Abstract
The levels of blood glucose (BG) in humans tend to increase with age deviating from the norm specified for the young adults. Such elevation is often considered as a factor contributing to an increase in risks of disease and death. The proper use of intervention strategies coping with or preventing consequences of BG elevation requires understanding the roles of external forces and intrinsic senescence in this process. To address these issues, we performed analyses of longitudinal data on BG collected in the Framingham Heart Study using methods of descriptive statistics and statistical modeling. The approach allows us to separate effects of persistent external disturbances from “normal” aging related changes due to the senescence process. We found that the BG level corresponding to the lowest mortality risk tends to increase with age. The changes in the shape of the mortality risk with age indicate the aging related decline in resistance to stresses affecting the BG level. The results show that analyzing longitudinal data using advanced methods may substantially increase our knowledge on factors and mechanisms responsible for aging related changes in humans.
Keywords: physiological norm, aging, allostasis, mortality risk, stress resistance, longitudinal data
1. Introduction
Glucose utilization is the core metabolic process in most living organisms. To monitor and control this process in humans, several indices have been introduced. One of them, the fasting blood glucose (FBG), is routinely measured in clinical facilities as well as in various studies of aging, health, and longevity. It represents glucose concentration in a blood (or serum) sample taken after the overnight fast. Its high levels (hyperglycemia) indicate improper glucose homeostasis and are associated with a number of pathological conditions. An impaired (elevated) FBG is one of the key indicators of metabolic disorder leading to type 2 diabetes mellitus (T2DM) and accelerated onsets of other chronic conditions, including cardiovascular and renal disorders.
Decreased glucose tolerance in aging individuals is noted in numerous epidemiological studies (Sinclair and Finucane, 2001). In adults aged 50 years and older the levels of FBG rise by 0.06 mmol/l per decade (Scheen, 2005). This increase is a result of increase in insulin resistance (mainly due to life-style loads) and decline in insulin secretion (to be believed as inevitable consequences of aging) (Chang and Halter, 2003). To what extent can an increase in FBG be considered as “normal”, i.e., resulted from inevitable senescence-related changes? And what is the contribution of preventable external conditions (e.g., inadequate nutrition, inappropriate life style, stresses of life, or other persisting harmful exposures) to increase in insulin resistance? How do these two aging-associated components interact to affect age-specific mortality risk? How should one maintain the level of FBG during the life course to maximize chances of exceptional survival? Addressing these questions requires analyzing the age-trajectories of both the normal and pathological (allostatic) components of the FBG levels in connection with the all-cause mortality risk function.
Although the aging-related changes in the FBG levels have been intensively discussed in scientific literature for more than 40 years (Andres, 1971; 1981; Elahi et al., 2002; Muller et al., 1996), no systematic attempts have been performed either to separately evaluate effects of allostatic response and “normal” changes on the observed age trajectories of the FBG, or to evaluate the dynamic contribution of each component to the all-cause mortality. Furthermore, it is often argued that decision on normality or abnormality of the FBG levels should be based on distribution of the values of this index in a given population. More adequate characterization can be made from longitudinal studies when subjects with different levels of FBG are followed for development of cardiovascular diseases and death (Andres, 1981).
In this paper, we develop an approach, which allows for addressing some of the raised questions and evaluating allostatic and “normal” components of changes in FBG from longitudinal data collected in the Framingham Heart Study (FHS). We start with descriptive analyses of longitudinal data on BG to develop insights and ideas concerning the dynamic properties of this variable. Then, more specific properties dealing with effects of allostatic adaptation, the age dependence of BG norms, as well as the decline in stress resistance are investigated using methods of statistical modeling described in Yashin et al. (2007).
2. Data and Method
The Framingham Heart Study Data
The Framingham Heart Study (FHS) was set up more than 60 years ago to evaluate the relationships between potential risk factors determined in individuals, who had not yet developed CHD or suffered a heart attack or stroke, and the subsequent development of the disease or death. In the 1948, the study recruited 5209 non-institutionalized white subjects (2336 males and 2873 females, with 993 surviving participants as of February 28, 1999) between the ages of 28 and 62 in the town of Framingham, Massachusetts. A total of 5128 of 5209 these participants were described as free of “overt CHD.” Since then, the participants of the original cohort have been reexamined biennially for a physical examination, laboratory tests, a detailed medical history, and an extensive testing for cardiovascular risk factors.
The level of BG is identified and widely accepted CHD risk factor. This variable is measured in the Framingham study together with age, sex and other physiological indices (Govindaraju et al., 2008; Terry et al., 2005). In this paper, we considered the data on blood glucose using 19 exams (i.e., 1–4, 6, 8–10, 13–23) of the FHS study. The ideal approach to studying glucose metabolism would involve a glucose tolerance testing and measuring fasting glucose at each examination, but at the time the study was planned, this did not appear practicable (Dawber, 1980). Glucose tolerance testing was not systematically performed in the study and in the most of exams blood glucose measurements were casual (i.e., randomly fasting or non-fasting) (Kannel et al., 1979; Port et al., 2006). To get values of random blood glucose closer to fasting blood glucose levels, the measurements were taken at the end of many hours of examination. The analyses performed in Port et al. (2006) suggest the possibility of using random glucose levels as a surrogate measure for fasting blood glucose in this study. Taking this into account, we equated measurements of random glucose with those of fasting glucose in the analyses and used the term “blood glucose” (BG) for all these measurements to avoid the confusion. The amount of glucose was determined in the subject’s whole blood, in milligrams per 100 milliliters.
Representation of effects of allostatic adaptation in individual age trajectory of BG
The concept of allostasis was originally introduced by Sterling and Eyer (1988) to describe how the cardiovascular system adjusts to resting and active states of the body. Then, this notion became popular to describe the adaptive response of an organism to the environmental and psychosocial situations, commonly referred to as “stresses.” Allostasis means that an organism maintains “stability” of some functions by changing the others. Unlike homeostasis, allostasis refers to the organism’s ability to cope physiologically, behaviorally, and emotionally with specific environmental challenges while maintaining the regulatory control of the homeostatic systems that operate within narrow parameters (McEwen and Wingfield, 2003). The body pays the price (allostatic load) for being forced to adapt to persistent environmental and psychosocial challenges. The allostatic load depends on how inefficient the response is, and/or how many stressful challenges an individual experiences. Over time, allostatic load can accumulate and the overexposure to neural, endocrine, and immune stress mediators can have adverse effects on various organ systems leading to the development of a disease and increasing mortality risk. Thus, studying effects of allostatic adaptation provides us with important indicators of physiological pre-disease pathways.
