC1 and other TRPC channels fulfill these criteria. STIM1 binds to TRPC1, TRPC2, TRPC4 and TRPC5 but not to TRPC3, TRPC6 and TRPC7, and STIM1 regulates TRPC1 channel activity. Structure-function analysis reveals that the C-terminus of STIM1 contains the binding and gating function of STIM1. The ERM domain of STIM1 binds to TRPC channels and a lysine-rich region participates in the gating of SOCs and TRPC1. Knock-down of STIM1 by siRNA and prevention of its translocation to the plasma membrane inhibit the activity of native SOCs and TRPC1. These findings support the conclusion that TRPC1 is a SOC. Similar studies with other TRPC channels should further clarify their regulation by STIM1 and function as SOCs.
Introduction
The receptor-evoked Ca2+ signal entails Ca2+ release from the endoplasmic reticulum (ER) that is followed by activation of Ca2+ influx channels at the plasma membrane. Activation of Ca2+ influx in response to Ca2+ release from internal stores lead to the definition of these Ca2+ influx channels as store-operated channels or SOCs [1]. Ca2+ influx through SOCs is a key component of the receptor-evoked Ca2+ signal. SOCs-mediated Ca2+ influx controls numerous physiological functions on a time scale ranging from milliseconds to many hrs [2]. In the absence of Ca2+ influx the Ca2+-mediated effects terminate within few minutes due to exhaustion of the finite intracellular Ca2+ stores. The molecular identity of the SOCs and how they sense the Ca2+ content in the ER has been a mystery for many years. Several recent breakthroughs that are the focus of this special issue lead to major inroads in addressing these questions. The best characterized SOC is the channel mediating the Icrac current. Icrac is activated by stimulation of receptors that signal through an increase in IP3 and by passive depletion of ER Ca2+ [1, 3]. A long awaited finding recently identifies the Orai proteins as the pore forming channels that mediate Icrac [4–9]. This topic is covered by several reviews in this issue. Another group of channels that function as SOCs are the TRPC channels. However, which of the TRPC channels function as SOC is still controversial, with evidence for and against for almost each of the channels. The important discovery of STIM1 as the sensor of ER Ca2+ content that conveys it to the SOCs [10, 11] should greatly help in expanding the definition of SOCs and in clarifying which channel behaves as SOCs. Describing the regulation of TRPC1 channels by STIM1 and its relevance to the function of TRPC channels as SOCs is the aim of this short review.
TRPC channels as SOCs
TRPC channels are non-selective, Ca2+-permeable cation channels that are activated by stimulation of G proteins-coupled and Tyrosine phosphorylated receptors. There is evidence indicating that several TRPC channels function as SOCs. However, the majority of the evidence relies on deletion of specific TRPC isoforms or their silencing by antisense or siRNA, rather than demonstrating directly their function as SOCs. For example, HEK cells express native TRPC1, TRPC3, TRPC4, TRPC6 and TRPC7. Treatment with antisense to specifically inhibit transcription of the endogenous TRPC1 [12–15] and TRPC3 [12, 13] reduces Ca2+ influx when activated either by receptor stimulation or passive store depletion. Knockdown (KD) of the same channels by siRNA [16] also inhibits SOC activity. An important finding was that comparable inhibition of Ca2+ influx occurs by KD of several individual TRPC channels. KD of TRPC1, TRPC3, or TRPC7 by siRNA markedly inhibits SOCs activated by passive depletion of ER Ca2+, whereas suppression of TRPC4 or TRPC6 has no effect. Moreover, the concomitant KD of TRPC1+TRPC3, TRPC1+TRPC7, TRPC3+TRPC7, or TRPC1+TRPC3+TRPC7 reduces SOCs similar to the KD of TRPC1 alone [16]. Interestingly, the same study showed that agonist-stimulated Ca2+ influx was inhibited by suppression of any of the TRPC channels expressed in HEK cells [16]. The implication of these findings is that the complement of cellular TRPC channels is assembled into a single or limited number of complexes that form at least part of the SOCs.
