Abstract
Objectives
To investigate the effect of dantrolene, a drug generally used to treat Malignant Hyperthermia (MH), on the Ca2+ release and cardiomyocyte function in failing hearts.
Background
The N-terminal (N: 1-600) and Central (C: 2000-2500) domains of the ryanodine receptor (RyR), harbor many mutations associated with MH in skeletal muscle RyR (RyR1) and polymorphic ventricular tachycardia in cardiac RyR (RyR2). There is strong evidence that inter-domain interaction between these regions plays an important role in the mechanism of channel regulation.
Methods
Sarcoplasmic reticulum (SR) vesicles and cardiomyocytes were isolated from dog LV muscles (normal or rapid ventricular pacing for 4 weeks), for Ca2+ leak, transient, and spark assays. To assess the zipped or unzipped state of the interacting domains, the RyR was fluorescently labeled with methylcoumarin acetate in a site-directed manner. We employed a quartz-crystal microbalance technique to identify the dantrolene binding site within the RyR2.
Results
Dantrolene specifically bound to domain 601-620 in RyR2. In the SR isolated from pacing-induced dog failing hearts, the defective inter-domain interaction_(domain unzipping) has already occurred, causing spontaneous Ca2+ leak. Dantrolene suppressed both domain unzipping and the Ca2+ leak, showing identical drug concentration-dependence (IC50=0.3 μmol/L). In failing cardiomyocytes, both diastolic Ca2+ sparks and delayed afterdepolarization were frequently observed, but 1 μmol/L dantrolene inhibited both events.
Conclusions
Dantrolene corrects defective inter-domain interactions within RyR2 in failing hearts, inhibits spontaneous Ca2+ leak, in turn improves cardiomyocyte function in failing hearts. Thus, dantrolene may have a potential to treat heart failure, specifically targeting the RyR2.
Keywords: heart failure, calcium, sarcoplasmic reticulum
The skeletal muscle type-1 and cardiac type-2 ryanodine receptors (RyR1 and RyR2, respectively) are intracellular channels that mediate Ca2+ release from the sarcoplasmic reticulum (SR). More than 70 point mutations in RyR1 have been reported to cause malignant hyperthermia (MH) and central core disease (CCD), potentially lethal genetic disorders of skeletal muscle (1). As widely recognized, MH mutations are not randomly distributed along the RyR1 sequence. The majority of them are localized in two restricted regions: the N-terminal (Cys35-Arg614), and the central (Asp2129-Arg2458) domains, while a third, C-terminal region (Ile3916-Gly4942) contains fewer MH mutations (1). All the RyR1 Ca2+ release channels with MH mutations are characterized by leaky SR Ca2+ channel (1, 2).
Based on the distribution of these mutations, Ikemoto and colleagues proposed the hypothesis that the interactions between the N-terminal domain and the central domain of RyR1 are involved in Ca2+ channel regulation as an intrinsic regulator of the Ca2+ channel, and these interacting domains comprise a ‘domain switch’ (3, 4). According to this hypothesis, in the resting or non-activated state, the N-terminal and central domains make close contact at several sub-domains (domain zipping). The conformational constraints imparted by the “zipped” configuration of these two domains stabilize and maintain the closed state of Ca2+ channel. Stimulation via excitation-contraction (e-c) coupling or pharmacological agents weakens these critical inter-domain contacts, resulting in a reduction of conformational constraints (domain unzipping), thereby lowering the energy barrier for Ca2+ channel opening. Weakening of these inter-domain interactions may also occur via mutation or with the use of synthetic domain peptides.
To date, more than 60 RyR2 missense mutations have been found to be linked with two inherited forms of sudden cardiac death: i.e. catecholaminergic polymorphic ventricular tachycardia (CPVT), and arrhythmogenic right ventricular cardiomyopathy type 2 (ARVC2) (1). All RyR2 mutations cluster into three regions of the channel that correspond to the three MH/ CCD mutation regions (N-terminal domain, central domain and channel forming domain) of RyR1. Particularly, some mutations of RyR2, which have been reported in the cardiac disease patients, are located in the regions corresponding to the skeletal N-terminal and central domains harboring most of MH mutations that cause an increased Ca2+ leak (5). This suggests that RyR2 shares a common domain-mediated channel regulation mechanism with RyR1, and that the increased Ca2+ leak of diseased RyR2 channels may be attributable to defective modes of inter-domain interaction. We recently reported that the defective inter-domain interaction within the RyR2 (viz. domain unzipping) destabilizes the channel gating of the RyR2, associated with FKBP12.6 dissociation from RyR2 and Ca2+ leak, causing contractile dysfunction of cardiomyocyte (6). Based on these findings, we have proposed that the weakened inter-domain interaction is one of the key mechanisms underlying the pathogenesis of ARVC2, CPVT and heart failure and that normal operation of the domain switch is essential for proper function of both RyR1 and RyR2 channels.
Parness and colleagues have identified the dantrolene-binding site within RyR1 as Leu590-Cys609 (named DP1) using [3H]azidodantrolene, a pharmacologically active photoaffinity analog of dantrolene (7).
Recently, we demonstrated a novel mechanism by which dantrolene improves hyper-sensitization of channel gating to Ca2+ seen in MH channels (8). Upon adding dantrolene to defective RyR1 Ca2+ release channel mimicking MH, dantrolene was found to produce a local conformational change, resulting in reinforcement of the inter-domain interactions (8).
Because the amino acid sequence of the DP1 region is exactly identical between RyR1 and RyR2, we hypothesized that dantrolene would be equally effective to correct the defective inter-domain interaction in failing hearts. Here we report that dantrolene in fact corrects defective inter-domain interaction within RyR2, inhibits Ca2+ leak, and improves cardiomyocyte function in failing hearts.
Methods
Materials
Dantrolene was purchased from Sigma.
Animal disease model
In beagle dogs, heart failure was induced by chronic rapid RV pacing (Online appendix).
Preparation of SR vesicles
SR vesicles were prepared from normal or 4w-paced-failing dog LV, as described previously (9).
Peptides used and peptide synthesis
Domain peptide (DPc10), corresponding to amino acids Gly2460- Pro2495 of RyR2 were synthesized and used for analysis of domain-domain interaction within RyR2 (Online appendix).
Construction and expression of RyR2 fragments
The peptides corresponding to various regions of RyR2 (1-610, 494-1000, 741-1260, 1245-1768, 1741-2270, 1981-2520 and 2234-2750) were PCR amplified (Online appendix).
Quartz crystal microbalance ( QCM ) measurements
Binding of dantrolene to RyR2 fragments was detected by using a 27-MHz quartz-crystal microbalance (QCM) (Initium, Inc., Japan) (Online appendix).
Ca2+-uptake and -leak assays
Ca2+-uptake and the following Ca2+ leak assays were carried out as described previously (9).
Site-directed fluorescent labeling of the RyR2
Specific fluorescent labeling of the RyR2moiety of the SR membrane was performed using the cleavable hetero-bifunctional cross-linking reagent sulfosuccinimidyl 3-((2-(7-azido-4-methylcoumarin-3-acetamido) ethyl) dithio) propionate (SAED) from PIERCE, with DPc10 as a site-specific carrier following the site-directed fluorescent labeling method (6).