The effects of allostatic adaptation contribute to the individual age trajectories of BG (and other physiological indices) and have to be reflected in the mathematical model describing age dynamics of this index. Following Yashin et al. (2007), we describe dynamic changes in the BG using the diffusion type stochastic differential equations:
| (1) |
Here Yt is the value of BG level at age t in an arbitrarily chosen individual, describes the effect of allostatic adaptation, which individual’s BG is forced to follow by homeostatic forces. In the absence of external disturbances , where ft is the optimal age trajectory of the BG, minimizing mortality risk. The strength of homeostatic forces is characterized by the negative feedback coefficient, at. The coefficient bt characterizes the contribution of external disturbances described by a Wiener process, Wt. The random variable Y0 describes the variability of the initial value of physiological index, which is assumed to be independent of Wt for each t ≥ 0. The introduction of and at into the model facilitates the biological interpretation of the results of statistical analyses of longitudinal data using such a model. The discrete-time versions of this model used in data analyses are described in the Appendix.
Normal BG values and conditional hazard
The value of persistent deviation of the BG, Yt, from the norm depends on deviation of allostatic component from the “normal” age trajectory of the BG, ft, where the “norms” are defined as the values of BG minimizing all-cause-mortality risk for a given age, and are estimated using methods of statistical modeling applied to the FHS data. The approach exploits the fact that mortality risk considered as a function of BG is J- or U shaped. We use the modification of the quadratic hazard model, which explicitly incorporates the notion of the normal physiological state into the quadratic hazard (Yashin et al., 2007). The function ft is defined above. The term shows the total mortality rate for individuals whose BG followed the optimal trajectory ft. It is convenient for evaluating the gain in life expectancy which could be reached if the aging dependent changes in BG will be under control (i.e., will be kept around the norm). An important advantage of this approach is that changes in the shape of this function with age can be evaluated from the data. The coefficient μt shows how the steepness of the quadratic hazard curve changes with age: when μt is an increasing function, the U-function of mortality risk is getting narrower with age.
Since the increments of mortality risk resulting from the deviations of the same magnitude to the right and to the left from the norm may differ, it is convenient to consider a non-symmetric form of the quadratic hazard:
| (2) |
Here I(.) denotes the indicator function, which is 1 when the condition in the parentheses is true and 0 otherwise.
The coefficient shows how the steepness of the quadratic hazard curve located to the left from the optimal (normal) point changes with an increasing age. An increase in this function with age indicates the decline in the resistance to stresses associated with deviations of the physiological index to the left from its age-specific “norm” with age. The coefficient shows how the steepness of the quadratic hazard located to the right from the optimal point changes with age. An increase in this function with age indicates the decline in the resistance to stresses associated with deviations of the physiological index to the right from its age-specific “norm” with age.
3. Results of descriptive analyses
Average age trajectories of BG
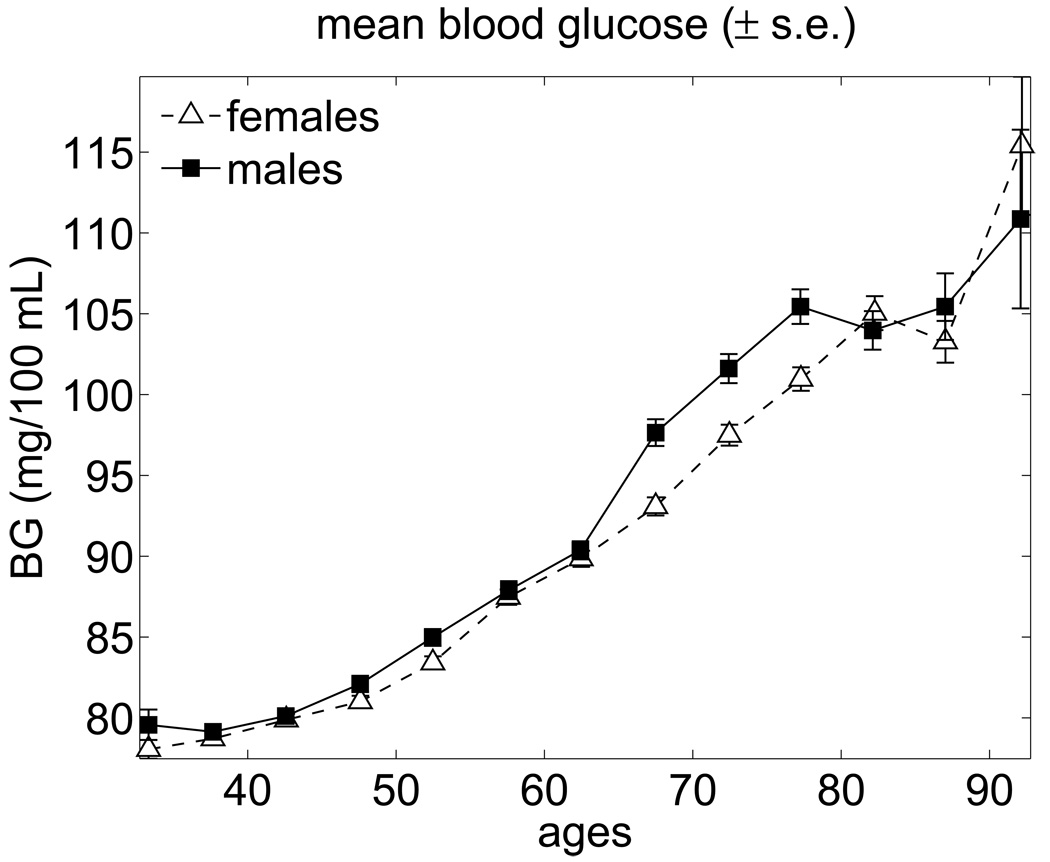
The average age trajectories of BG observed in the pooled sample of the FHS participants are shown in Fig. 1 for males and females. This figure shows that the average trajectories of BG monotonically increase with age until 77 years for males and 82 years for females and are virtually the same for males and females with slightly higher values for males. After these ages, the BG concentration slightly declines and then increases after the age of 87 years (see Barbieri et al., 2001, where similar effect was reported). It is important to note that the BG trajectories shown in Fig. 1 do not represent the average biological changes in the BG concentration. This is because compositional changes due to the mortality selection may affect the dynamic properties of these curves. Since the BG level is an established risk factor, individuals whose BG levels are too high (i.e., substantially deviate from the “normal” level) have high chances of dying first. Hence, these individuals drop out of the averaging procedure affecting the average BG age trajectories. Thus, the curves shown in Fig. 1 reflect the effects of both the biological adaptation of the BG level to other aging-related changes developing in a human body and changes in the population composition due to the mortality selection.