Additional evidence for the function of TRPC channels as SOCs 1s provided by the finding that KD of TRPC1 reduces Isoc current in the smooth muscle cell line A7r5 cell [17] and reduces SOC activity in human keratinocytes [18]. KD of TRPC3 reduces SOC activity in rat prostate smooth muscle cells [19]. Disruption of the trpc4 gene in mice revealed that TRPC4 functions as SOC in endothelial cells [20, 21]. KD of TRPC4 reduces SOCs activity in mouse mesangial cells [22] and human corneal epithelial cells [23]. Disruption of the interaction between protein 4.1 and TRPC4 inhibits Isoc current in endothelial cells [24]. An interesting behavior was observed with TRPC7. When it is expressed at high levels, TRPC7 functions as a store-independent channel that is inhibited by high concentration of Gd3+. By contrast, at low expression level TRPC7 behaves as a SOC, its activity depends on the presence of IP3 receptors and it is highly sensitive to inhibition by Gd3+ [25–27]. Hence, the function of TRPC7 as SOCs depends on its expression level. TRPC3 function is also affected by its expression level. When it is expressed at low levels, it can be partially activated by passive store depletion [28]. However, at high expression levels TRPC3 behaves as store- and IP3 receptors-independent Ca2+ influx channel [29, 30]. Altered TRPC channels behavior based on their expression level may account for some of the variable results reported in the literature. The combined findings from the knockout and knockdown approaches indicate that TRPC channels form one type of SOCs and mediate a large fraction of the receptor-stimulated Ca2+ influx in many cell types and for many receptors.
For a long time, SOCs were equated only with Icrac. That the properties of native and topically expressed TRPC channels are different from those of Icrac forms the major objection in accepting the TRPC channels as SOCs. For example, an Icrac-like current that is highly selective for Ca2+ and is activated by passive store depletion can be found in several cell types. However, when TRPC3 is overexpressed in HEK 293 cells, it behaves as a non-selective monovalent cation permeable channel with a large conductance [28, 31, 32]. Additional uncertainty was raised by contradictory reports on the activation of TRPC3 by store depletion [28–30, 32]. The dependence of TRPC channels behavior on their expression level discussed above and the discovery of the Orais and STIM1 may provide an explanation to some of these observations. It is now clear that Orai1 [4–7, 9, 33–35], and perhaps Orai2 and Orai3 [34, 36] are the proteins that mediate Icrac. However, recently it was shown that Orai1 forms a complex with STIM1 and TRPC1 [37]. Of particular significance is the finding that KD of TRPC1 partially inhibited SOCs-mediated Ca2+ influx and Isoc, whereas KD of Orai1 completely inhibited Ca2+ influx and Isoc [37]. These findings are consistent with multiple forms of SOCs that are related to Orai1, TRPC channels and Icrac. Indeed, KD with siRNA and western blot show that both Orai1 and several TRPC channels are expressed in HEK cells and both channel types are regulated by STIM1 [9, 33–35, 38]. The properties of channel regulation by STIM1 lead to an expanded and a molecular definition of SOCs and allow further examination of the SOC properties of TRPC channels.