Fluorescence quenching of the MCA fluorescence attached to the DPc10 binding site
The zipped and unzipped states of RyR were evaluated by chemical quencher analysis, as described previously (4, 6, 8) (Online appendix).
Isolation of cardiac cardiomyocytes
Cardiomyocytes were isolated from the LV free wall of normal or 4w-paced failing dogs, as described previously (6) (Online appendix).
Cell shortening and Ca2+ transient measurement
Measurements of cardiomyocyte cell shortening and intracellular Ca2+ were performed using fura-2 AM, as described previously (6) (Online appendix).
Analysis of Ca2+ sparks with laser scanning confocal microscopy
Ca2+ sparks were measured on a laser scanning confocal microscope (Online appendix).
Measurement of membrane potential with laser scanning confocal microscopy
Measurements of membrane potential were performed on a laser scanning confocal microscope following the same protocol for the measurement of Ca2+ sparks (Online appendix).
Statistics
Unpaired t-test was used for statistical comparison of data between the two states. We also used ANOVA for repeated measures with a post hoc Scheffe’s test for statistical comparison of concentration-dependent data. Data are expressed as means±SD. We accepted a p value less than 0.05 as statistically significant.
Results
Effect of dantrolene on in vivo cardiac function
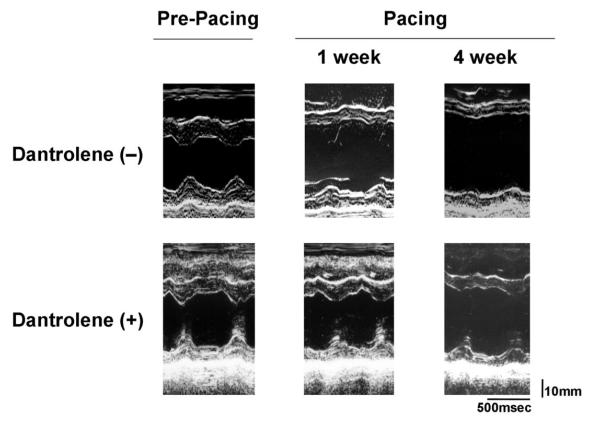
In the dantrolene-treated dogs with long-term RV pacing, both LV end-diastolic and LV end-systolic diameters were smaller than in dantrolene-untreated dog, and fractional shortening was significantly improved (Table 1, Figure 1A). This indicates that the development of heart failure by pacing was attenuated by the treatment with dantrolene.
Table 1.
Hemodynamic Data
| HR, bpm | SBP, mmHg | DBP, mmHg | LVDd, mm | LVDs, mm | LVFS, % | |
|---|---|---|---|---|---|---|
| Untreated (n=6) | ||||||
| Prepacing | 116±12 | 130±16 | 81±5 | 31.1±2.1 | 20.5±2.8 | 34.6±7.3 |
| 1w-pacing | 119±13 | 116±19 | 82±5 | 36.7±2.1a | 29.7±1.7 a | 19.0±3.6 a |
| 4w-pacing | 121±14 | 120±11 | 83±10 | 39.6±1.4 a | 34.4±1.6 a | 13.0±2.8 a |
| Dantrolene-treated (n=4) | ||||||
| Prepacing | 115±7 | 129±5 | 84±5 | 31.2±1.6 | 20.4±2.1 | 34.8±6.4 |
| 1w-pacing | 119±2 | 123±6 | 80±9 | 32.0±1.8 b | 23.8±0.5 a, b | 25.6±5.5 b |
| 4w-pacing | 116±7 | 127±10 | 85±6 | 35.0±2.3 a, c | 27.8±2.2 a, c | 20.6±6.2 a, c |
HR indicates heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVFS, left ventricular fractional shortening. Each datum point represents the mean ± SD. The number of experiments is shown in the parentheses. Unpaired T-test was employed to determine the statistical significance of the data (p value).
p<0.05 vs prepacing
p<0.05 vs 1w-pacing (untreated)
p<0.05 vs 4w-pacing (untreated)
Figure 1. Effects of dantrolene on cardiac function.
Representative M-mode echocardiogram.
Specific binding of dantrolene to domain 601-620 of RyR2
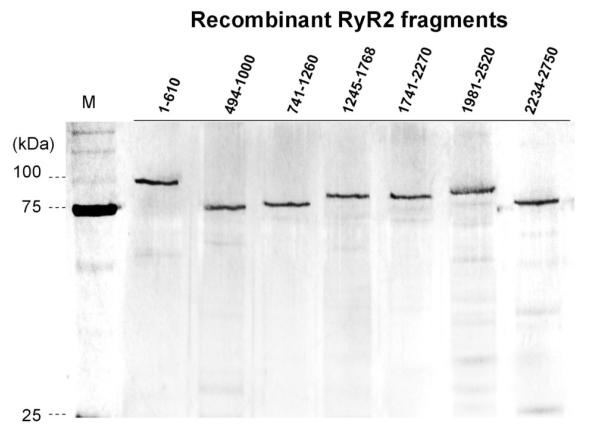
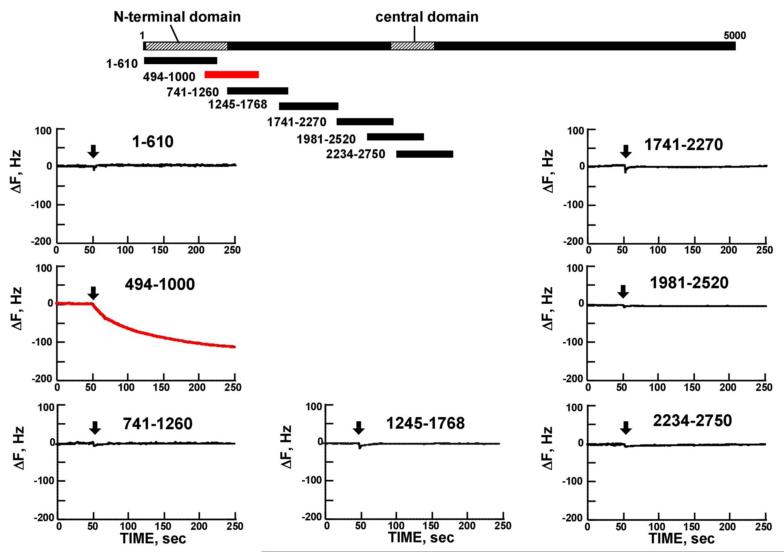
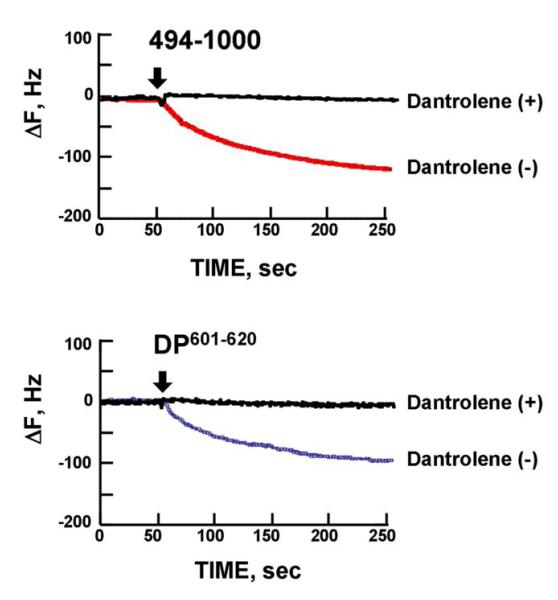
Dantrolene was previously reported to bind to the domain Leu590-Cys609 in the case of RyR1 (7). We investigated whether dantrolene also binds to the corresponding domain 601-620 of RyR2 using a quartz crystal microbalance technique (a highly sensitive mass-measuring technique). In order to identify the dantrolene-binding region, we screened several recombinant fragments corresponding to various regions covering the area from the N-terminus to residue 2750, which include both of the aforementioned critical regulatory domains {N-terminal (1-600) and central (2000-2500) domains}. Figure 2A shows the gel image of these recombinant fragments. As shown in Figure 2B, of all recombinant fragments we tested, only the fragment494-1000 showed the signal of dantrolene binding.