Fig. 1.
Average trajectories of blood glucose for females and males in the FHS (pooled data for exams 1–25).
Making biological changes visible
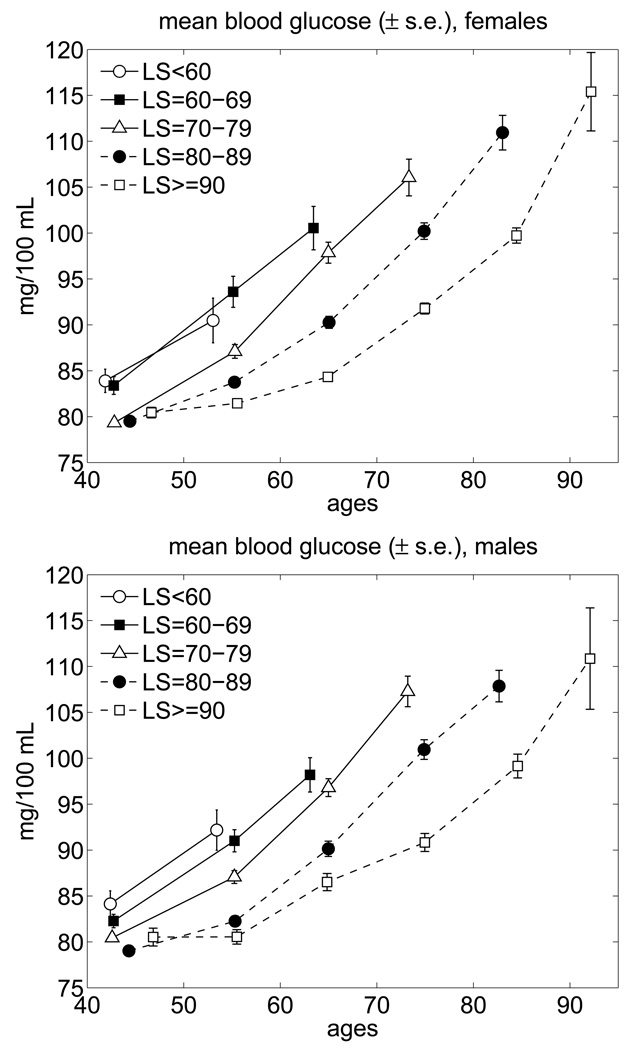
The biological changes can be made visible if one is able to consider the BG trajectories for individuals died at different ages. Fig. 2 shows the average age trajectories of BG for the FHS sub-cohorts died in different age intervals. Individuals living relatively short lives (SL) are classified into five groups (i.e., died before the age of 60 years, between 60 and 69, 70 and 79, and 80 and 89 years of age). The long lived (LL) individuals are those whose age at death exceeded 90 years. Until the age of 90, the average LL trajectories describe the average biological changes in BG among these individuals.
Fig. 2.
Average trajectories of blood glucose for long-lived and short-lived females and males in the FHS (pooled data for exams 1–25; “LS” means lifespan).
One can see from this figure that for all groups the average age trajectories are the increasing functions of age. All trajectories for the SL individuals are higher than that for the LL ones for both genders. An important finding from these analyses is that the average levels of BG in the LL individuals increases relatively slow till the ages of about 55–60 years. After these ages, an increase in the BG continues with a much higher rate.
T2DM makes the most difference
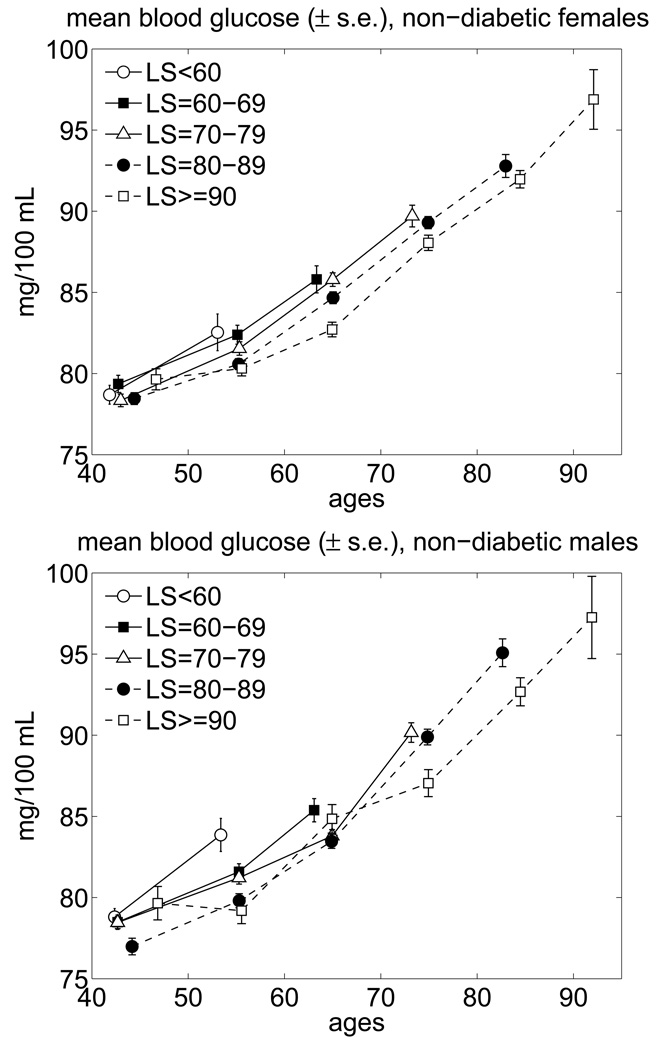
Note that the high average age trajectories of BG in the SL and LL individuals shown in Fig. 2 are likely to reflect the contribution of individuals with Type II Diabetes Mellitus (T2DM), which have a high BG level by the definition of this disease. To evaluate this contribution to the age pattern of average BG, we removed individuals having T2DM from the averaging procedure. The results are shown in Fig. 3.