Expanded and molecular definition of SOCs
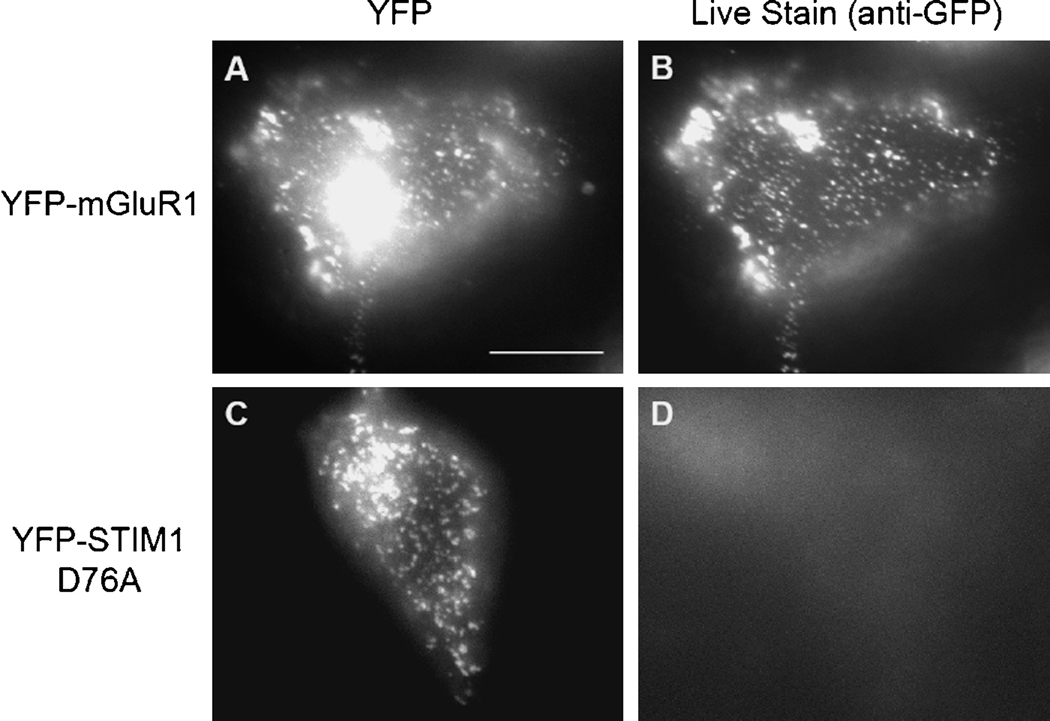
The classical definition of SOCs is channels that are similarly activated by agonist-mediated and agonist-independent Ca2+ release from the ER [1]. The discovery of STIM1 and its mode of action allow a more general and molecular definition of the SOCs. The most fundamental property of STIM1 is that it is a Ca2+ binding protein that migrates within the ER membrane to regions that are very close to the plasma membrane and reorganizes in punctae that associate with Ca2+ influx channels and activate them [10, 11, 34, 38–42]. This is also illustrated in Fig. 1, which shows a Total Internal Fluorescence (TIRP) images of cells expressing YFP-mGluR1 (A, B) or YFP-(D76A)STIM1 (C, D) and stained with anti-GFP. In the case of YFP-mGluR1 the intrinsic (A) and surface fluorescence (B) are similar, indicating the insertion of YFP-mGluR1 into the plasma membrane. By contrast, the punctate intrinsic fluorescence of the constitutively active YFP-(D76A)STIM1 is not observed on the cell surface, indicating the (D76A)STIM1 is not inserted into the plasma membrane, although it activates TRPC1. Since depletion of Ca2+ from the ER is obligatory for the translocation of STIM1 and for the activation of SOCs and Icrac [11, 38, 39, 41, 42], an expanded and molecular definition of SOCs became possible. SOCs are channels that are regulated by STIM1 and require the translocation of STIM1 from the ER towards the plasma membrane to form punctae in response to depletion of ER Ca2+. We consider all channels that fulfill these criteria to be SOCs. These criteria are sufficient to exclude STIM1-regulated channels that are not SOCs. For example, the arachidonate-regulated Ca2+-selective channel (ARC) is regulated by STIM1 [43]. However, this channel is regulated by plasma membrane resident STIM1 and does not require translocation of STIM1 from the ER to the plasma membrane [43].
Fig. 1.
Majority of the YFP-STIM1 D76A punctae are not on the plasma membrane. HEK293 cells were transfected with YFPmGluR1 together with Homer1 and Shank3 to get the YFP-mGluR1 to the plasma membrane (A, B) or YFP-STIM1 D76A (C, D). A and C are intrinsic YFP signals. B and D are images of surface live staining with an anti- GFP rabbit polyclonal antibody. The intense signal in A is from Golgi. Scale bar, 10 µm.
The STIM1 domains and their potential functions
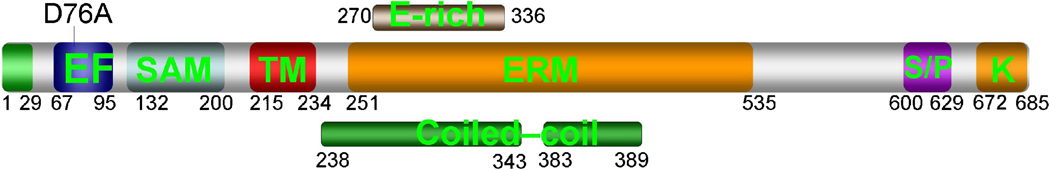
STIM1 is a Ca2+ binding protein with several functional domains (Fig. 2).
Fig. 2.