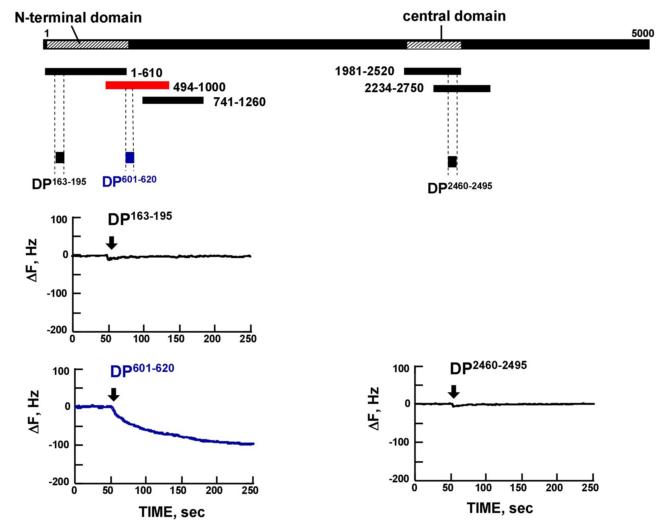
Figure 2. Identification of dantrolene binding site within RyR2.
A. Gel image of the recombinant fragments corresponding to various regions covering the area from the N-terminus to residue 2750. B, C, D. Screening of the dantrolene binding site within the RyR2 with recombinant RyR2 fragments and synthetic peptides, using quartz-crystal microbalance (QCM). Dantrolene was immobilized on the sensor chip surface as described in “Materials and Methods.” The traces represent the time course of the binding of various peptides or RyR2 fragments to dantrolene fixed on the sensor chip. Note that DP601-620 and RyR2 fragment 494-1000 specifically bound to the dantrolene (Fig. 2B and Fig. 2C), but not in the presence of excess added dantrolene (Fig.2D).
In order to test the hypothesis that RyR2 and RyR1 share the identical dantrolene binding sequence (see ‘Introduction’), DP601-620 (a synthetic peptide corresponding to DP1 described above) was subjected to the QCM binding assay. As shown in the QCM recording of Figure 2C, there was a rapid binding of dantrolene to DP601-620. There was no detectable drug binding to the central domain peptide; DPc10 (2460-2495) and to the N-terminal domain peptide: DP163-195. As shown in Figure 2D, the binding of dantrolene to fragment494-1000 or DP601-620 was abolished in the co-presence of excess dantrolene (30μmol/L).
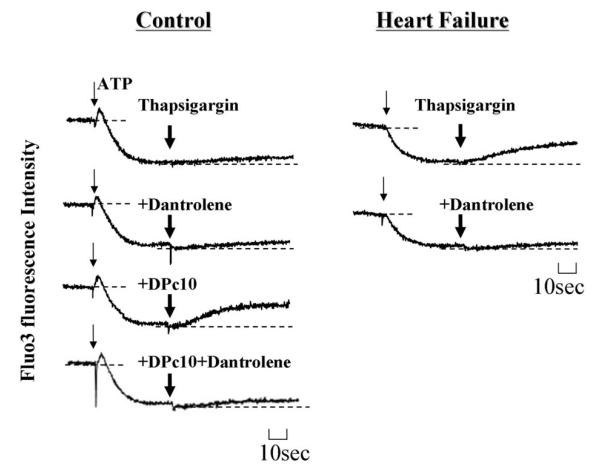
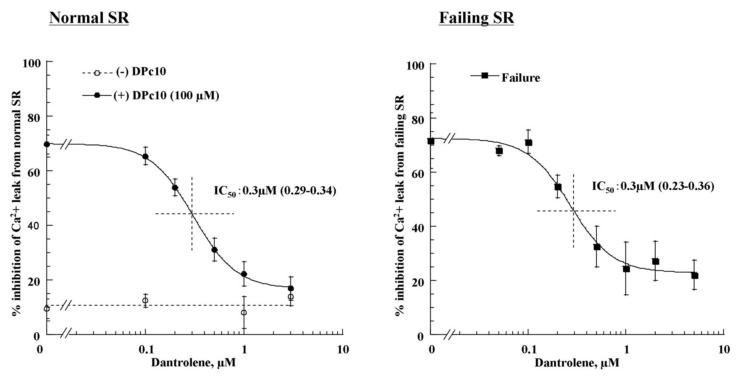
Dantrolene inhibited both DPc10-induced Ca2+ leak from the normal SR and spontaneous Ca2+ leak from the failing SR
Addition of 0.3 μmol/L thapsigargin to normal SR vesicles at a steady-state of ATP-dependent Ca2+ uptake produced little Ca2+ leak, while addition of 100 μmol/L DPc10 together with 0.3 μmol/L thapsigargin produced a pronounced leak. However, the Ca2+ leak was almost completely suppressed by 1 μmol/L dantrolene (Figure 3A). In failing SR, 0.3 μmol/L thapsigargin produced spontaneous Ca2+ leak. Dantrolene (1 μmol/L) abolished the spontaneous Ca2+ leak that otherwise would have occurred in the failing SR (Figure 3A). As shown in Figure 3B, the concentration dependence of dantrolene inhibition of DPc10-induced Ca2+ leak in normal SR occurs in the concentration range of 0.1 – 1.0 μmol/L with the concentration of a half-maximal inhibition (IC50) of 0.3 μmol/L. The concentration dependence and IC50 (0.3 μmol/L) of dantrolene inhibition of the spontaneous Ca2+ leak in failing SR (Figure 3C) are identical to those of DPc10-induced Ca2+ leak in the normal SR. We also evaluated the effect of dantrolene on Ca2+ leak induced by PKA phosphorylation of RyR2 (by cAMP) (Figure 3D). In the presence of 1μmol/L dantrolene, cAMP-induced Ca2+ leak was completely inhibited. Dantolene (0.1-1 μM) had no effect on the Ca2+ uptake time course (data not shown).
Figure 3. Effect of dantrolene on SR Ca2+ leak.
