Abstract
OBJECTIVES
To determine whether fragmented sleep in nursing home patients would improve with increased exposure to bright light.
DESIGN
Randomized controlled trial.
SETTING
Two San Diego–area nursing homes.
PARTICIPANTS
Seventy-seven (58 women, 19 men) nursing home residents participated. Mean age ± standard deviation was 85.7 ± 7.3 (range 60–100) and mean Mini-Mental State Examination was 12.8 ± 8.8 (range 0–30).
INTERVENTIONS
Participants were assigned to one of four treatments: evening bright light, morning bright light, daytime sleep restriction, or evening dim red light.
MEASUREMENTS
Improvement in nighttime sleep quality, daytime alertness, and circadian activity rhythm parameters.
RESULTS
There were no improvements in nighttime sleep or daytime alertness in any of the treatment groups. Morning bright light delayed the peak of the activity rhythm (acrophase) and increased the mean activity level (mesor). In addition, subjects in the morning bright light group had improved activity rhythmicity during the 10 days of treatment.
CONCLUSION
Increasing exposure to morning bright light delayed the acrophase of the activity rhythm and made the circadian rhythm more robust. These changes have the potential to be clinically beneficial because it may be easier to provide nursing care to patients whose circadian activity patterns are more socially acceptable.
Keywords: dementia, sleep, light, circadian rhythms, nursing home, older
Wandering at night, agitation, disturbed sleep, and night-day reversal are common problems in those with dementia. Patients who wander around at night or try to leave the house in the middle of the night present a major problem for caregivers; in addition, many of these patients spend their days sleeping.1 Families often manage these problems by having patients institutionalized.2,3 Learning more about these sleep disturbances and examining techniques to improve these symptoms might postpone, if not avoid, institutionalization. This has the potential to save billions of healthcare dollars annually,4 decrease caregiver stress, and provide opportunities for those with dementia to live at home for longer periods of time.
The disturbed sleep seen in nursing home residents may be due to changes in circadian rhythms. Human circadian rhythms are biological cycles of about 24 hours that include sleep/wake, body temperature, and melatonin secretion cycles. Circadian rhythms are controlled by the supra-chiasmatic nucleus (SCN) of the hypothalamus. In normal aging and in Alzheimer's disease (AD), the SCN deteriorates, contributing to alterations in circadian rhythms.5–9
A second reason for sleep disturbances in this population may be decreased exposure to bright light. Bright light (≥2,000 lux) appears to be one of the most powerful synchronizers of circadian rhythms and directly influences secretion of melatonin, sleep/wake patterns, and other circadian rhythms.10 Young adults are, on average, exposed to approximately 58 minutes of bright light a day (≥2,000 lux),11 healthy older people to 60 minutes a day,12 and AD patients living at home to 30 minutes a day.12 Our early research showed that nursing home residents were exposed to 11 to 19 minutes of light brighter than 1,000 lux and that none were exposed to light above 2,000 lux.13 More recently, Shochat et al.14 reported that nursing home residents were exposed to a median of 10 minutes of light over 1,000 lux per day during baseline, and that lower illumination levels were related to subsequent nighttime sleep fragmentation and to more phase-advanced circadian activity rhythms. In patients with dementia specifically, the median time of bright light exposure was only 1 minute, and 47% were never exposed to more than 1,000 lux.1 Bliwise et al.15 found similar results. With decreased light exposure, changes seem to occur in sleep and activity rhythms.
Bright light can be used to change the timing of circadian rhythms (the circadian phase), and new research suggests that bright light exposures at certain times may increase the amplitude of circadian rhythms without necessarily affecting the phase of the rhythm.16 These data are complemented by evidence that evening bright light can augment melatonin secretion later in the night, thus increasing the amplitude of the melatonin circadian rhythm.17
Studies conducted in the field and under controlled conditions,9,18–21 although not all laboratory studies,21 have shown that circadian rhythms in older adults are phase advanced, that is, that the rhythms are shifted to an abnormally early time, resulting in their falling asleep and waking up earlier than what is socially appropriate. Others have reported that some AD patients have phase-delayed activity22 compared with nondemented controls, that is, rhythms are shifted to an abnormally late time. Evening bright light has been shown to delay circadian rhythms, whereas early morning light has been shown to advance circadian rhythms. As a result, advanced rhythms, such as those seen in older adults, might be beneficially delayed with exposure to evening light, whereas a phase delay may be beneficially advanced with exposure to morning light.23
Several groups of investigators have studied the effect of bright light on sleep in demented people, but no clear-cut conclusions can yet be drawn. Many of the studies had small sample sizes, used subjective ratings of sleep and behavior as outcome measures, and did not include control conditions. Also, different light levels were administered in each study, at different times of the day, and for varying amounts of time.24–33
Using objective data in a larger sample of nursing home residents, this study examined the effect of light treatment for sleep fragmentation and circadian rhythm abnormalities, with the assumption that the fragmented nighttime sleep seen in nursing home residents may be the result of impaired circadian activity rhythms and that the impaired rhythm may be the result of low light exposure. This model is compatible with other models of circadian sleep regulation.34–36 It was hypothesized that increasing daily illumination exposure would improve nighttime sleep quality and amplify and resynchronize circadian rhythms.