Fig. 3.
Average trajectories of blood glucose for long-lived and short-lived non-diabetic females and males in the FHS (pooled data for exams 1–25; “LS” means lifespan).
One can see from this figure that the exclusion of individuals with T2DM decreases the mean levels of BG especially at advanced ages and makes the average BG trajectories of the LL and SL individuals closer to each other. The latter indicates that the factors independent of the level of the BG concentration are the major contributors to the differences in life spans (LSs) for the remaining individuals. This suggests increasing the dimension of the risk factors included in the description of the risk. One can also see that the average age trajectories for the SL and LL non-diabetic individuals increase with age. This increase, however, is within the range considered as normal FBG values. We hypothesize that the age trajectories of BG for the non-diabetic part of the LL individuals in the Framingham cohort may be close to the FBG curves which minimize the total mortality risk. To test this assumption, we applied the extended version of the stochastic process model of human mortality and aging (Yashin et al., 2007) to the FHS data on BG.
4. Results of more advanced statistical analyses
We applied the discrete-time variant of equation (1)–equation (2) (see description in Appendix) to the analysis of data on BG for females and males in the FHS. The model allows one to evaluate age-trajectories of all coefficients involved in equations (1)–(2) including the effects of allostatic adaptation,, the physiological norms, ft, the baseline hazards, the coefficients characterizing the resistance to stresses (deviations from the norm to the right and to the left) with age and test the hypotheses about the significance of their dependence on age.
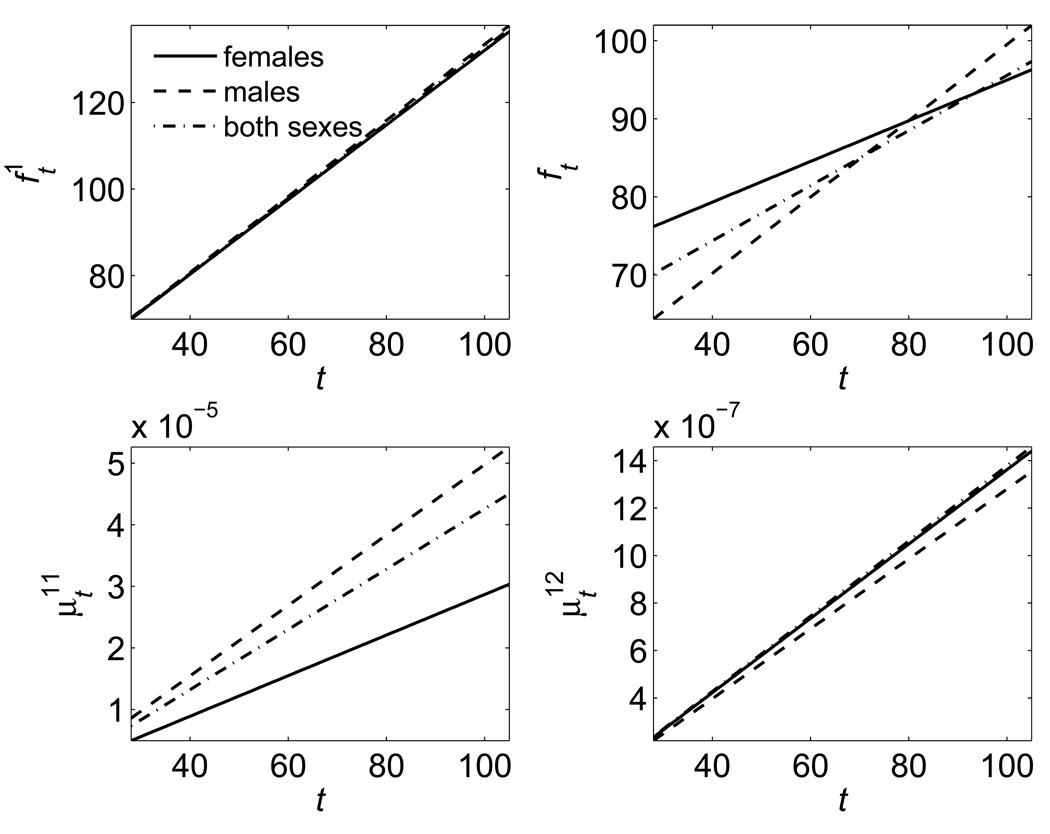
Fig. 4 shows the graphs of respective estimates for males and females separately and for both sexes together as linear functions of age, as suggested by the results of empirical analyses in Fig. 3. One can see in the upper left panel of this figure that the effect of allostatic adaptation is represented by the increasing linear functions, which look similar for both sexes.
Fig. 4.
The quadratic hazard model applied to data on blood glucose in the FHS: Estimates of the mean allostatic states , the physiological norms (ft), and the quadratic hazard terms for different ages (t).
The BG norms shown in the upper right panel are also the increasing functions of age for each sex. It is interesting to note that this increase is developing within the limits suggested by the American Diabetic Association (ADA) as the normal levels of FBG.
The coefficients characterizing the sensitivity of all-cause mortality risk to deviations to the right (the lower right panel) and to the left (the lower left panel) from the age-specific norm are estimated as increasing functions of age. This means that the range of tolerable deviations from the norm is getting narrower with an increasing age or the “price” of the same deviation from the norm measured by the increment in mortality risk increases at the old ages compared to that for the young adults. This indicates that the resistance to stresses declines with an increasing age. The likelihood ratio test performed for the FHS BG data shows that the coefficients responsible for the age dependence of allostatic adaptation and physiological norms ft are significantly different from zero.
Since the function ft corresponds to the minimum mortality, it could be used as a target for intervention strategies aiming at reducing mortality risk by affecting the BG level. However, the use of the trajectory of ft for targeting medical interventions may be impractical because it may be enough to reach the values of mortality risk close to its minimal value in appropriate vicinity of the normal trajectory. In other words, the notion of the “normal range” of BG levels rather than the “normal” value of BG may be more practical for applications. To pursue this idea, we evaluated the age-dependent ranges of possible deviations of BG from the norm which can be considered as “acceptable” levels of increase in mortality risk.