A. Representative time courses of Ca2+ uptake and Ca2+ leak in normal and failing SR vesicles in the presence or absence of dantrolene. B. Concentration dependence of dantrolene inhibition of the DPc10 (100 μmol/L)-induced Ca2+ leak in normal SR vesicles. C. Concentration dependence of dantrolene inhibition on spontaneous Ca2+ leak in failing SR vesicles. Each datum point is a mean of 4 to 6 measurements ± SD. D. Effect of dantrolene on cAMP-induced Ca2+ leak in normal SR vesicles. Thapsigargin (0.3 μmol/L) was added following ATP-dependent Ca2+ uptake after preincubation of the SR vesicles with 30 μmol/L cAMP plus 1 μmol/L okadaic acid.
Dantrolene reversed an abnormal domain unzipping of the domain switch
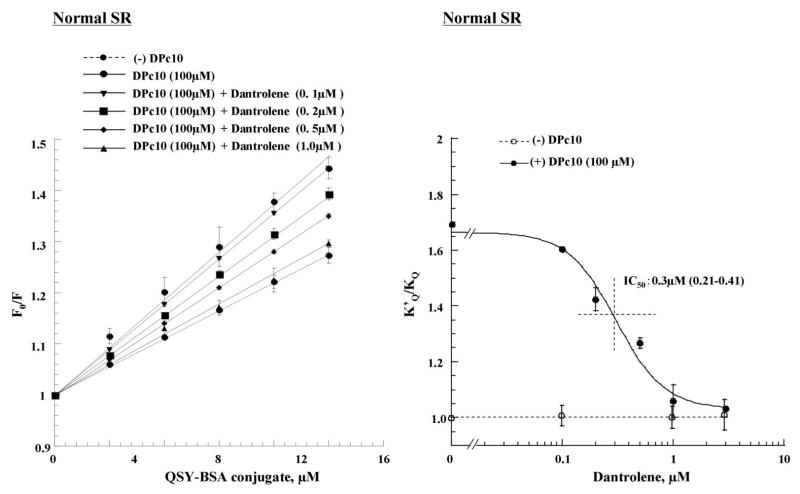
In order to investigate the effect of dantrolene on the mode of inter-domain interaction between the N-terminal domain and the central domain (two regulatory domains of the domain switch, see ‘Introduction’), we utilized the MCA fluorescence quenching technique we have developed to determine the extent of unzipping of the domain switch (see “Methods” section). The slope of the plot, which is equal to the Stern-Volmer quenching constant (KQ), is a measure of the degree of domain unzipping.
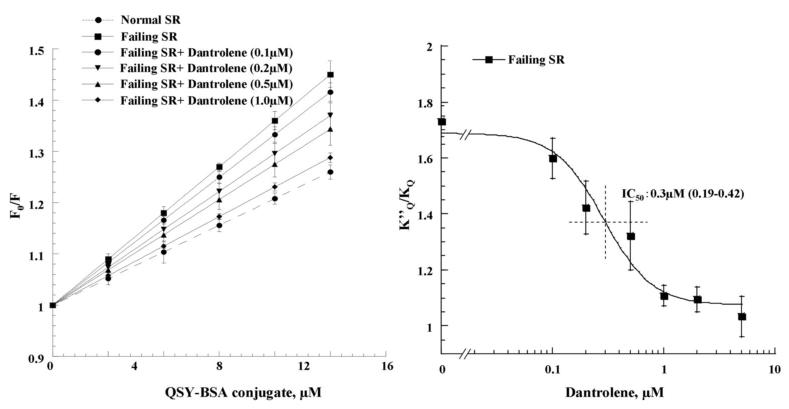
As shown in our previous report (6), KQ increased with increasing concentrations of DPc10 and leveled off at about 100 μmol/L peptide. In the experiment shown in Figure 4A, we investigated the effect of increased concentrations of dantrolene on the KQ that had reached a maximal level by 100 μmol/L peptide. As shown in Figure 4B, the K’Q/ KQ value (where K’Q is the quenching constant in the DPc10-treated SR and KQ is the quenching constant in the untreated control SR) was reduced with an increase of dantrolene concentration, and at concentrations higher than 1 μmol/L it reached the control level (K’Q/ KQ = 1). Importantly, the IC50 (0.3 μmol/L) of dantrolene inhibition of domain unzipping shown here is identical to that of dantrolene inhibition of DPc10-induced Ca2+ leak shown in Figure 3B. Dantrolene had no appreciable effect on KQ unless DPc10 was added (Figure 4B). This suggests that in the normal SR the domain switch is in a fully zipped state. In contrast, the KQ value of failing SR was comparable with the value of normal SR that had been treated with 100 μmol/L DPc10, suggesting that in the failing RyR2 the domain switch had already been unzipped. Dantrolene reduced KQ with a concentration dependence identical to what we have seen in the DPc10-added normal SR (Figure 4C, 4D). Here again, the IC50 value (0.3 μmol/L) of dantrolene inhibition of domain unzipping is identical with that of spontaneous Ca2+ leak in failing SR. These results indicate that dantrolene reverses domain unzipping and re-stabilizes the zipped configuration of the domain switch, thereby inhibiting Ca2+ leak.
Figure 4. Stern-Volmer plots of the fluorescence quenching data.
A. The Stern-Volmer plots of fluorescence quenching in the presence of various concentrations of dantrolene. Domain unzipping was produced by adding 100 μmol/L DPc10 to normal SR.
B. Concentration dependence of dantrolene inhibition of domain unzipping (K’Q/ KQ) in DPc10-added and untreated normal SR. Note that dantrolene reduced the K’Q/ KQ value (where K’Q is the quenching constant in DPc10-added normal SR and KQ is the quenching constant in normal SR) in DPc10-added normal SR in a concentration dependant manner (IC50: 0.3 μmol/L), while dantrolene had no appreciable effect in untreated normal SR.
C. The slope of Stern-Volmer plots of fluorescence quenching as a function of various concentrations of dantrolene added to failing SR.
D. Concentration dependence of dantrolene inhibition of K”Q/ KQ (where K”Q is the quenching constant in failing SR and KQ is the quenching constant in normal SR) in failing SR. Note that dantrolene reduced on the K”Q/ KQ in failing SR in a concentration dependant manner (IC50: 0.3 μmol/L). Each datum-point is a mean of 4 to 6 measurements ± SD.