METHODS
Subjects
Medical records of 268 patients were reviewed. Of these, 89 (33%) were inappropriate for study (bed-bound, deemed too ill by nursing or research staff, having severe visual impairments, or unable to communicate), 61 (23%) refused consent or families did not want their parent “bothered” or their schedule disrupted, and 118 (44%) gave consent for participation. Twenty-five of the 118 (21%) withdrew during the initial data collection before randomization. Five additional patients (4%) refused treatment and were dropped from the study. Eleven patients (9%) dropped out because of health reasons (died before beginning study, strokes) or because the family or the patient did not wish to continue.
Seventy-seven patients (65% of the original 118 consenting; 58 women, 19 men), completed the protocol. Of these 77, 31% were able to give their own informed consent (i.e., were competent); for the remaining 69%, a guardian's signature and the patient's verbal assent were obtained. Each patient's physician also gave verbal consent. The University of California at San Diego Committee on the Investigation of Human Subjects approved this study.
Data for this study were collected at two private San Diego–area nursing homes. Fifty-five patients lived on a skilled nursing wing, 18 lived on an intermediate care wing, and four lived on an assisted-living wing. The mean age ± standard deviation (SD) of patients was 85.7 ± 7.3 (range 60–100). Eighty percent of patients were not depressed according to the Geriatric Depression Scale (GDS)37 (scored <15; mean ± SD = 10.6 ± 5.9, range 1–27). The mean score on the Mini-Mental State Examination (MMSE)38 was 12.8 ± 8.8 (range 0–30); 71% had a MMSE score less than 20. No ophthalmic examinations were performed. Data were lost on five patients; results presented are on the remaining 72 complete data sets.
Apparatus
Sleep/wake activity was measured with an Actillume recorder (Ambulatory Monitoring, Inc., Ardsley, NY). The Actillume is a small device worn on the wrist. It contains a piezoelectric linear accelerometer, a microprocessor, 32K-byte random access memory, and associated circuitry for the purpose of recording intensity and frequency of movement. This results in two variables, the sum (average of all activity movements per minute) and the maximum activity (the largest or maximum movement per minute). The device is fully described elsewhere.39 The Actillume also contains a log-linear photometric transducer, and the illumination measurements are approximately log-linear from a range below full moonlight to the brightest summer day at noon. The Action3 software package (Ambulatory Monitoring, Inc.) was used to score the sleep/wake based on sum and maximum activity and to compute bright light exposure.
Determination of sleep/wake inferred from wrist activity has been previously validated,40 including in patients with dementia.39 The correlation for total sleep time between the criterion standard of brain waves (electroencephalogram (EEG)) and Actillume (based on sum activity) was .91 (P < .001) in patients with dementia.39 Waking EEG records in patients with dementia show much diffuse slow activity, similar to that seen in sleep, which makes it difficult to differentiate sleep from wake.41 The Actillume therefore, is a more viable, reliable alternative for studying wake and sleep in this population.
Light was administered via a Brite-Lite box (Apollo Light System, Orem, UT). The Brite-Lite uses cool-white fluorescent bulbs with a special ballast and reflectors to augment its brightness. Boxes were placed 1 meter from a patient's head within a 45 visual field, often on top of the television. Patients could read, eat, converse, or watch television during light treatment sessions.
Procedure
Each patient first wore the Actillume for one 24-hour period to determine the extent of sleep fragmentation and the extent of daytime sleepiness. A Sleep Spread Score (SSS) was computed, based on the variance of the distribution of sleep over time, divided into quartiles. Block-stratified randomization using preassignment by order of entry was used. Stratification was by gender and by quartiles of the categorical SSS distribution. This information was used to assign patients randomly to one of four treatments: evening bright light, morning bright light, evening dim red light, and daytime sleep restriction (DSR).
Patients in the evening bright light group were exposed to 2,500 lux from 5:30 p.m. to 7:30 p.m. Although this is earlier than traditionally suggested for phase delaying circadian rhythms,42,43 this 2-hour period represented the time immediately before bedtime in this sample. The goals of the evening bright light were to increase total daily illumination exposure and to delay the circadian rhythm, which was hypothesized to be abnormally phase advanced. Patients in the morning bright light group were exposed to 2,500 lux from 9:30 a.m. to 11:30 p.m. The goals of the morning light were to increase total daily illumination exposure and to increase the synchronization of circadian rhythms without necessarily shifting the phase. Patients in the dim light group were exposed to less than 50 lux red light from 5:30 p.m. to 7:30 p.m. This was to serve as a control condition for the bright light groups. A staff member sat with the patients during each 2-hour light treatment period to ensure that the patients remained awake and did not wander away from the light box.
For patients in the DSR group, one staff member accompanied each patient for 6 hours during the day, 9:00 a.m. to noon and after lunch from 2:00 p.m. to 5:00 p.m. The task of the staff members was to ensure that patients did not doze off or fall asleep during this time, thereby restricting sleep during the day.
Each protocol lasted 18 days. The Actillume was worn for the entire protocol, except for brief removal for showers and for downloading and reinitializing at the end of the 3-day baseline period and after each 5 days of treatment.
During the first 3 days, baseline Actillume measures were taken, and the GDS and MMSE were administered. Medical charts were abstracted and medications noted. Treatment began on Day 4 and lasted for 10 days. Posttreatment follow-up data were collected for 5 additional days.