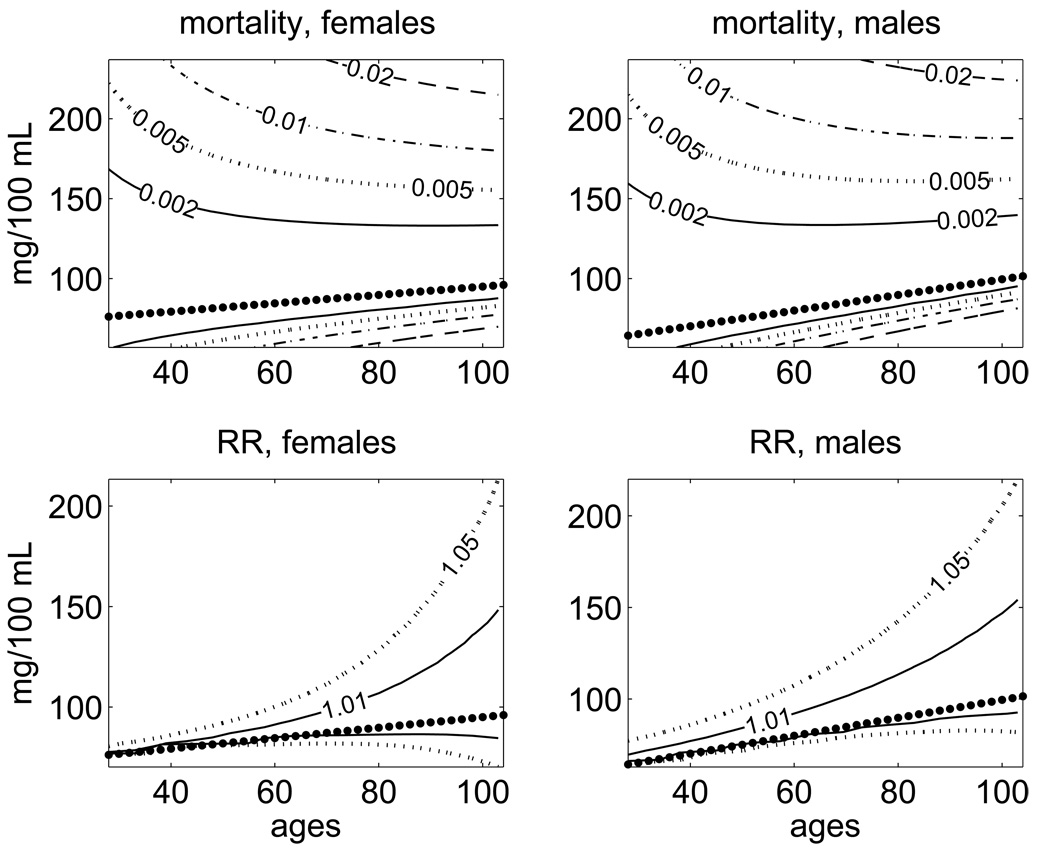
Fig. 5 (upper panels) shows the age-specific ranges of values of BG with the contour lines corresponding to the selected levels of increase in the absolute mortality risks. Together with the graphs for the norms ft, the figure displays the contour lines corresponding to an increase in the absolute values of the evaluated mortality risk by 0.002, 0.005, 0.01, and 0.02. This means that, for example, the contour line , corresponding to a 0.01 increase in the absolute risk from its optimal level, is calculated from the equality: . For the absolute value of risk, we also draw the contour lines .These lines capture the general pattern of changes in the ranges of values with “acceptable” levels of increase in the mortality rate observed for BG as a risk factor: the ranges of “acceptable” values for this index become narrower with age. This corresponds to the narrowing U-shape of the quadratic term of the hazard with age. The non-symmetric shape of the quadratic term in equation (2) allows one to reveal differences in an increase of mortality due to deviations of BG to the left and to the right from the age trajectories of the physiological norm ft.
Fig. 5.
Age-specific ranges of values of BG with minimal mortality rates (upper panels) and minimal relative risks (RR) of death (lower panels) estimated for females and males in the FHS data using the quadratic hazard model. The labels on the lines denote the values of an increase in the mortality rate (respectively, in the relative risk) compared to the minimal values at respective ages (ft). The dotted lines correspond to the age trajectory of the normal level of BG, ft, approximated by a linear function.
It is important to note that the age pattern of changes in the relative risk shows the opposite tendency: in contrast with the narrowing U-shape for the absolute mortality risk, the U-curve of the relative risk is getting wider with age. Fig. 5 (lower panels) shows the age-specific ranges of values of BG with different levels of increase in the relative risk of death. The figure displays the contour lines with the values of the relative risk 1.01 and 1.05, i.e., the lines corresponding to the 1% and 5% increase in the relative risk of death . The figure illustrates the general pattern of changes in the ranges of values with “acceptable” levels of increase (say, 1%) in the relative risk of death: the ranges of “acceptable” values of BG become wider with age. Note that this pattern is opposite to that observed for the tolerable range of BG calculated using the values of the absolute mortality risk. The difference is caused by a fast increase in the baseline mortality with age. Such an increase means that the other influential factors which are summarized in (e.g., senescence itself) become more important with age than the increase in mortality risk due to deviation of a particular physiological index from the optimal age trajectory. On the contrary, at younger ages, where the mortality rate is lower, even small deviations from ft produce a substantial increase in the relative (but not in the absolute) risk of death.
The reason why the dynamic properties of the relative risk differ from those of the absolute risk is clearly seen when comparing mathematical representation of the absolute risk (in a simplified case of a symmetric quadratic hazard term)
with that of the relative risk
One can see from this comparison that the ratio may decline with age when increases faster than . This decline is associated with the widening of the respective U-curve with age.