STIM1 and its domains
STIM1 was originally identified as a tumor suppressor [44] that affects proliferation of B-cells [45] and several tumor cell lines [44, 46]. STIM1 asumed center stage in Ca2+ signaling research when two independent screens for regulators of Ca2+ influx channels, one in drosophila S2 cells [10] and one in HEK cells [11], identified STIM1 as a regulator of Ca2+ influx channels. Domain analysis revealed that STIM1 has an N-terminal EF hand Ca2+-binding domain, a sterile α motif (SAM) domain, a single transmembrane domain (TM), an ezrin–radixin–moesin (ERM) domain, a serine-proline-rich region (S/Pregion) and a lysine-rich region. Part of the ERM domain is predicted to have two coiledcoil domains and to contain a glutamate-rich region (E-region). The orientation of STIM1 places the EF hand and the SAM domains in the ER lumen when STIM1 is in the ER and in the extracellular space when STIM1 is at the plasma membrane.
Recent structure-function studies revealed some of the role of these domains. The Ca2+ binding EF hand and SAM domains regulate aggregation of STIM1 and its translocation from the ER to the plasma membrane [42, 47]. Thus, Ca2+ release from the ER and mutations in the Ca2+ binding pocket of the EF hand to prevent Ca2+ binding results in translocation of STIM1 towards the plasma membrane, formation of punctae and activation of SOCs [10, 11, 38, 40–42, 48]. Accordingly, the EF hand resident domain has relatively low Kd for Ca2+ of about 0.2–0.6 mM [47]. When bound with Ca2+ the EF hand and SAM domains exist as monomers, whereas the Ca2+ free domains form multimeric aggregates [47]. In addition, the SAM domain is required for coalescing STIM1 into punctae upon Ca2+ release from the ER [42]. Interestingly, the C terminal domain of STIM1 was sufficient for activation of SOCs, although it was not as active as full length STIM1 [38]. This would suggest that the translocation of STIM1 from the ER towards the plasma membrane and gating of SOCs by STIM1 are mediated by two different sectors of STIM1.
Based on the effect of deletion of the SAM together with the coiled-coil domains and of the S/P-region Baba et al concluded that these domains are required for activation of SOCs by STIM1 [42]. However, we reported that deletion of the E-region that forms part of the first coiled-coil domain and of the S/P-region failed to prevent activation of SOCs by the C-terminal half of STIM1 [38]. There are at least two explanations for the contradictory findings. First, we and Baba et al deleted different sequences. For deletion of part or the entire coiled-coil domain we deleted amino acids 270–336 and Baba et al deleted amino acids 249–390. It is possible that different deletions results in different effects. However, it is more likely that the different results are due to the different assays used to evaluate SOC activity. Baba et al used direct measurement of Ca2+ influx [42], whereas we used the NFAT nuclear translocation assay that requires long incubation of the cells with the constructs [38]. If the deletion constructs of the constitutively active C-terminus STIM1 retain even small activity it would be sufficient to drive NFAT into the nucleus, resulting in an apparent lack of effect of the deletion. Measurement of Ca2+ influx is more likely to reflect the function of a particular domain. Therefore, it is likely that the coiled-coil domain and S/P-region are required for activation of SOCs and perhaps TRPC channels by STIM1.