Data Analyses
Time to bed at night and time out of bed in the morning were not recorded, because research staff were not always present to note the time. Because sleep was so disrupted, it was not possible to tell bedtime and uptime from Actillume data. Actillume recordings were therefore divided into “day intervals” (defined as 7:00 a.m. to 7:00 p.m.) and “night intervals” (defined as 7:00 p.m. to 7:00 a.m.) for analysis of sleep/wake measures. Because this period may have included some time out of bed in the morning, analyses were also computed based on night being defined as 10:00 p.m. to 6:00 a.m., an interval when almost all the patients were likely to be in bed. Analyses were conducted in parallel for both sum and maximum activity.
For the purpose of estimating circadian activity rhythms, the mean activity level per minute was used. Periodic functions for activity, based on a five-parameter extension of the traditional cosinor analysis for circadian rhythms, were estimated separately for each subject in each condition of the experiment using the logarithms of the raw data. This five-parameter extension model has been described in detail elsewhere.44 In brief, data sets that are most rhythmic or have the strongest rhythms will yield the largest model F statistics and the smallest standard errors.
A subject's data were included in the circadian rhythm analyses only if the F test for the pretreatment activity rhythm showed a statistically significant rhythm (F > 3.41). The decision to exclude subjects from analysis if their activity patterns lacked a circadian rhythm was made before other analyses and was based on the idea that a lack of circadian rhythm in activity results from extreme neurodegeneration.45 Nine patients were excluded because of lack of circadian activity rhythms at baseline.
Parametric analyses of the parameter estimates and their normal transformations were performed. Treatment effects were assessed by computing changes from pretreatment to the second treatment week (possible improvement) and from the second treatment week to the posttreatment week (possible relapse). Between-treatment comparisons of the effectiveness of the treatments were based on these change scores, along with repeated measures analysis of variance accompanied by preplanned comparisons. Analyses of the group parameter estimate variances revealed that the variances were not statistically different across groups; therefore, the between-treatment comparisons of the normal-transforms of the parameter changes were used to assess statistical significance. Although a large number of analyses were performed, they were all based on a priori hypotheses originally outlined in our grant proposal to conduct this study. All statistical tests with P-values < .05 are reported as statistically significant; every test with P-values between .05 and .10 is reported as a trend. All computations and graphing were performed using SAS.46,47
RESULTS
Compliance with Actillume Recorders
Fifty-one patients (66%) never removed their Actillume during the 18-day protocol. Twenty patients (26%) removed the Actillume only one to three times for brief periods during the 18 days. Only six patients (8%) removed the Actillume between four and six times in 18 days. Because our staff spent several hours each day with the patients, and because the nursing home staff were trained to be aware of the Actillume, little Actillume data were lost.
Compliance with Treatments
Patients stayed in front of the light box for an average of 107.8 minutes per 120-minute treatment session and were awake for 86% of that time. This resulted in patients receiving an average of 92 minutes of treatment (median 102 minutes) per 120-minute treatment session. There were no important differences in compliance across light treatment conditions.
For patients in the DSR group, the mean percent time awake increased from 65% during baseline to 68% during treatment (P < .074), indicating that the treatment was successful in keeping subjects awake more of the time. Although a 3% increase may not seem like much, this translated into a mean increase of 22 minutes.
Nighttime Sleep
There were no significant changes in sleep or activity level at night in any of the treatment groups.
Daytime Alertness
There were no significant changes in sleep or activity level during the day in any of the treatment groups.
Circadian Rhythms
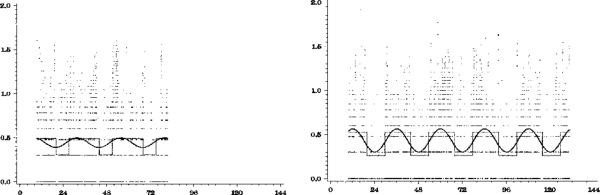
Sample graphs of the activity data from one patient are presented in Figure 1.
Figure 1.
Raw data and model fit for one subject before and during morning light therapy. Raw data are plotted as small dots, the traditional cosine curve is plotted as a wide, smooth line, and the extended model is plotted as a thin, dotted line. The pretreatment activity data (left-hand panel) seem to have no rhythm. The peaks of the curve clearly do not match the peaks in the data. The activity data in the treatment phase (right-hand panel) are clearly more rhythmic. The extended cosine model is clearly a better representation of the data than the cosine model. For this subject, activity rhythmicity improved after treatment, and the activity acrophase estimate shifted to a later time after treatment.