5. Application of semiparametric Cox model
Alternatively, we investigated a semiparametric Cox-type model of the effects of BG on the total mortality risk with no assumptions about the form of age dependence of the normal curve. Besides, the effect of the BG level as a mortality risk factor is estimated for the whole remaining period of life after the measurement. The mortality rate considered as a function of age t and the level of BG x is:
where I (x-x0) is the indicator function (i.e., I(y) = 1 for y > 0 and I (y) = 0 otherwise). Five parameters to be estimated include bi (i=1,…,4)and x0. The level of BG, i.e., x, is the last measurement before a certain age t0. Age t runs from t0 to the age at death (possibly censored). Therefore, the distinct choice of t0 (e.g., t0 ∈{35, 40,…,80} ) produces separate datasets. Denoting an individual’s risk factor measured at t0i (t0i <≈t0) as xi (t0), we get the likelihood corresponding to the data from one dataset:
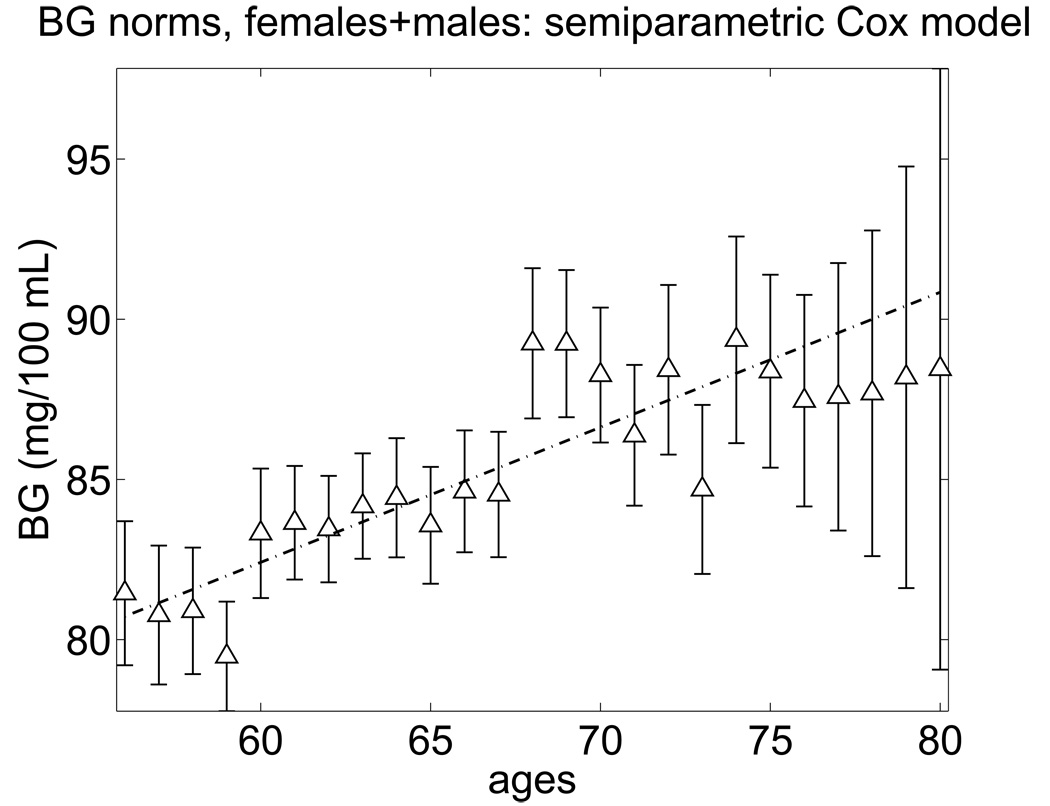
Here ti is the age at death (possibly censored) and δi is the censoring indicator. The estimates of x0 calculated for each dataset with specific t0 allow us to evaluate the age dependence of the BG norm x0. These estimates calculated for the FHS cohort (two sexes combined) are presented in Fig. 6 together with the linear regression line. Parameter estimates of the regression line a + b t0 shown in Fig. 6 are a = 57.1 +/− 3.7 (mg/100 mL); b = 0.42 +/− 0.06 (mg/100 mL * year−1).
Fig. 6.
The norm of blood glucose for females and males in the FHS estimated by a semiparametric extended Cox-type model.
One can see from this figure that the BG norm can be approximated by an increasing linear function of age similar to that evaluated using the quadratic hazard model. The difference of the value of the slope of this linear function from zero is statistically significant. Note that a linear increase in the normal BG level is just a statistical approximation. There is no biological theory suggesting any shape of the normal curve. It might be possible that the normal BG curve follow a more complicated age trajectory. The age pattern of the point estimates of BG minimizing the all-cause mortality risk for individuals of different age groups shown in Fig. 6 suggests that a logistic curve may provide a better fit to the data than a linear function. However, comparison of quadratic hazard models with logistic and linear curves revealed that the latter provides a better fit as defined by the Akaike Information Criterion (Akaike, 1974). The main message from these analyses, however, is that the BG norm may be consistently defined for individuals of each age category and it is likely that this norm changes with age.
6. Discussion
Exceptional survival could happen by chance despite the presence of factors making substantial contribution to mortality risk. This conclusion follows from models of conditional survival widely used in demography, epidemiology and biostatistics. When conditions are unfavorable, these chances are small. They could increase if the values of risk factors will be kept closer to values minimizing mortality risk during individual’s life course. Although not all risk factors could be controlled to such extent, some of them could. The values of many physiological indices affecting mortality risk, including the level of BG, may be shifted in the favorable direction by the proper use of drugs, moderate exercises, adequate nutrition, life style, etc. This fact creates potential opportunity to maximize chances of exceptional survival by properly maintaining physiological state during the life course.
Maintaining the proper level of blood glucose (BG) is often recommended for having a long and healthy life span (Burge, 2001; McAulay et al., 2001; Menzin et al., 2001; Willcox et al., 2006). In order to follow this recommendation, a quantitative definition of the “proper” or “normal” FBG levels is needed. Although several such definitions already exist and are widely used in research and medical practices, the continuing debates about them indicate that the consensus has not yet been reached. The current definitions of the “normal” FBG typically use the information on its population distribution or on the relationship between FBG and the risk of diabetes and, recently, also CVD (Levitan et al., 2004; WHO, 2006). While these definitions are based on clinical experience, they are often linked to a specific outcome measure (e.g., the risk of a particular disease), and therefore can differ for different such measures.
Such practice can be completely justified in situations with known clinical conditions in which the risk of death from CVD or other cause is high. When no information about such conditions is available considering the total mortality risk as an outcome measure and defining the BG norm as a value of this index with minimizes mortality risk at a given age seems to be an appropriate strategy. The results of our analyses indicate that the BG norm, defined this way, may be a convenient indicator of average aging related changes caused by intrinsic (senescence) forces which can be estimated from the data. For each gender, these functions increase with age from about 70–80 mg/dl at age 40 to about 95–100 mg/dl at age 100 years, thus staying approximately within the traditionally “normal” range of 70–100 mg/dl. Two other approaches used in the analyses of these data (analyses of the age trajectories of BG in the LL individuals and the use of the Cox’s type model) also confirmed the similar pattern of age dependence of the BG norm. The effects of external disturbances on the age trajectory of BG are also estimated from the data.
