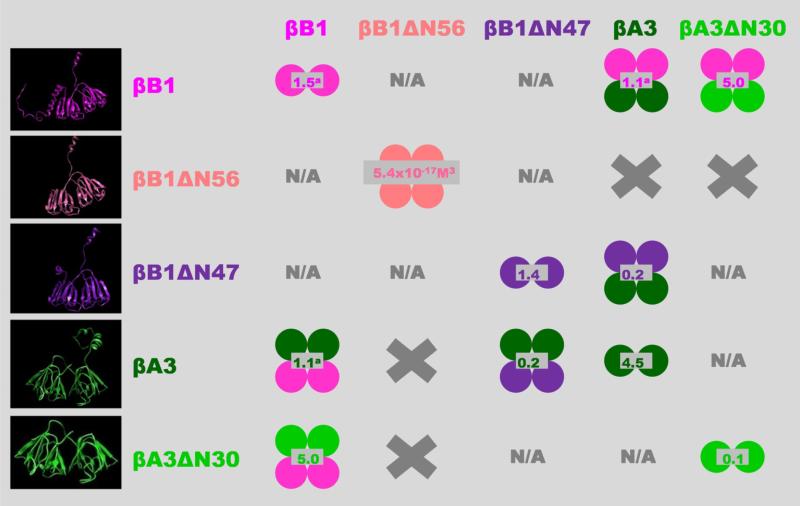
Figure 10. Overview of β-crystallin homo- and hetero-association.
The indicated shifts in the oligomeric equilibria as a result of N-terminal truncations may also occur in the lens but this is difficult to prove directly due to the high in-vivo protein concentrations. Some support for this, however, is that the proteolytic processing of crystallins causes cataract formation in mice (40). Single circles represent monomeric units; homo-oligomers are shown in single colors; hetero-oligomers bicolored; values in gray indicate dissociation constants (Kd) in μM (except for βB1ΔN56); left panel show β-crystallins models from top to bottom: βB1 full length and βB1 with N-terminal truncation of 56 (βB1ΔN56) and 47 (βB1ΔN47) residues; βA3 full length and with N-terminal truncation of 30 residues (βA3ΔN30). X, no association between indicated β-crystallins and N/A. interaction not characterized.
a Values previously determined (34).