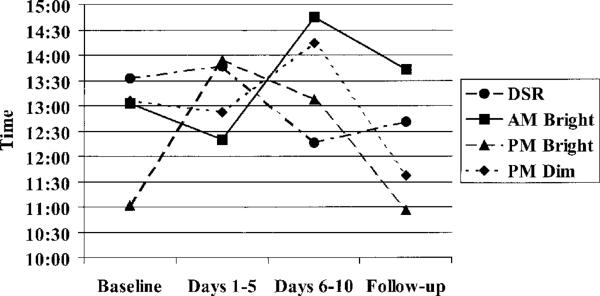
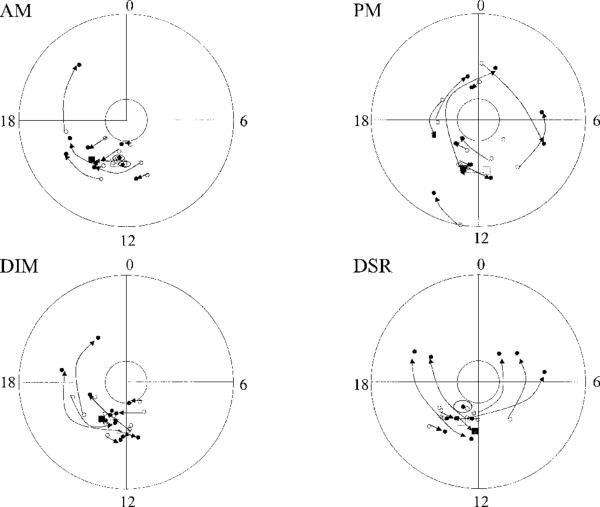
In the morning light group, there was a significant 1 hour 46 minute delay (SD = 1.36) in the activity rhythm acrophase (P = .022) and a significant increase in the mesor (P = .027) from baseline to treatment Week 2 (see Table 1 and Figure 2). There were no other significant changes in activity rhythms. Although evening bright light delayed the rhythm, the change was not significant, likely because of the large variability. In the morning light group, acrophase was delayed in every individual (see Figure 3).
Table 1.
Circadian Activity Rhythm Measures for Each Treatment Group by Week of Study
| Treatment Group | Pretreatment | Treatment Week 1 | Treatment Week 2 | Posttreatment |
|---|---|---|---|---|
| Daytime sleep restriction | ||||
| n | 12 | 10 | 12 | 11 |
| Amplitude, mean ± SD | 0.359 ± 0.192 | 0.416 ± 0.299 | 0.309 ± 0.185 | 0.511 ± 0.265 |
| Mesor, mean ± SD | 0.690 ± 0.172 | 0.713 ± 0.174 | 0.790 ± 0.206 | 0.732 ± 0.230 |
| Acrophase, mean ± SD | 1332 hr ± 1:52 | 1347 hr ± 5:29 | 1216 hr ± 5:40 | 1241 hr ± 2:58 |
| Morning bright | ||||
| n | 10 | 10 | 10 | 9 |
| Amplitude, mean ± SD | 0.393 ± 0.173 | 0.328 ± 0.127 | 0.341 ± 0.140 | 0.333 ± 0.149 |
| Mesor, mean ± SD | 0.728 ± 0.212 | 0.768 ± 0.173 | 0.844 ± 0.232* | 0.779 ± 0.222 |
| Acrophase, mean ± SD | 1300 hr ± 2:04 | 1214 hr ± 3:03 | 1445 hr ± 2:54† | 1354 hr ± 2:39 |
| Evening bright | ||||
| n | 11 | 9 | 11 | 11 |
| Amplitude, mean ± SD | 0.358 ± 0.270 | 0.284 ± 0.130 | 0.401 ± 0.384 | 0.462 ± 0.816 |
| Mesor, mean ± SD | 0.829 ± 0.326 | 0.678 ± 0.192 | 0.822 ± 0.290 | 0.779 ± 0.356 |
| Acrophase, mean ± SD | 1116 hr ± 6:14 | 1392 hr ± 6:29 | 1314 hr ± 6:46 | 1039 hr ± 4:46 |
| Evening dim | ||||
| n | 13 | 12 | 13 | 11 |
| Amplitude, mean ± SD | 0.527 ± 0.312 | 0.533 ± 0.459 | 0.479 ± 0.245 | 0.559 ± 0.444 |
| Mesor, mean ± SD | 0.704 ± 0.149 | 0.704 ± 0.193 | 0.709 ± 0.202 | 0.705 ± 0.159 |
| Acrophase, mean ± SD | 1252 hr ± 2:03 | 1252 hr ± 4.43 | 1414 hr ± 3.04 | 1137 hr ± 3.32 |
Mesor Treatment Week 2 versus baseline, P = .027.
Acrophase Treatment Week 2 versus baseline, P = .022.
SD = standard deviation.
Figure 2.
Treatment effects on acrophase time. Acrophase was delayed during treatment in the morning light group (P = .022). DSR = daytime sleep restriction.
Figure 3.
Circular plot of acrophase shift after treatment in the four treatment groups. Note that, in the morning light group, all arrows from baseline (open circles) to treatment days (closed circles) point clockwise (i.e., delayed or moved later in the day). In the evening light group, acrophase advanced (arrows pointing counterclockwise) for some patients and delayed (arrows pointing clockwise) for others. DSR = daytime sleep restriction.
Overall model F statistics are presented in Table 2. This is the most reliable measure for making inferences about the strength of the circadian rhythm. There was a trend for improvement in model fit (increase in rhythmicity) for morning bright (P = .064) and a significant improvement in evening dim light (P = .010) during treatment Week 2 relative to baseline.
Table 2.