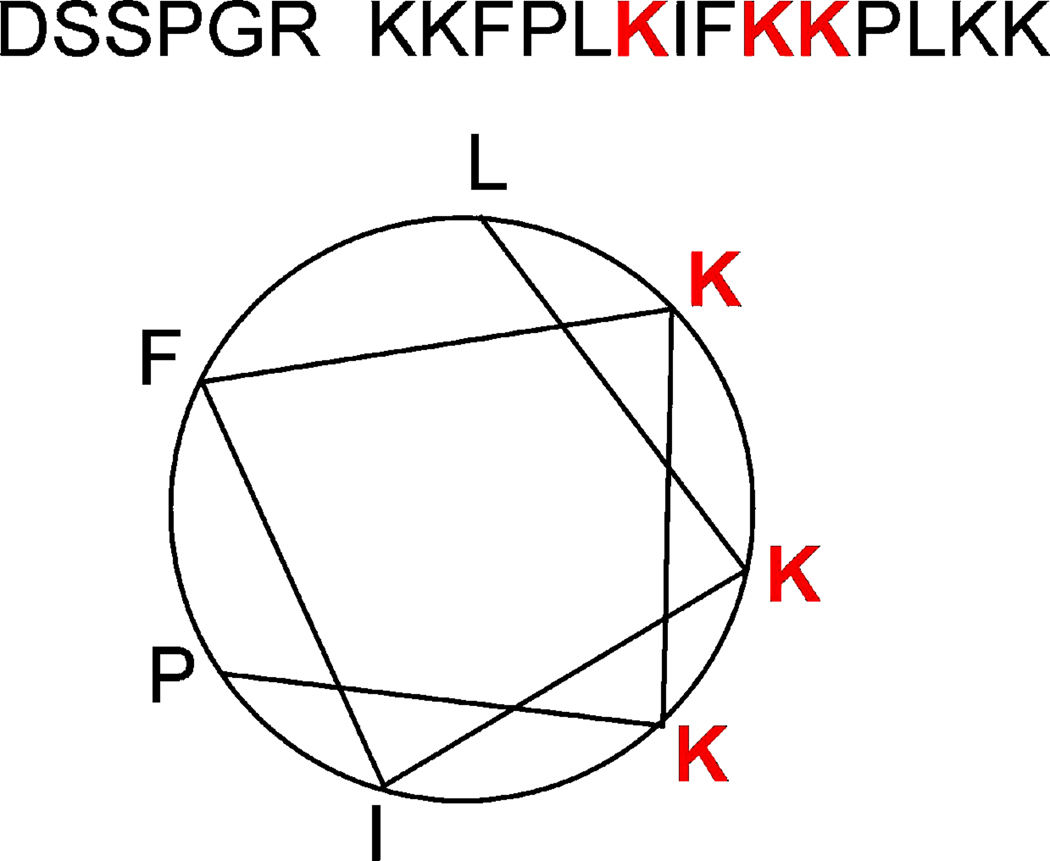
Another important domain is the lysine-rich region at the C terminal tail of STIM1. Fig. 3 shows that the 14 amino acids of the lysine-rich region are predicted to form a two-turn helix with the three terminal lysines on one surface. Deletion of this lysine-rich region prevents gating of the native SOCs and TRPC1 by STIM1 [38]. The potential role of the lysine-rich region in the function of STIM1 is discussed further below in relation to activation of TRPC channels by STIM1.
Fig. 3.
The predicted two-turn helix of the C-terminal tail of STIM1.
Regulation of TRPC channels by STIM1
The participation of TRPC channels in receptor-mediated Ca2+ influx and SOCs prompted examination of whether they interact and are regulated by STIM1. We have examined the interaction of all mammalian TRPC channels with topically expressed STIM1 and found that STIM1 binds TRPC1, TRPC2, TRPC4 and TRPC5, but not TRPC3, TRPC6 and TRPC7 [38]. In addition, it was shown that STIM1 binds to the native TRPC1 in platelets [49] and is present in a complex with TRPC1-STIM1-Orai1 [37].
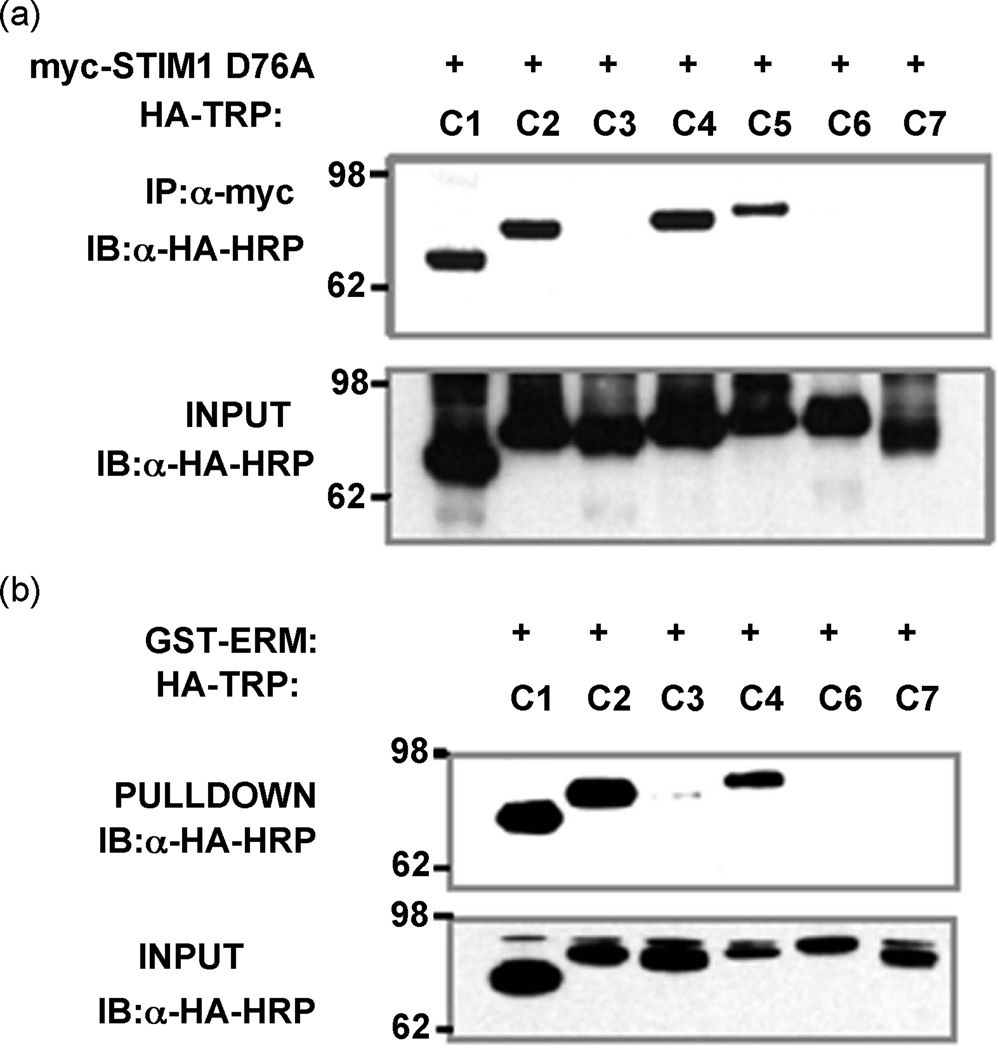
This is illustrated in Fig. 4a, which shows that TRPC channels interact with STIM1. Deletion analysis revealed that the STIM1-ERM domain mediates the binding of STIM1 to the TRPC channels. Fig. 4b and [38] show that the STIM1-ERM domain binds TRPC1, TRPC2 and TRPC4, but not TRPC3, TRPC6 and TRPC7, similar to the findings with the full-length STIM1, suggesting that the STIM1-ERM domain binds to a sequence or structural motif that is similar in TRPC1, TRPC2, TRPC4 and TRPC5.