Effects of dantrolene on Ca2+ transient, cell shortening, Ca2+ spark and membrane potential of normal and failing cardiomyocytes
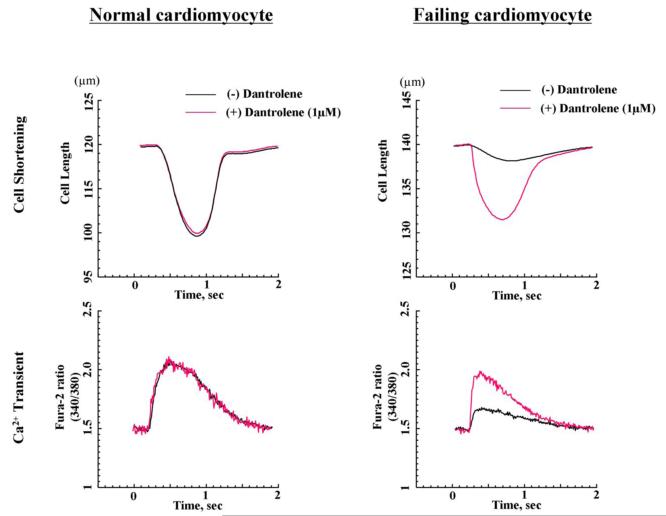
In order to investigate the effect of dantrolene on the isolated normal and failing cardiomyocytes, we measured both Ca2+ transient and cell shortening simultaneously in these cardiomyocytes. As shown in Figure 5A and as summarized in Table 2, Ca2+ transient and cell shortening in the cardiomyocytes isolated from the pacing-induced failing dog hearts were deteriorated, compared with those in normal cardiomyocytes. Both Ca2+ transient and cell shortening were partially restored towards normal by dantrolene (1.0 μmol/L). In normal cardiomyocytes, there was no detectable change in Ca2+ transient and cell shortening after addition of dantrolene.
Figure 5. Effect of dantrolene on Ca2+ transient and cell shortening.
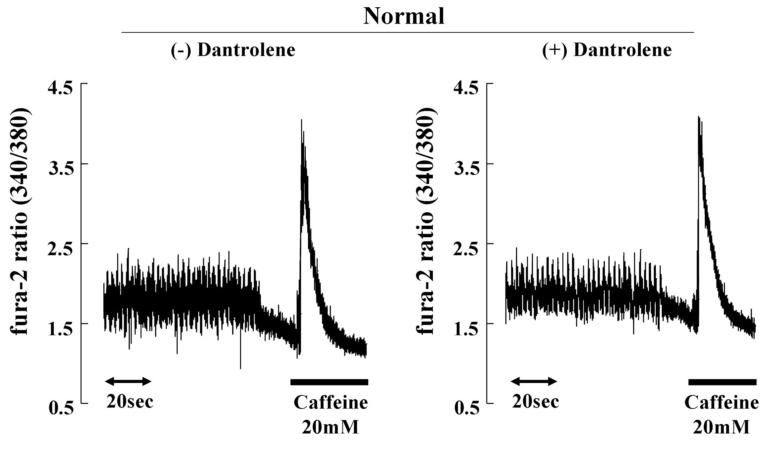
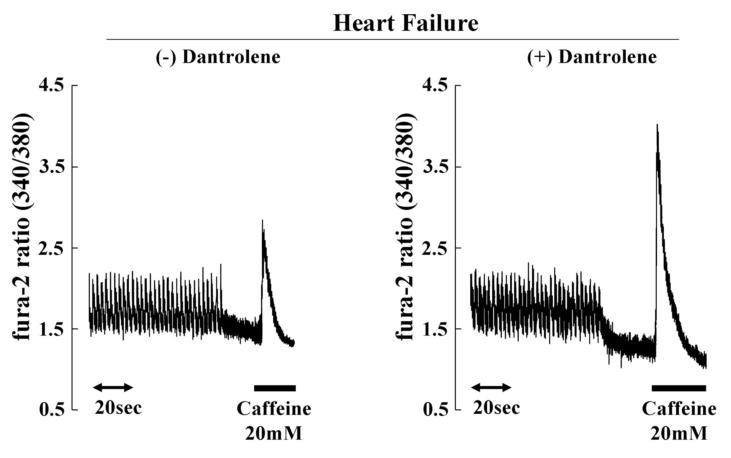
A. Ca2+ transient and cell shortening in normal and failing cardiomyocytes in the presence or the absence of dantrolene (1.0 μmol/L). B. Ca2+ transients before and after addition of caffeine in normal and failing cardiomyocytes. Caffeine-induced increase in Ca2+ transients reflecting SR Ca2+ load was measured after a train of stimuli at 0.5 Hz by rapidly switching the perfusing solution to the one containing 20 mmol/L caffeine for 5 to 6 seconds. Note that the peak Ca2+ transient after addition of caffeine(indicative of SR Ca2+ load), was higher in normal cardiomyocyte than in failing cardiomyocyte, and that dantrolene treatment ameliorated the SR load by inhibiting SR Ca2+ leak only in failing cardiomyocyte.
Table 2.
Effect of dantrolene on cell shortening and Ca2+ transient in normal and failing cardiomyocytes.
| Number of cell (n) |
Cell shortening (%) |
Peak of Ca2+ transient (%) |
Time from peak to 80% decline (x 10-2sec) |
|
|---|---|---|---|---|
| Normal (n=6) | ||||
| Untreated | 30 | 8.7±1.2 | 30.2±4.5 | 83.9±22.6 |
| Dantrolene (1 μM) | 30 | 8.6±2.3 | 30.5±6.6 | 85.7±12.7 |
| Heart Failure (n=4) | ||||
| Untreated | 30 | 1.7±0.5 a | 14.5±3.6 a | 126.9±18.9 a |
| Dantrolene (1 μM) | 38 | 5.8±2.6 b | 26.2±7.8 b | 99.2±16.9 b |
Cell shortening: max % cell-length change from diastolic (baseline) level. Peak of Ca2+ transient: max % fluorescence change from diastolic (baseline) level. Each datum point represents the mean ± SD. Number of preparations is shown in the parentheses. At least 4 cells were evaluated in each preparation. Unpaired t-test was employed to determine the statistical significance of the data (p value).
p<0.01 vs untreated (normal)
p<0.01 vs untreated (heart failure)
Since intracellular peak Ca2+ transient strongly depends on the SR Ca2+ loading, SR Ca2+ content was assessed by measuring peak Ca2+ transient after addition of caffeine. In normal cardiomyocytes, dantrolene was without effect on peak Ca2+ transient after addition of caffeine (Figure 5B). In failing cardiomyocytes, peak Ca2+ transient after addition of caffeine was reduced, whereas it was restored towards normal in the presence of dantrolene (1 μmol/L) (Figure 5C). These findings suggest that prevention of Ca2+ leak by dantrolene is effective to increase cardiomyocyte contractility by increasing SR Ca2+ loading.