Measures of Rhythmicity (Based on Extended Cosine Model) of Activity for Each Treatment Group by Week of the Study
| Treatment Group | Baseline | Treatment Week 1 | Treatment Week 2 | Posttreatment |
|---|---|---|---|---|
| Daytime sleep restriction | ||||
| n | 12 | 10 | 12 | 11 |
| F-value, mean ± SD | 80.4 ± 120.1 | 92.6 ± 144.0 | 135 ± 228 | 119 ± 171 |
| Morning bright | ||||
| n | 10 | 10 | 10 | 9 |
| F-value, mean ± SD | 41.3 ± 52.6 | 67.6 ± 77.3 | 70.5 ± 60.9* | 68.6 ± 85.0 |
| Evening bright | ||||
| n | 11 | 9 | 11 | 11 |
| F-value, mean ± SD | 31.8 ± 20.6 | 27.6 ± 35.6 | 29.6 ± 34.3 | 19.0 ± 20.6 |
| Evening dim | ||||
| n | 13 | 12 | 13 | 11 |
| F-value, mean ± SD | 131 ± 178 | 167 ± 218 | 273 ± 392† | 340 ± 554 |
Note: F statistics have 4 numerator degrees of freedom and at least 4,000 denominator degrees of freedom.
Treatment week 2 versus baseline, P = .064.
Treatment week 2 versus baseline, P = .010.
SD = standard deviation.
DISCUSSION
Increased light exposure, whether in the morning or evening, did not improve measures of nighttime sleep or daytime alertness. However, results suggested that morning bright light might delay circadian rhythms and improve circadian rhythm quality in nursing home residents.
It is not clear why light treatment in our study produced results different from those of other investigators, but there are several possible explanations. First, the level of dementia in the current study may have been more severe than those in some other studies. Our patients were severely demented, with a mean MMSE score of 12.8 (median = 9). Other studies that examined the effect of light treatment in dementia used moderately and severely demented patients, but did not report the precise level of impairment. In addition, no distinction was made between patients with AD and those with other types of dementia in the current study. It is possible that light treatment might improve sleep in some types of dementia but not others. We are currently replicating this study in a more homogenous group of patients with possible or probable AD.
In other studies of light treatment, subjective ratings often resulted in improvements, whereas objective ratings, all based on wrist activity, showed improvements in both inter and intra-daily variability but did not consistently show improvements in percentage of time asleep or awake. In one of the first studies of the effect of light on sleep in AD, Okawa et al. conducted a series of studies in which patients were exposed to anywhere from 3,000 to 8,000 lux in the morning from 9:00 a.m. to 11:00 p.m.24,26–28,32 Two of the studies used nurses’ ratings of sleep and resulted in half the patients showing an improvement in ratings in the first study26 and a significant improvement in ratings of sleep in the second study.28
Two other studies also used staff ratings of sleep. Lyketsos et al.,33 administered 2,500 lux of light to eight demented and agitated nursing home patients in the morning for 4 weeks. Nursing staff completed sleep logs that indicated that nighttime sleep improved significantly in these patients compared with 4 weeks with dim light exposure. Koyama et al.,29 studied six nursing home patients with dementia with 4,000 lux administered from 9:30 a.m. to 11:30 p.m. for half the subjects and from 11:00 a.m. to noon for the other three subjects. Nursing staff observations and sleep log records indicated that three of six subjects had significant improvements in rated percentage of nighttime sleep and two of six had significant improvements in rated percentage of daytime wakefulness.
In a more recent study by Okawa et al. using wrist actigraphs, only patients with vascular dementia showed a decrease in nighttime activity after light treatment. There were no improvements in the patients with AD.32 Satlin et al.25 exposed patients with AD to evening bright light, and, in 80% of the patients studied, staff's ratings of sleep/wake improved; moreover, based on wrist activity, there was a significant decrease in intradaily variability, percentage of nocturnal activity, and severity of sundowning behavior and a significant increase in the amplitude of the rhythm.
A second study also found changes in variability/stability. Van Someren et al.30 exposed 22 demented patients to all-day indirect bright light with an mean of 1,136 lux. After 4 weeks of treatment, wrist activity data indicated a significant increase in interdaily stability (suggesting an increase in the strength of the coupling between the rhythm and environmental cues) and decreased intradaily variability (indicating less fragmentation).
One additional study examined the effect of light on AD patients living in the community. Five patients were exposed to 2 hours of morning light at 2,000 lux between 7:00 a.m. and 9:30 a.m. using light visors for 10 days. This study found no significant changes in any sleep or rhythm parameters.31
The only previous study to administer evening bright light was Satlin et al.,25 in which light treatment was administered from 7:00 p.m. to 9:00 p.m., with a resulting improvement in sleep. When this protocol was initially planned, evening bright light was also to be administered from 7:00 p.m. to 9:00 p.m., and afternoon light was to be administered between 2:00 p.m. and 4:00 p.m., a time expected to have no “phase shifting” effects on circadian rhythms. However, when the protocol commenced, it became clear that this group of nursing home patients went to bed by 7:30 p.m., and treatment time was therefore shifted to occur immediately before bedtime, between 5:30 p.m. and 7:30 p.m. This forced original afternoon treatment time to be moved to the morning before lunch, between 9:30 a.m. and 11:30 a.m. These untraditional times may account for some of the discrepancies in results.