Fig. 4. Interaction of STIM1 with TRPC channels.
Panel (a) shows to co-IP of STIM1 and the indicated TRPC channels expressed in HEK cells. Panel (b) shows the pull-down of the indicated TRPC channels by GST-ERM domain of STIM1.
Regulation of TRPC channels activity by STIM1 has been demonstrated only for TRPC1 so far [38, 49]. KD of STIM1 or over-expression of the dominant negative ΔERM-STIM1( D76A) inhibit TRPC1 channel activity. Expression of the EF hand mutant (D76A)STIM1, that constitutively assembles into punctae at near plasma membrane domains, activates TRPC1 and increases its spontaneous activity [38]. A critical question in the mechanism by which STIM1 regulates SOCs is whether STIM1 is a channel subunit. This question is highlighted in the finding that STIM1 is obligatory for the function of Orai1 [9, 33–35, 42] and Orai2 [34] as Icrac channels. Hence, expression of Orai1 alone does not result in Icrac current and suppresses the native SOC activity, whereas co-expression of Orai1 and STIM1 results in a large Icrac current. STIM1 does not play a similar permissive role with TRPC channels. Thus, expression of high levels of WT-STIM1 and the constitutively active (D76A)STIM1 only marginally increase or have no effect of TRPC1 current density [38]. That STIM1 is unlikely to function as a channel subunit is further suggested by the finding that the cytoplasmic C terminal domain of STIM1 (CT-STIM1), which lacks the transmembrane domain, is sufficient to activate TRPC1 even when native STIM1 was effectively KD [38].