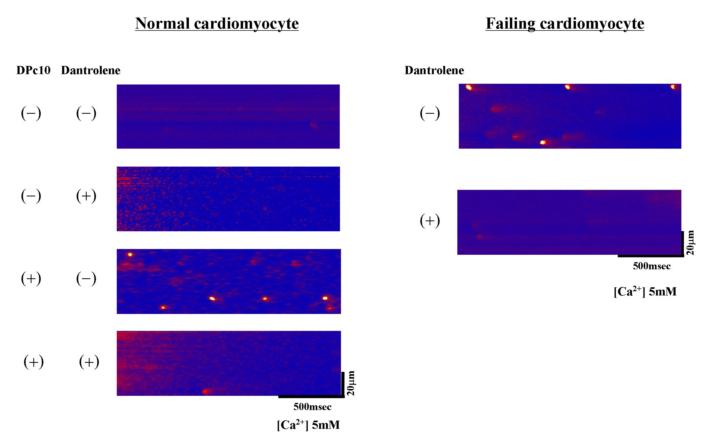
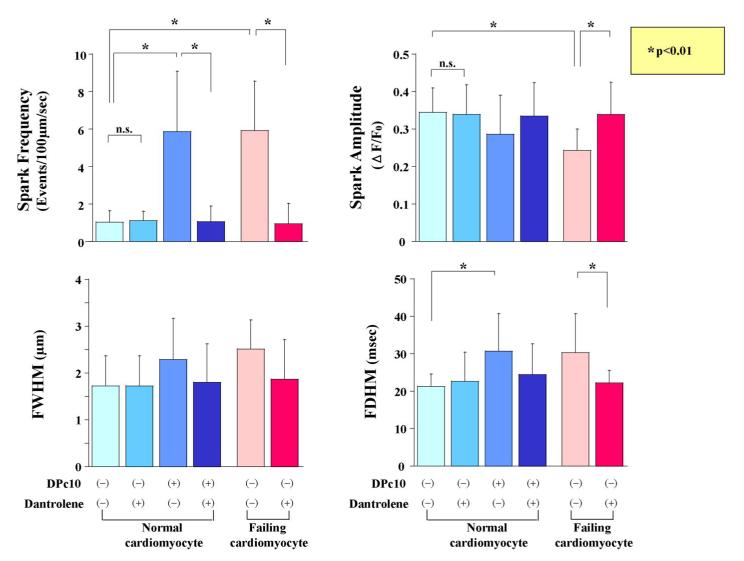
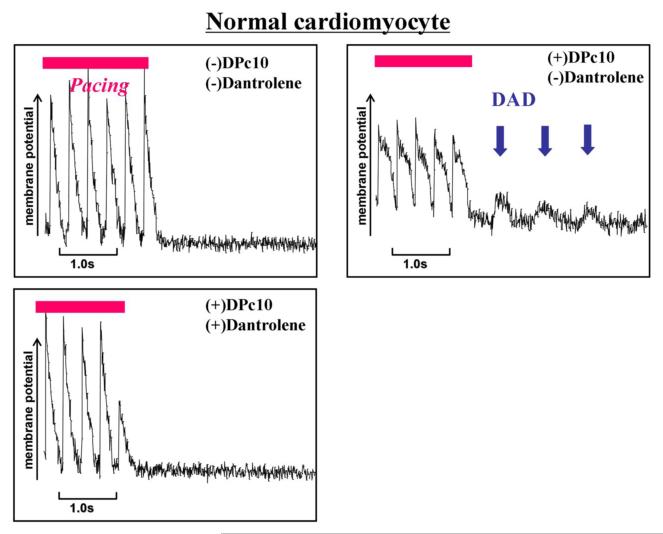
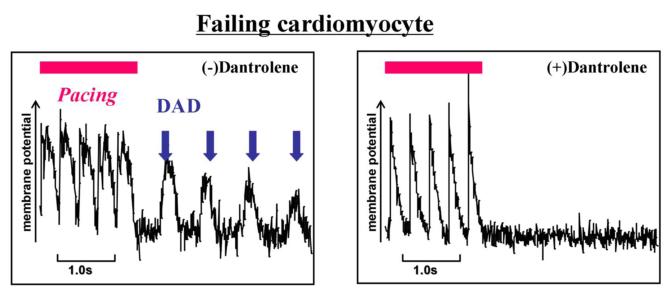
To further explore the effects of dantrolene on the SR Ca2+ release mechanism, we investigated elementary Ca2+ release events (Ca2+ sparks) in normal and failing cardiomyocytes (Figure 6A, 6B). In normal cardiomyocytes, spontaneous Ca2+ sparks occurred at a very low frequency. In DPc10-introduced cardiomyocytes, however, the frequency of spontaneous Ca2+ sparks significantly increased, but it was reduced to a normal level in the presence of dantrolene (1 μmol/L). In cardiomyocytes from failing hearts, the spark frequency was markedly increased as in the DPc10-introduced normal cardiomyocyte, whereas it decreased to normal level in the presence of dantrolene (1 μmol/L). We further investigated the effect of dantrolene on the membrane potential of isolated cardiomyocytes. In normal cardiomyocytes, no fluctuation of membrane potential was observed even in the presence of 100 nM forskolin after 3Hz pacing (Figure 7A), while the fluctuations of the membrane potential, which seem to represent delayed after depolarization, were observed in the DPc10-incorporated cardiomyocytes (Figure 7A). However, the fluctuations of membrane potential seen in the DPc10-incorporated cardiomyocytes disappeared after addition of dantrolene (Figure 7A). As shown in Figure 7B, the spontaneous fluctuations of membrane potential were observed in failing cardiomyocytes, while they were prevented from occurring in the presence of dantrolene (1 μmol/L). The spontaneous fluctuations of membrane potential seen in DPc10-incorporated or failing cardiomyocytes were not induced by lower pacing rate:1 Hz (not shown), but induced only after the application of higher pacing rate:3 Hz.
Figure 6. Effect of dantrolene on diastolic Ca2+ sparks in DPc10-incorporated normal cardiomyocytes and in failing cardiomyocytes.
A. Representative line-scan images of Ca2+ sparks. B. Bar graphs summarize the various values on Ca2+ spark: frequency, amplitude, FWHM (full width at half maximum), FDHM (full duration at half maximum), for the same groups of cells. Each datum point represents the mean ± S.D. The number of cells for each group is 20 to 30.
Figure 7. Effect of dantrolene on the post-pacing fluctuation of membrane potential (delayed after depolarization) in DPc10-incorporated normal cardiomyocytes and in failing cardiomyocytes.
Pacing was performed at higher pacing rate (3Hz) in the presence of forscolin (100 nM) for a few seconds and stopped. Post-pacing fluctuation of the membrane potential were observed in DPc10-induced or failing cardiomyocytes, but not in normal cardiomyocytes. Representative line-scan plots of membrane potential recorded in normal cardiomyocytes (A) and in failing cardiomyocytes (B).
Discussion
Defective inter-domain interactions within RyR2 play a key role in the abnormal channel gating of RyR2 in failing hearts (6). Therefore, correction of the defective inter-domain interaction seems to be a new therapeutic strategy against heart failure and possibly cardiac arrhythmia. In our recent reports (10, 11), we have shown that both K201 and antioxidant edaravone prevents Ca2+ leak through RyR2 by correcting the defective inter-domain interaction within RyR2, in turn improving intracellular Ca2+ handling and cardiac function, and attenuating LV remodeling during development of pacing-induced heart failure.