To determine whether sleep improves at night, one needs a reliable definition of “night,” (i.e., a measure of bedtime and uptime). Other studies have not reported how they defined night and day. In this study, because the patients were sleeping on and off throughout the day and night, it was not possible to tell when they actually went to bed; therefore, times had to be chosen to define night. It is possible that, had we known the true bedtimes, the data may have appeared somewhat different. We are currently replicating this protocol with observed bedtimes and uptimes.
The current study included two “control” groups, one as a control for staff interaction during light (DSR) and one as a control for the light itself (dim red light). Only two other studies had control conditions, one of which only used observations33 and the other which found no results in AD patients.32 Therefore, in all the other studies, it may not have been the light alone that accounted for the findings.
Our patients were fairly compliant, but, even with staff accompanying them during treatment, the average duration of light therapy was only 92 minutes. It is not easy to encourage demented older people to stay awake and to sit continuously for 2 hours in front of a light box. Having the light on for 2 hours is no guarantee that the patients are awake and actually sitting in front of the light for the full time. Although other studies reported the duration of light therapy sessions (the length of time the light was on), no other study reported patient compliance with treatment. It is therefore unknown whether patients in these other studies received less or more light exposure than our sample.
The compliance issue raises another important point. As mentioned, it was difficult to keep patients sitting in front of light boxes. Even with constant companionship, patients still had a tendency to wander away or fall asleep. In the clinical setting, staff would rarely have the time to sit with patients during treatment. Van Someren et al.30 had ceiling-mounted high-intensity lights placed in the living rooms of the nursing homes. Colenda et al.31 had patients wear bright light visors rather than using bright light boxes. Although the patients tolerated the visors, the intervention resulted in no significant improvements in measures of sleep or circadian rhythms. In facilities that wish to shift patients’ activity rhythms with the use of bright light, the most clinically relevant recommendation would be to install bright lights in the ceiling with controls that would allow them to be regulated for lux level, timing, and duration.
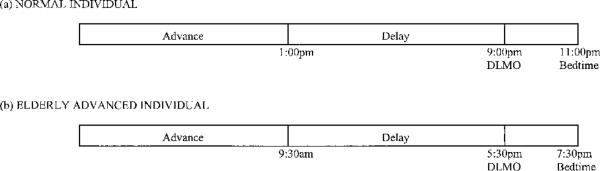
Another unexpected finding was that morning bright light delayed circadian activity rhythms in this study. It is important to note that the acrophase was delayed in every patient in this treatment group. This was not simply the result of aggregating data across subjects. In younger adults, morning light generally advances circadian rhythms,23 but the phase delay seen here suggests that the rhythms of demented older individuals may be different. Light exposure of the same intensity, at different times of the day, results in different phase shifts. This is called the phase response curve (PRC).48 The PRC is linked to the time that intrinsic melatonin secretion begins (dim light melatonin onset (DLMO)). In normal adults, DLMO typically occurs at 9:00 p.m. Based on Lewy's theory of the PRC to light,48 bright light exposure between 1:00 p.m. and 9:00 p.m. will delay the circadian rhythm. This occurs 8 hours before the DLMO and is called the delay portion of the PRC. The advance portion of the PRC falls before 1:00 p.m. (see Figure 4, Panel A). Because older people are phase advanced relative to younger adults,6–9 DLMO would also be phase advanced. Lewy's theory would suggest that DLMO in these older people who are phase advanced occurs at perhaps 5:30 p.m. rather than at 9:00 p.m., which would place the delay portion of the PRC between approximately 9:30 a.m. and 5:30 p.m. (Figure 4, Panel B). This was the time of the morning light treatment. Therefore, if these older people are phase advanced, the phase delay zone of the light PRC may have been stimulated with the morning bright light, thus resulting in a change in circadian rhythms opposite of what would be expected in young adults. There may also be other theories to explain this finding. Additional research still needs to be done.
Figure 4.
Dim light melatonin onset (DLMO) and phase response curve in normal older people (a) and older people who are phase advanced (b). In normal middle-aged adults, DLMO is at approximately 9:00 p.m., 2 hours before bedtime. The delay portion of the phase response curve (PRC) would begin at about 1:00 p.m. In the older patients in this study, bedtime was 7:30 p.m., and DLMO was likely to be around 5:30 p.m. This would place the beginning of the delay portion 8 hours earlier, or at 9:30 a.m., the time of the morning light treatment.
In summary, although none of the treatments explored improved sleep at night or alertness during the day in these older, primarily demented individuals, increasing exposure to morning bright light delayed the acrophase of the activity rhythm and made the circadian rhythm more robust. These changes have the potential to be clinically beneficial, because it may be easier to provide nursing care to patients who have more socially acceptable circadian activity patterns. Morning bright light has also been shown to improve agitated behavior in a small subsample of this same group of subjects.49
The results of this study are consistent with the viewpoint that light treatment in the nursing home is difficult to apply and results are not robust. This is different from studies of bright light treatment of depression,50 or delayed sleep phase,51 where there are large, robust improvements with groups consisting of as few as 10 subjects. Although the sample size in the current study was relatively large for a light therapy trial, it is rather small for standard treatment trials. For example, studies of treatment of depression with antidepressant medications are no more robust than the current study and require 40 to 50 subjects in each group, treated for 8 to 16 weeks, to find a significant treatment effect.52 The possibility that treatment effects exist in this study should not be discounted; rather, additional larger trials are still needed to determine whether light administered at different times to patients with known AD will have a beneficial effect on sleep.