It follows from the definition of the norm that BG levels which are above or below the normal values for a given age are associated with an increased relative mortality risk at this age. What is important, the range of deviations from the norm that only barely affect the relative risk of death increases with advancing age and, for the elderly, it may even include pre-diabetic and diabetic (according to the ADA and WHO definitions, Genuth et al., 2003; WHO, 1999; WHO, 2006) values of 126 mg/dl or higher. This, of course, does not mean that these diseases should not be treated. This indicates that the risk of death due to other causes,, in which senescence plays an important role, increases with age faster than the risk associated with a fixed deviation of BG from its optimal trajectory (measured by functions and , respectively). The fact that the age trajectories of optimal (normal) age related changes in BG are within the limits of the normal range for BG established by the ADA definition (Genuth et al., 2003) indicates that the model used in our analyses does not contradict the experience of medical practitioners, which is a good sign for any model used in the analyses of data on aging, health and longevity.
The graphs in Fig. 5 show that deviations of the BG to the levels about 70–80 mg/dl (levels that are optimal for men younger than 50) actually increase mortality risk in the oldest old males. This observation is consistent with the general gerontological concept that the same problems (e.g., a high BG level) in the young and the old persons may require different treatments. Our results thus strongly suggest the importance of studying changes in the optimal BG trajectories as well as respective age related changes in the U-shape form of the mortality risk function.
The strength of the approach is that the FHS longitudinal data on the BG concentration may be well described using the stochastic process model of aging-related changes in BG homeostasis as well as aging-related changes in the coefficients describing the contribution of the BG into mortality risk. An important advantage of this model is that it allows for taking into account and evaluating from the data the effects of hidden persistent external disturbances using the recently developed concept of allostasis and allostatic adaptation.
Considering the BG dynamics together with respective mortality risk in a simple one-dimensional physiological space has one more scientific merit. It allows for important observation that any procedure of BG normalization does not diminish the mortality risk to zero. This is because alternative pathways capable of returning the BG to its normal age trajectory may end up with pathological or even lethal values of other (unobserved) physiological/biological variables and, respectively, with an unpredictable increase in mortality risk conditional on these variables. Considering a one-dimensional case makes this situation clear.
The results of this study indicate the possibility of evaluating effects of allostatic adaptation to external disturbances on the BG age trajectories and separating them from the effects resulting from compensatory adaptation and remodeling in response to changes caused by senescence process inevitably developing even in the absence of any external disturbances. The approach developed in this paper allows for systematically addressing research questions about factors and mechanisms involved in regulation of aging related changes and longevity in humans, which is difficult to address using standard statistical tools.
The analysis also shows that the U-shaped function of all-cause mortality risk gets narrower with age. This indicates that the resistance to stresses, which effects are manifested through the BG variation, is likely to decline with age. In contrast, the U-shape of the relative risk is a widening function of age. Our analyses indicate the need for a multidimensional definition and investigation of the notion of a physiological norm using all available data.
One limitation of this approach is related to the fact that in most exams of the FHS blood glucose measurements were randomly fasting or non-fasting. To reduce the bias, and make the results of blood measurement closer to those of fasting measurements, blood was drawn at the end of many hours of examination. A recent study (Port et al., 2006) verified that the age- and sex-adjusted deciles of the fasting and random glucose distributions in examinations, where fasting specimen was available, are very close to the random ones, being slightly lower. So, one can expect that the use of random (instead of fasting) glucose data will have a little impact on our analyses compared to the use of the FBG data.
Another serious methodological issue deals with the data most appropriate for studying aging related changes in BG metabolism. The BG data available from the FHS do not take into account an increase of glucose intolerance with aging, which means that post-meal glucose levels increase with increasing age. Therefore, there may be an age effect on random glucose levels which affects estimates of the BG norm obtained in this study.
One more limitation is that the approach uses a one-dimensional description of physiological state. Therefore the estimates of the normal BG levels should be used with care. This is because contemporary researchers and medical practitioners usually have information about other indices characterizing individuals’ physiological state and health/well-being status in addition to the level of BG. This means that a proper procedure of BG normalization should involve adjustments in other indices as well, and, hence, the notion of physiological norm should be extended to include more than one index (i.e., it should be calculated by minimizing mortality risk considered as a function of many physiological indices). At the same time, a substantial deviation of the BG from its one-dimensional norm may serve as an important indicator of a possible pathological development signaling that individual’s health requires a careful investigation which has to involve other physiological indices. Thus the need for multidimensional analyses is an important message stemmed from this study.
The results raise several methodological issues for further studies. One deals with connection of the BG level with mortality from specific causes. There is a substantial body of evidence linking elevated glucose levels to specific microvascular and macrovascular conditions, which have a direct impact on CVD mortality. Introducing and studying population heterogeneity in susceptibility to different causes of death might be an important step in clarifying this issue.
A more technical issue is that blood glucose levels are consistently lower than serum glucose reflecting the contribution of lower glucose content of red blood cells. To the extent that older subjects have a lower red blood cell count, which is likely, this may also skew the results of analysis. More studies are needed to consider multidimensional age dynamics of biological and physiological indices and their effects on morbidity and mortality risks.
Acknowledgements
The research reported in this paper was supported by the National Institute on Aging grants R01AG027019, R01AG028259, and 5P01AG008761. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. The Framingham Heart Study (FHS) is conducted and supported by the NHLBI in collaboration with the FHS Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the FHS or the NHLBI.
Appendix
General model (discrete time)
Let Xt, Yt be two stochastic processes describing the life history of an individual. The process Xtk equals zero if an individual died in the interval [tk, tk+1), and it equals one if he/she survived until the age tk+1. The process Yt is a discrete time stochastic process describing observations of BG. We assume that this process satisfies the following equation:
| (A1) |
where .Note that (A1) is a discrete-time variant of equation (1). Time intervals between tk and tk+1 could be non-equal for different k.