An interesting finding is that deletion of part of the first coiled-coil domain that includes the glutamate-rich region and deletion of the S/P-rich region of the CT-STIM1 do not prevent binding of CT-STIM1 to TRPC1 [38]. However, similar deletions inhibits activation of SOCs by STIM1 [42]. Moreover, deletion of these domains impairs the translocation of STIM1 towards the plasma membrane [42], but they are not required for activation of TRPC1 by CT-STIM1 [38]. The combined findings suggest that the first coiled-coil domain and the S/P-rich region do not participate in the gating of the SOCs. Rather, they mediate the translocation of STIM1 from the ER towards the plasma membrane in response to depletion of ER Ca2+.
A region of STIM1 that is important in gating of SOCs is the lysine-rich domain. Deletion of the lysine-rich region (ΔK-STIM1) or K/E and K/A substitution of all lysines in this region inhibits the ability of STIM1 to activate TRPC1 and the native SOCs [38]. Importantly, the ΔK-STIM1 and the K/E mutants act as dominant negative constructs and inhibit the function of the native STIM1. On the other hand, the lysine-rich region is not required for binding of STIM1 to TRPC1 [38]. Together, these findings implicate the lysine-rich region of STIM1 in gating of TRPC channels and other SOCs. How the lysine-rich region participates in the action of STIM1 to gate the channels remains to be elucidated.
Although it has not been examined thoroughly, it will be important to test whether other TRPC channels are regulated by STIM1 and to examine their mode of regulation by STIM1. Since TRPC2, TRPC4 and TRPC5 bind to the ERM domain of STIM1, it is expected that STIM1 does regulate the activity of these channels, and the regulation is in a mechanism analogous to that of the regulation of TRPC1 by STIM1 [38]. Surprisingly, a recent study reported that a combined KD of TRPC1, 3, 4, 5, 6 channels in HEK cells did not affect agonist-stimulated Ca2+ influx [50]. However, the actual KD of the TRPCs and the extent of the KD were not examined in this work. The lack of binding of STIM1 to TRPC3, TRPC6 and TRPC7 predicts that STIM1 does not directly regulate these channels. Rescue of Ca2+ oscillations by TRPC3 in HEK cells in which STIM1 was KD was taken to suggest that TRPC3 is not regulated by STIM1 [50]. However, since cells express multiple TRPC channels [16, 22, 23, 51], and several TRPC channels interact with each other or are present in the same Ca2+ signaling complexes [44–46, 52–58], it is necessary to examine the role of STIM1 in the activity of each of the TRPC channels before it is known which of these channels are regulated by STIM1 and how STIM1 gates each of the channels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 4.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 5.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 6.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. 10 STIM1, an essential and conserved component of store-operated Ca2+channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Babnigg G, Villereal ML. Functional significance of human trp1 and trp3 in store-operated Ca(2+) entry in HEK-293 cells. Am J Physiol Cell Physiol. 2000;278:C526–C536. doi: 10.1152/ajpcell.2000.278.3.C526. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Zagranichnaya TK, Gurda GT, Eves EM, Villereal ML. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J Biol Chem. 2004;279:43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- 14.Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaca L, Sampieri A. Calmodulin modulates the delay period between release of calcium from internal stores and activation of calcium influx via endogenous TRP1 channels. J Biol Chem. 2002;277:42178–42187. doi: 10.1074/jbc.M204531200. [DOI] [PubMed] [Google Scholar]
- 16.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 17.Brueggemann LI, Markun DR, Barakat JA, Chen H, Byron KL. Evidence against reciprocal regulation of Ca2+ entry by vasopressin in A7r5 rat aortic smooth-muscle cells. Biochem J. 2005;388:237–244. doi: 10.1042/BJ20041360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu CL, Chang W, Bikle DD. Phospholipase cgamma1 is required for activation of store-operated channels in human keratinocytes. J Invest Dermatol. 2005;124:187–197. doi: 10.1111/j.0022-202X.2004.23544.x. [DOI] [PubMed] [Google Scholar]
- 19.Thebault S, Zholos A, Enfissi A, Slomianny C, Dewailly E, Roudbaraki M, Parys J, Prevarskaya N. Receptor-operated Ca2+ entry mediated by TRPC3/TRPC6 proteins in rat prostate smooth muscle (PS1) cell line. J Cell Physiol. 2005;204:320–328. doi: 10.1002/jcp.20301. [DOI] [PubMed] [Google Scholar]