ACKNOWLEDGMENTS
The project could not have been completed without cooperation of the administration, staff, and patients at the San Diego Hebrew Home and Seacrest Village and without the help of Denise Williams Jones, Lorraine Almendarez, Idalia Canez, Rena Fox, Roberta Lovell, William Mason, Linda Parker, and the student volunteers who spent countless hours with the patients.
Supported by National Institute on Aging Grants AG08415, NIA AG02711, NCI CA85264, and NHLBI HL44915; the Sam and Rose Stein Institute for Research on Aging; the Department of Veterans Affairs VISN-22 Mental Illness Research, Education and Clinical Center; the University of California at San Diego Cancer Center; and the Research Service of the Veterans Affairs San Diego Healthcare System.
Footnotes
Parts of this manuscript were presented at the 12th Annual Meeting of the Society of Light Treatment and Biological Rhythms, Evanston, Illinois, 2000.
REFERENCES
- 1.Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep and light exposure related to dementia in nursing home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- 2.Sanford JRA. Tolerance of debility in elderly dependents by supporters at home: Its significance for hospital practice. BMJ. 1975;3:471–473. doi: 10.1136/bmj.3.5981.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollak CP, Perlick D, Linsner JP, et al. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990;15:123–135. doi: 10.1007/BF01321316. [DOI] [PubMed] [Google Scholar]
- 4.Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22:S386–S393. [PubMed] [Google Scholar]
- 5.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 6.Nicolau GY, Haus E, Lakatua DJ, et al. Differences in the circadian rhythm parameters of urinary free epinephrine, norepinephrine and dopamine between children and elderly subjects. Romanian J Med-Endocrinol. 1985;23:189–199. [PubMed] [Google Scholar]
- 7.Prinz PN, Christie C, Smallwood R, et al. Circadian temperature variation in healthy aged and in Alzheimer's disease. J Gerontol. 1984;39:30–35. doi: 10.1093/geronj/39.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Touitou Y, Sulon J, Bogdan A, et al. Adrenal circadian system in young and elderly human subjects: A comparative study. J Endocrinol. 1982;93:201–210. doi: 10.1677/joe.0.0930201. [DOI] [PubMed] [Google Scholar]
- 9.van Coevorden A, Mockel J, Laurent E, et al. Neuroendocrine rhythms and sleep in aging men. Am J Physiol. 1991;260:E651–E661. doi: 10.1152/ajpendo.1991.260.4.E651. [DOI] [PubMed] [Google Scholar]
- 10.Wever RA, Polasek J, Wildgruber CM. Bright light affects human circadian rhythms. Pflügers Arch. 1983;396:85–87. doi: 10.1007/BF00584704. [DOI] [PubMed] [Google Scholar]
- 11.Espiritu RC, Kripke DF, Ancoli-Israel S, et al. Low illumination by San Diego adults: Association with atypical depressive symptoms. Biol Psychiatry. 1994;35:403–407. doi: 10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 12.Campbell SS, Kripke DF, Gillin JC, et al. Exposure to light in healthy elderly subjects and Alzheimer's patients. Physiol Behav. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Jones DW, Hanger MA, et al. Sleep in the nursing home. In: Kuna ST, Suratt PM, Remmers JE, editors. Sleep and Respiration in Aging Adults. Elsevier Press; New York, NY: 1991. pp. 77–84. [Google Scholar]
- 14.Shochat T, Martin J, Marler M, et al. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–380. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 15.Bliwise DL, Carroll JS, Lee KA, et al. Sleep and sundowning in nursing home patients with dementia. Psychiatry Res. 1993;48:277–292. doi: 10.1016/0165-1781(93)90078-u. [DOI] [PubMed] [Google Scholar]
- 16.Jewett M, Kronauer R, Czeisler C. Phase-amplitude resetting of the human circadian pacemaker via bright light: A further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- 17.Bunnell DE, Treiber SP, Phillips NH, et al. Effects of evening bright light exposure on melatonin, body temperature and sleep. J Sleep Res. 1992;1:17–23. doi: 10.1111/j.1365-2869.1992.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Gillin JC, Kripke DF, Campbell SS. Ambulatory measures of activity, light, and temperature in elderly normal controls and patients with Alzheimer disease. Bull Clin Neurosci. 1989;54:144–148. [Google Scholar]
- 19.Duffy JF, Dijk DJ, Klerman EB, et al. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 20.Carrier J, Monk TH, Reynolds CF, et al. Are age differences in sleep due to phase differences in the output of the circadian timing system? Chronobiol Int. 1999;16:79–91. doi: 10.3109/07420529908998714. [DOI] [PubMed] [Google Scholar]
- 21.Touitou Y, Haus E. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol Int. 2000;17:369–390. doi: 10.1081/cbi-100101052. [DOI] [PubMed] [Google Scholar]