Let , k = 1,…,n be a random vector of observations of the process Yt at ages t1,…, tk Denote by the conditional probability of death in the interval [tk, tk+1) of an individual given an observed trajectory . We assume that this probability depends only on values of Ytk as follows:
| (A2) |
where is similar to equation (2):
| (A3) |
For the likelihood function, we need conditional distributions of Ytk given the observations .From (A1),
| (A4) |
where
| (A5) |
for k > 2, and
| (A6) |
Consider N independent observations of individuals in the above described scheme. Denote by the observed trajectories of the process Yt for ith individual, where ni is the number of observations of the process Yt for ith individual. Let δi=1 if ith individual died in the interval , δi=0 if he/she survived until the age and δi=2 if the individual is lost to follow up at the last observation (censored at the age ). The contribution of ith individual into the likelihood function is
| (A7) |
where the respective probabilities are given by (A2)–(A6). The likelihood function is a product of , i = 1…N.
In applications to the FHS data, we used the following specifications of functions in (A1), (A3): (i) the Gompertz baseline mortality rate; (ii) linear functions for and ;(iii) constant feedback coefficient and diffusion, at =aY, bt=σ1; (iv) linear optimal age-trajectories,ft =af+bft; (v) a linear function describing the effects of allostatic adaptation, .Parameters to be estimated in the model: aμ0, bμ0, aμ11, bμ11, aμ12, bμ12, aY, σ0, σ1, af1, bf1, af and bf. The likelihood maximization was performed using the constrained optimization procedure of MATLAB’s optimization toolbox (MathWorks Inc., 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H. A new look at the statistical model identification. ITAC AC-19. 1974;(6):716–723. [Google Scholar]
- Andres R. Aging and diabetes. Med. Clin. North Am. 1971;55(4):835–846. doi: 10.1016/s0025-7125(16)32479-8. [DOI] [PubMed] [Google Scholar]
- Andres R. Aging, diabetes, and obesity: standards of normality. Mt. Sinai J. Med. 1981;48(6):489–495. [PubMed] [Google Scholar]
- Barbieri M, Rizzo MR, Manzella D, Paolisso G. Age-related insulin resistance: is it an obligatory finding? The lesson from healthy centenarians. Diabetes. Metab. Res. Rev. 2001;17(1):19–26. doi: 10.1002/dmrr.178. [DOI] [PubMed] [Google Scholar]
- Burge MR. Lack of compliance with home blood glucose monitoring predicts hospitalization in diabetes. Diabetes Care. 2001;24(8):1502–1503. doi: 10.2337/diacare.24.8.1502. [DOI] [PubMed] [Google Scholar]
- Chang AM, Halter JB. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metabol. 2003;284(1):E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- Dawber TR. The Framingham Study: The Epidemiology of Atherosclerotic Disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- Elahi D, Muller DC, Egan JM, Andres R, Veldhuis J, Meneilly GS. Glucose tolerance, glucose utilization and insulin secretion in ageing. Endocrine Facets of Ageing. 2002;242:222–242. [PubMed] [Google Scholar]
- Genuth S, Alberti KGMM, Bennett P, Buse J, DeFronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- Govindaraju DR, Cupples LA, Kannel WB, O'Donnell CJ, Atwood LD, D'Agostino RB, Fox CS, Larson M, Levy D, Murabito J, Vasan RS, Splansky GL, Wolf PA, Benjamin EJ, Jeffrey CH. Genetics of the Framingham Heart Study population. Adv. Genet. 2008;62:33–65. doi: 10.1016/S0065-2660(08)00602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Gordon T, Castelli WP. Obesity, lipids, and glucose intolerance. The Framingham Study. Am. J. Clin. Nutr. 1979;32(6):1238–1245. doi: 10.1093/ajcn/32.6.1238. [DOI] [PubMed] [Google Scholar]
- Levitan EB, Song YQ, Ford ES, Liu SM. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch. Intern. Med. 2004;164(19):2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- MathWorks Inc. Optimization Toolbox™ 4 User's Guide. Natick, MA: The MathWorks, Inc.; 2008. [Google Scholar]
- McAulay V, Deary IJ, Ferguson SC, Frier BM. Acute hypoglycemia in humans causes attentional dysfunction while nonverbal intelligence is preserved. Diabetes Care. 2001;24(10):1745–1750. doi: 10.2337/diacare.24.10.1745. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Menzin J, Langley-Hawthorne C, Friedman M, Boulanger L, Cavanaugh R. Potential short-term economic benefits of improved glycemic control: A managed care perspective. Diabetes Care. 2001;24(1):51–55. doi: 10.2337/diacare.24.1.51. [DOI] [PubMed] [Google Scholar]
- Muller DC, Elahi D, Tobin JD, Andres R. The effect of age on insulin resistance and secretion: A review. Semin. Nephrol. 1996;16(4):289–298. [PubMed] [Google Scholar]
- Port SC, Boyle NG, Hsueh WA, Quinones MJ, Jennrich RI, Goodarzi MO. The predictive role of blood glucose for mortality in subjects with cardiovascular disease. Am. J. Epidemiol. 2006;163(4):342–351. doi: 10.1093/aje/kwj027. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. Diabetes mellitus in the elderly: insulin resistance and/or impaired insulin secretion? Diabetes Metab. 2005;31(Sp Iss 2):S27–S34. doi: 10.1016/s1262-3636(05)73649-1. [DOI] [PubMed] [Google Scholar]
- Sinclair A, Finucane P. Diabetes in old age. 2nd ed. Chichester, New York: Wiley; 2001. [Google Scholar]
- Sterling P, Eyer J. Allostasis: A New Paradigm to Explain Arousal Pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- Terry DF, Pencina MJ, Vasan RS, Murabito JM, Wolf PA, Hayes MK, Levy D, D'Agostino RB, Benjamin EJ. Cardiovascular risk factors predictive for survival and morbidity-free survival in the oldest-old Framingham Heart Study participants. J. Am. Geriatr. Soc. 2005;53(11):1944–1950. doi: 10.1111/j.1532-5415.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- WHO. Diagnosis and classification of diabetes mellitus and its complications. Geneva, Switzerland: WHO; Report of a WHO Consultation. 1999
- WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva, Switzerland: WHO; Report of a WHO/IDF Consultation. 2006
- Willcox BJ, He QM, Chen R, Yano K, Masaki KH, Grove JS, Donlon TA, Willcox DC, Curb JD. Midlife risk factors and healthy survival in men. JAMA. 2006;296(19):2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Akushevich I, Kulminski A, Akushevich L, Ukraintseva SV. Stochastic model for analysis of longitudinal data on aging and mortality. Math. Biosci. 2007;208(2):538–551. doi: 10.1016/j.mbs.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]