The most important aspect of the present study is the finding that dantrolene, which might have been thought to be a skeletal muscle-specific muscle relaxant, works on the cardiac muscle as well. It is used to treat MH by correcting defective inter-domain interaction within RyR1. Dantrolene is now found to bind to domain 601-620 of RyR2 and correct defective inter-domain interaction within RyR2 in failing hearts. This in turn inhibits spontaneous Ca2+ leak/ Ca2+ sparks, and improves cardiomyocyte function in failing hearts. This conclusion is based on the following findings. 1) DPc10, which is the synthetic peptide used as a domain switch unzipping agent, induced domain unzipping (Figure 4A, 4B). Both DPc10-induced domain unzipping and the subsequent Ca2+ leak were inhibited by dantrolene. Dantrolene inhibition of domain unzipping and that of Ca2+ leak showed an identical concentration dependence (IC50:0.3μmol/L) (Figures 3 and 4). 2) In failing SR, the RyR is already in an unzipped state, as it occurs in normal SR after treatment with DPc10. Dantrolene restored the zipped state in a concentration dependant manner (IC50:0.3μmol/L) (Figure 4D). Similarly, dantrolene inhibited spontaneous Ca2+ leak from failing SR with the same concentration dependence (IC50:0.3μmol/L) (Figure 3C). Dantrolene also inhibited the cAMP-induced Ca2+ leak in normal SR. 3) Incorporation of DPc10 produced diastolic Ca2+ sparks in normal cardiomyocytes, while these sparks were inhibited by dantrolene. In failing cardiomyocytes, the magnitude of the intracellular Ca2+ transient decreased by 52% and its decay time was prolonged by 51%, but these abnormalities were normalized by 1μmol/L dantrolene. 4) The SR Ca2+ content, assessed by rapid application of caffeine, was reduced in failing cardiomyocytes, but it was restored by dantrolene, suggesting that the inhibition of SR Ca2+ leak by dantrolene increased SR Ca2+ content and thereby improved Ca2+ transient and cell shortening (Figure 5A, 5B). 5) In the failing cardiomyocytes, diastolic Ca2+ sparks were observed, but they were eliminated almost completely by 1 μmol/L dantrolene. 6) Importantly, less than 1 μmol/L dantrolene had no appreciable effect on normal SR and cardiomyocyte functions in all experiments. It is also reported that dantrolene restores normal inter-domain interactions within RyR1 (viz. zipped state) in DP4-treated RyR1 channel which is analogous to MH channel (8). Taken together, all of these data strongly suggest that the stable inter-domain interaction of regulatory domains within RyR1 or RyR2 is an essential requirement for normal channel function. Defective inter-domain interaction (viz. domain unzipping) causes channel dysfunction in RyR1 of MH and in RyR2 of failing heart; thus correction of the defective inter domain interaction restores normal channel gating and hence prevents diseased conditions.
Recently, Yang et al (12) reported that DPc10 lowered the [Ca2+] threshold for spontaneous Ca2+ release in permeabilized cardiomyocytes. That is, DPc10 mimicked the abnormal channel gating seen in CPVT/ARVC2. Consistent with these data, abnormal diastolic Ca2+ sparks were observed in DPc10-incorporated cardiomyocytes, while dantrolene suppressed the diastolic Ca2+ sparks (Figure 6A, B). Moreover, in DPc10-incorporated cardiomyocytes, the fluctuation of membrane potential, which represents delayed afterdepolarization (DAD), was observed, and dantrolene prevented it from occurring (Figure 7A). Thus, the restoration of the domain interaction from defective unzipped state to normal zipped state by dantrolene may be a new strategy for the treatment of polymorphic VT and post-translationally developed heart failure as well.
Although it is widely recognized that dantrolene has an appreciable effect on RyR1, there is a controversy as to whether dantrolene modulates channel gating of RyR2. Fruen and colleagues (13) did not find any dantrolene inhibition of Ca2+ release in the RyR2 expressed in CHO cells. In contrast, it is reported that dantrolene improved the inotropic response to norepinephrine in human failing heart (14), and reduced Ca2+ oscillations, thereby decreasing the magnitude of diastolic intracellular [Ca2+] in post-infracted rat myocardium (15). The present finding that dantrolene inhibited cAMP-induced Ca2+ leak may partly explain the improvement of the beta-adrenergic responsiveness by dantrolene. Taken together, dantrolene appears to have much more appreciable effect on Ca2+ handling in failing (Ca2+ overloaded) hearts than in normal hearts, because of its inhibition of diastolic Ca2+ release that can be seen only in failing hearts. Dantrolene was found to enhance the Ca2+ uptake rate in rat cardiac SR, but also showed mild negative inotropic effect in parallel with a decrease in peak Ca2+ transient (16). In that study, however, the concentration of dantrolene was 50 μmol/L, 50 times higher than that used in the present study. In the present study, dantrolene (0.1-1 μmol/L) had no effect on the SR Ca2+ uptake time course, Ca2+ transient, and cell shortening in normal hearts. Low concentrations of dantrolene (0.1-1 μM) used in this study seem to have no significant effect on SR Ca2+ ATPase and Ca2+-induced Ca2+ release in normal hearts.
The present data also showed that dantrolene inhibited DPc10-induced Ca2+ leak in normal RyR2, and spontaneous Ca2+ leak in failing RyR2, whereas it had no appreciable effect on normal RyR2, in which inter-domain interactions between N-terminal and central regulatory domains are still in the zipped state. These findings suggest that the effect of dantrolene on channel gating depends on the conformational state of the regulatory domains. That is, dantrolene may be effective for stabilizing RyR2 only in the unzipped state, but not in the zipped state. The report that [3H]dantrolene-binding to the rabbit RyR2 could be seen only in particular conditions (in the presence of a very high concentration of EGTA) (17) supports the idea that dantrolene binding to RyR2 is indeed dependent on a particular conformational state of RyR2 that takes place in disease conditions.
To identify the dantrolene binding site within the RyR2, we screened recombinant constructs corresponding to the region from the N-terminus to the residue 2750 using the QCM assay, and found that only the recombinant construct corresponding to aa494-1000 and the synthetic peptide corresponding to aa601-620 bound with dantrolene. This indicates that the aa601-620 region of RyR2 represents the site of dantrolene binding. However, we cannot exclude the possibility that there might be another drug binding region in the carboxyl half of the RyR2, because our screening did not extend beyond the residue 2750. We cannot also exclude the possibility that there might be additional drug binding sites, with a low affinity of binding, even in the N-terminal half, because some of the recombinant constructs may have failed to retain the native tertiary structure required for high-affinity drug binding. Altered intracellular Ca2+ handling is one of the causative mechanisms in the generation of lethal arrhythmia in the patients with heart failure and CPVT. Diastolic Ca2+ leak from SR via RyR2 will initiate DAD and triggered activity, leading arrhythmia. Therefore, stabilization of RyR2 may be a novel therapeutic strategy against heart failure and CPVT (10). The proposed molecular mechanism by which dantrolene corrects the defective channel gating in diseased RyR2 is summarized in the Figure 8A and 8B.