- 22.Satlin A, Volicer L, Stopa E, et al. Circadian locomotor activity and core-body temperature rhythms in Alzheimer's disease. Neurobiol Aging. 2000;16:765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- 23.Campbell SS, Terman M, Lewy AJ, et al. Light treatment for sleep disorders: Consensus report. V. Age-related disturbances. J Biol Rhythms. 1995;10:151–154. doi: 10.1177/074873049501000207. [DOI] [PubMed] [Google Scholar]
- 24.Okawa M, Mishima K, Hishikawa Y, et al. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep. 1991;14:478–485. doi: 10.1093/sleep/14.6.478. [DOI] [PubMed] [Google Scholar]
- 25.Satlin A, Volicer L, Ross V, et al. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer's disease. Am J Psychiatry. 1992;149:1028–1032. doi: 10.1176/ajp.149.8.1028. [DOI] [PubMed] [Google Scholar]
- 26.Okawa M, Hishikawa Y, Hozumi S, et al. Sleep-wake rhythm disorder and phototherapy in elderly patients with dementia. Biol Psychiatry. 1991;1:837–840. [Google Scholar]
- 27.Okawa M, Mishima K, Hishikawa Y, et al. Sleep disorder in elderly patients with dementia and trials of new treatments—enforcement of social interaction and bright light therapy. In: Kumar VM, Mallick HN, Nayar U, editors. Sleep-Wakefulness. Wiley Eastern Ltd.; New Delhi: 1993. pp. 128–132. [Google Scholar]
- 28.Mishima K, Okawa M, Hishikawa Y, et al. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiat Scand. 1994;89:1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 29.Koyama E, Matsubara H, Nakano T. Bright light threatment for sleep-wake distrubances in aged individuals with dementia. Psychiatry Clin Neurosci. 1999;53:227–229. doi: 10.1046/j.1440-1819.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Someren EJW, Kessler A, Mirmiran M, et al. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 31.Colenda CC, Cohen W, McCall WW, et al. Phototherapy for patients with Alzheimer disease with disturbed sleep patterns: Results of a community-based pilot study. Alzheimer Dis Associated Disord. 1997;11:175–178. doi: 10.1097/00002093-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer's type. Chronobiol Int. 1998;15:647–654. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- 33.Lyketsos CG, Lindell Veiel L, Baker A, et al. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- 34.Monk TH. Circadian rhythm. In: Roth T, Roehrs TA, editors. Clinics in Geriatric Medicine. W.B. Saunders Company; Philadelphia, PA: 1989. pp. 331–345. [Google Scholar]
- 35.Richardson GS. Circadian rhythms and aging. Biol Aging. 1990;13:275–305. [Google Scholar]
- 36.Brock MA. Chronobiology and aging. J Am Geriatr Soc. 1991;39:74–91. doi: 10.1111/j.1532-5415.1991.tb05909.x. [DOI] [PubMed] [Google Scholar]
- 37.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Ancoli-Israel S, Clopton P, Klauber MR, et al. Use of wrist activity for monitoring sleep/wake in demented nursing home patients. Sleep. 1997;20:24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jean-Louis G, Kripke DF, Cole RJ, et al. Sleep detection with an accelerometer actigraph: Comparisons with polysomnography. Physiol Behav. 2001;72:21–28. doi: 10.1016/s0031-9384(00)00355-3. [DOI] [PubMed] [Google Scholar]
- 41.Bliwise DL. Review: Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 42.Lewy AJ, Sack RL. Phase typing and bright light therapy of chronobiologic sleep and mood disorders. In: Halaris A, editor. Chronobiology and Psychiatric Disorders. Elsevier Science Publishing Co.; New York, NY: 1987. pp. 181–206. [Google Scholar]
- 43.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 44.Martin J, Marler MR, Shochat T, et al. Circadian rhythms of agitation in institutionalized Alzheimer's disease patients. Chronobiol Int. 2000;17:405–418. doi: 10.1081/cbi-100101054. [DOI] [PubMed] [Google Scholar]
- 45.Hofman MA. The human circadian clock and aging. Chronobiol Int. 2000;17:245–259. doi: 10.1081/cbi-100101047. [DOI] [PubMed] [Google Scholar]
- 46.SAS Institute Inc. SAS/STAT User's Guide (Version 6, Vols. 1 and 2) SAS Institute, Inc.; Cary, NC: 1989. [Google Scholar]
- 47.SAS Institute . ISAS/GRAPH Software (Version 6, Vols. 1 and 2) SAS Institute, Inc.; Cary, NC: 1990. [Google Scholar]
- 48.Lewy AJ, Bauer VK, Ahmed S, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 49.Lovell BJ, Ancoli-Israel S, Gevirtz R. The effect of bright light treatment on agitated behavior in institutionalized elderly. Psychiatry Res. 1995;57:7–12. doi: 10.1016/0165-1781(95)02550-g. [DOI] [PubMed] [Google Scholar]
- 50.Lam RW, Levitt AJ, editors. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. Clinical and Academic Publishing; Canada: 1999. [Google Scholar]
- 51.Campbell SS, Murphy PJ, van den Heuvel CJ, et al. Etiology and treatment of intrinsic circadian rhythm sleep disorders. Sleep Med Rev. 1999;3:179–200. doi: 10.1016/s1087-0792(99)90001-1. [DOI] [PubMed] [Google Scholar]
- 52.Khan A, Brown WA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: An analysis of the Food and Drug Administration database. Arch Gen Psychiatry. 2000;57:311–317. doi: 10.1001/archpsyc.57.4.311. [DOI] [PubMed] [Google Scholar]