- 20.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- 21.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol. 2004;287:C357–C364. doi: 10.1152/ajpcell.00068.2004. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Mergler S, Sun X, Wang Z, Lu L, Bonanno JA, Pleyer U, Reinach PS. TRPC4 knockdown suppresses epidermal growth factor-induced store-operated channel activation and growth in human corneal epithelial cells. J Biol Chem. 2005;280:32230–32237. doi: 10.1074/jbc.M504553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res. 2005;97:1164–1172. doi: 10.1161/01.RES.0000193597.65217.00. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez G, Bird GS, Mori Y, Putney JW., Jr Native TRPC7 channel activation by an inositol trisphosphate receptor-dependent mechanism. J Biol Chem. 2006;281:25250–25258. doi: 10.1074/jbc.M604994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lievremont JP, Bird GS, Putney JW., Jr Canonical transient receptor potential TRPC7 can function as both a receptor- and store-operated channel in HEK-293 cells. Am J Physiol Cell Physiol. 2004;287:C1709–C1716. doi: 10.1152/ajpcell.00350.2004. [DOI] [PubMed] [Google Scholar]
- 27.Lievremont JP, Numaga T, Vazquez G, Lemonnier L, Hara Y, Mori E, Trebak M, Moss SE, Bird GS, Mori Y, Putney JW., Jr The role of canonical transient receptor potential 7 in B-cell receptor-activated channels. J Biol Chem. 2005;280:35346–35351. doi: 10.1074/jbc.M507606200. [DOI] [PubMed] [Google Scholar]
- 28.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez G, Wedel BJ, Trebak M, St John Bird G, Putney JW., Jr Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J Biol Chem. 2003;278:21649–21654. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez G, Lievremont JP, St JBG, Putney JW., Jr Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc Natl Acad Sci U S A. 2001;98:11777–11782. doi: 10.1073/pnas.201238198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurst RS, Zhu X, Boulay G, Birnbaumer L, Stefani E. Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Lett. 1998;422:333–338. doi: 10.1016/s0014-5793(98)00035-0. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 33.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 34.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-Hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai family proteins. J Biol Chem. 2007 doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 37.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J Biol Chem. 2007 doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 39.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store-depletion or translocation to the plasma membrane. J Physiol. 2006 doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: a gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res. 1997;57:4493–4497. [PubMed] [Google Scholar]
- 45.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabbioni S, Veronese A, Trubia M, Taramelli R, Barbanti-Brodano G, Croce CM, Negrini M. Exon structure and promoter identification of STIM1 (alias GOK), a human gene causing growth arrest of the human tumor cell lines G401 and RD. Cytogenet Cell Genet. 1999;86:214–218. doi: 10.1159/000015341. [DOI] [PubMed] [Google Scholar]
- 47.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+Depletion-induced Oligomerization of Stromal Interaction Molecule 1 (STIM1) via the EF-SAM Region: AN INITIATION MECHANISM FOR CAPACITIVE Ca2+ ENTRY. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 48.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 50.Wedel B, Boyles RR, Putney JW, Bird GS. Role of the Store-operated Calcium Entry Proteins, Stim1 and Orai1, in Muscarinic-Cholinergic Receptor Stimulated Calcium Oscillations in Human Embryonic Kidney Cells. J Physiol. 2007 doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, Creighton JR, Townsley M, Goodman SR, Stevens T. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res. 2005;96:856–863. doi: 10.1161/01.RES.0000163632.67282.1f. [DOI] [PubMed] [Google Scholar]
- 52.Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP(3) pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- 55.Scelfo R, Sabbioni S, Barbanti-Brodano G, Negrini M. Subchromosomal assignment1 of the TSSC1 gene to human chromosome band 11p15.5 near the HBB gene cluster. Cytogenet Cell Genet. 1998;83:52–53. doi: 10.1159/000015123. [DOI] [PubMed] [Google Scholar]
- 56.Schilling WP, Goel M. Mammalian TRPC channel subunit assembly. Novartis Found Symp. 2004;258:18–30. discussion 30–43, 98–102, 263–266. [PubMed] [Google Scholar]
- 57.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- 58.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]