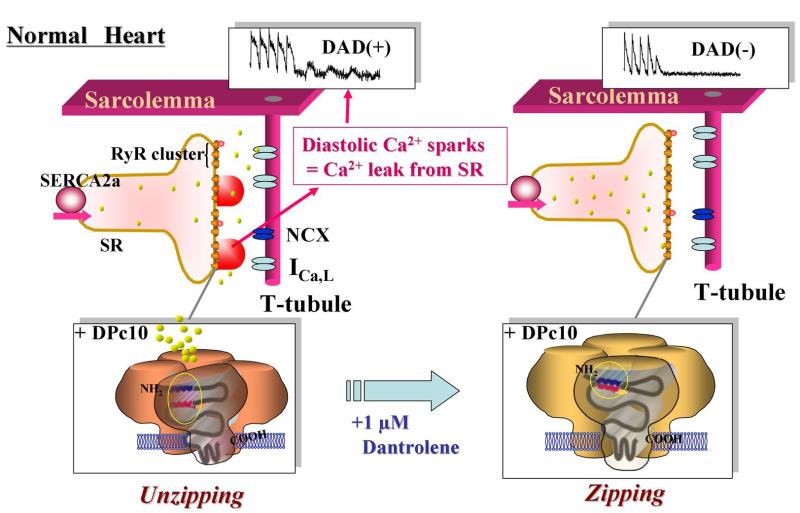
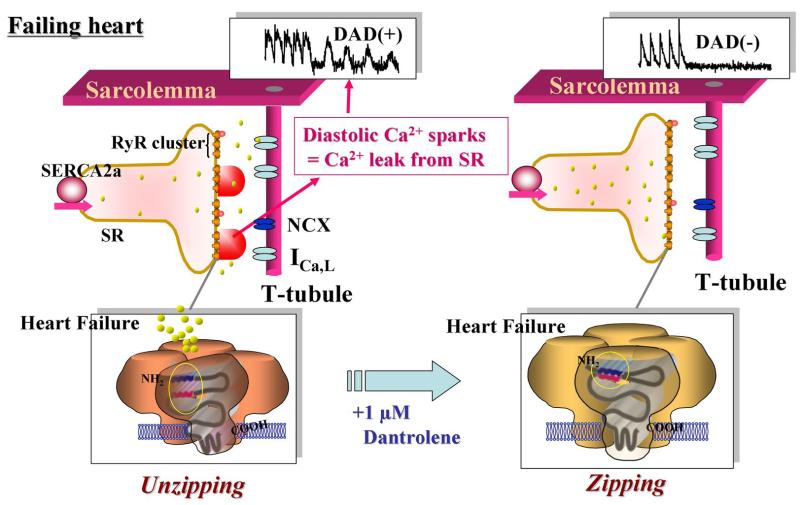
Figure 8. Schematic illustration of the effects of dantrolene on abnormal intracellular Ca2+ handling and membrane potential in cardiomyocytes.
A. In normal cardiomyocytes, domain unzipping between the N-terminal and central domains occurs upon incorporation of DPc10, thereby causing aberrant diastolic Ca2+ sparks. These Ca2+ sparks subsequently induce the delayed afterdepolarization (DAD) caused by inward Na+ current via Na+-Ca2+ exchange. Dantrolene inhibits all of these Ca2+ handling abnormalities. B. In failing cardiomyocytes, domain unzipping has already taken place, and it induces aberrant diastolic Ca2+ sparks and DAD. Dantrolene inhibits all of the Ca2+ handling abnormalities.
In conclusion, defective interactions of the regulatory domains in RyR2 seem to play a key role in the abnormal Ca2+ channel functions of RyR2 seen in the failing cardiomyocytes, such as increased Ca2+ spark frequency and DAD. The present finding that the defective domain interaction is corrected by dantrolene provides one with a new clue for the development of therapeutic strategy against heart failure and possibly lethal arrhythmia.
Supplementary Material
Acknowledgments
This work was supported by grants-in-aid for scientific research from The Ministry of Education in Japan (grant Nos. 18390234, 18659228 to MY, 18590777 to TY, 18591706 to SK) and the National Heart, Lung and Blood Institute (HL072841 to NI).
Abbreviations and Acronyms
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- MH
malignant hyperthermia
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- ARVC2
arrhythmogenic right ventricular cardiomyopathy type 2
- MCA
methylcoumarin acetate
- DAD
delayed afterdepolarization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None declared
References
- 1.Yano M, Yamamoto T, Ikemoto N, Matsuzaki M. Abnormal ryanodine receptor function in heart failure. Pharmacol Ther. 2005;107:377–391. doi: 10.1016/j.pharmthera.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Mickelson JR, Louis CF. Malignant hyperthermia: Excitation-contraction coupling, Ca2+ release channel, and cell Ca2+ regulation defects. Physiol. Rev. 1996;76:537–592. doi: 10.1152/physrev.1996.76.2.537. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, El-Hayek R, Ikemoto N. Postulated role of interdomain interaction within the ryanodine receptor in Ca2+ channel regulation. J Biol Chem. 2000;275:11618–11625. doi: 10.1074/jbc.275.16.11618. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T, Ikemoto N. Spectroscopic monitoring of local conformational changes during the intramolecular domain-domain interaction of the ryanodine receptor. Biochemistry. 2002;41:1492–1501. doi: 10.1021/bi015581z. [DOI] [PubMed] [Google Scholar]
- 5.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, Biasi MD, Wehrens XHT, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, Ohkusa T, Ikeda Y, Kobayashi S, Ikemoto N, Matsuzaki M. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- 7.Paul-Pletzer K, Yamamoto T, Bhat MB, Ma J, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, Ma J, Parness J. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem. 2002;277:34918–34923. doi: 10.1074/jbc.M205487200. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- 9.Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, Kobayashi S, Hisamatsu Y, Yamamoto T, Kohno M, Noguchi N, Takasawa S, Okamoto H, Matsuzaki M. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 10.Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Kohno M, Matsuzaki M. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 11.Yano M, Okuda S, Oda T, Tokuhisa T, Tateishi H, Mochizuki M, Noma T, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation. 2005;112:3633–43. doi: 10.1161/CIRCULATIONAHA.105.555623. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Ikemoto N, Lamb GD, Steele DS. The RyR2 central domain peptide DPc10 lowers the threshold for spontaneous Ca2+ release in permeabilized cardiomyocytes. Cardiovasc Res. 2006;70:475–685. doi: 10.1016/j.cardiores.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhao F, Li P, Chen SRW, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ channel; Molecular mechanism and isoform selectivity. J Biol Chem. 2001;276:13810–13816. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- 14.Meissner A, Min JY, Haake N, Hirt S, Simon R. Dantrolene sodium improves the force-frequency relationship and beta-adregenic responsiveness in failing human myocardium. Eur J Heart Fail. 1999;1(2):177–186. doi: 10.1016/s1388-9842(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 15.Min JY, Meissner A, Feng X, Wang JF, Simon R, Morgan JP. Dantrolene: effects on abnormal intracellular Ca2+ handling and inotropy in postinfarcted rat myocardium. Eur J Pharmacol. 2003;471:41–47. doi: 10.1016/s0014-2999(03)01816-8. [DOI] [PubMed] [Google Scholar]
- 16.Meissner A, Szymanska G, Morgan JP. Effects of dantrolene sodium on intracellular Ca2(+)-handling in normal and Ca2(+)-overloaded cardiac muscle. Eur J Pharmacol. 1996;316:333–42. doi: 10.1016/s0014-2999(96)00678-4. [DOI] [PubMed] [Google Scholar]
- 17.Paul-Pletzer K, Yamamoto T, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, Ma J, Parness J. Probing a putative dantrolene-binding site on the cardiac ryanodine receptor. Biochem J. 2005;387:905–909. doi: 10.1042/BJ20041336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.