Abstract
Background:
The purpose of this study was to evaluate the relationship between cognitive performance, risk factors for cardiovascular and cerebrovascular disease (CVD), and HIV infection in the era of highly active antiretroviral therapy.
Methods:
We evaluated the cognitive functions of men enrolled in the cardiovascular disease substudy of the Multicenter AIDS Cohort Study who were aged ≥40 years, with no self-reported history of heart disease or cerebrovascular disease. Results from comprehensive neuropsychological evaluations were used to construct composite scores of psychomotor speed and memory performance. Subclinical CVD was assessed by measuring coronary artery calcium and carotid artery intima-media thickness (IMT), as well as laboratory measures, including total cholesterol, fasting glucose, glycosylated hemoglobin, glomerular filtration rate (estimated), and standardized blood pressure and heart rate measures.
Results:
After accounting for education, depression, and race, carotid IMT and glomerular filtration rate were significantly associated with psychomotor speed, whereas IMT was associated with memory test performance. HIV serostatus was not significantly associated with poorer cognitive test performance. However, among the HIV-infected individuals, the presence of detectable HIV RNA in plasma was linked to lower memory performance.
Conclusions:
These findings suggest that HIV infection may not be the most important predictor of cognitive performance among older gay and bisexual men in the post–highly active antiretroviral therapy era, at least among those with access to medical care and to appropriate medications. Medical factors associated with normal aging are significantly associated with performance on neuropsychological tests, and good clinical management of these factors both in HIV-infected individuals and those at risk for infection may have beneficial effects in the short term and could reduce the risk of subsequent cognitive decline.
GLOSSARY
- BP
= blood pressure;
- CalCAP
= California Computerized Assessment Package;
- CES-D
= Center for Epidemiologic Studies–Depression scale;
- CVD
= cardiovascular and cerebrovascular disease;
- GFR
= glomerular filtration rate;
- HAART
= highly active antiretroviral therapy;
- HDL
= high-density lipoprotein;
- HAND
= HIV-associated neurocognitive disorder;
- Hb
= hemoglobin;
- IMT
= intima-media thickness;
- LDL
= low-density lipoprotein;
- MACS
= Multicenter AIDS Cohort Study;
- OR
= odds ratio;
- RAVLT
= Rey Auditory Verbal Learning Test.
The proportion of persons living with HIV/AIDS older than 50 years in the United States increased to 24% of all cases in 2005 from 17% in 2001. While the total number of infected persons increased 20% in that time period, the increase was 58% among individuals older than 50 years (http://www.cdc.gov/hiv/topics/over50/resources/factsheets/over50.htm). With increased use and success of highly active antiretroviral therapy (HAART), nearly three-quarters of all infected individuals older than 50 years die of non–HIV-related causes.1
Age is a significant risk modifier for HIV-associated neurocognitive disorder (HAND).2–4 There may be a differential effect of the APOE*4 allele in older HIV individuals,5 and diabetes is a critical comorbidity.6 HAART use may be associated with abnormal amyloid deposition in the brain7 and amyloid-β and tau levels in the CSF may be related to HIV-associated dementia.8 The pattern of neuropsychological deficits in HIV+ individuals is changing with HAART to include evidence of more cortical dysfunction.9 Thus, factors normally associated with age-related neuropsychiatric syndromes may become increasingly important in understanding the etiology of neurocognitive dysfunction occurring in older HIV+ patients.
The Multicenter AIDS Cohort Study (MACS) is a 4-site study of the natural and treated history of HIV infection among gay and bisexual men. Study participants were enrolled in 3 waves: 1984/1985 (1,813 HIV+ and 3,141 HIV−), 1987/1990 (382 HIV+ and 286 HIV−), and 2001/2003 (688 HIV+ and 662 HIV−). The MACS has tracked cognitive performance among the study participants for the past 24 years using screening tools, and a subcohort has been followed up with more detailed testing for approximately 20 years. In 2002, the MACS completed detailed evaluations of cardiovascular risk factors in a subgroup of infected and uninfected men; during the same time period, the entire cohort completed the detailed neuropsychological testing. This provided an opportunity to examine the relationship between cardiovascular risk factors and cognition, and the added effect of HIV serostatus on cognitive performance after accounting for the age-associated variables. This article reports results of this initial cross-sectional analysis. We predicted, based on available evidence, that cardiovascular risk variables would exert significant effects on cognition and that when these effects were considered in the analysis, HIV-related effects would be attenuated relative to observations before HAART.
METHODS
Standard protocol approvals, registrations, and patient consents.
The work described in this report was approved by the ethical standards committee on human experimentation at each of the MACS sites, and written informed consent was obtained from all participants.
Subjects.
The 945 participants in the MACS cardiovascular and cerebrovascular disease (CVD) substudy were used to analyze the relationship between cardiovascular risk and cognitive function. The MACS CVD substudy was initiated in 2004 and included men aged ≥40 years, with no self-reported history of heart disease (heart attack, heart surgery, other heart illness) or cerebrovascular disease. The baseline visits for the substudy were completed between July 2004 and January 2006. Of these men, 723 had been given a full MACS neuropsychological test battery within 1 year before or 2.5 years after the baseline CVD study visit. Of those, 641 men were also given the Trail Making Test, Symbol Digit Modalities Test, and the Center for Epidemiologic Studies–Depression scale (CES-D). Six men were subsequently excluded for not having relevant laboratory data within 21 weeks of their baseline CVD visit. In summary, neuropsychological and CVD data were available from 635 participants (figure e-1 on the Neurology® Web site at www.neurology.org). Demographic characteristics of the participants are shown in table 1.
Table 1 Characteristics of study sample
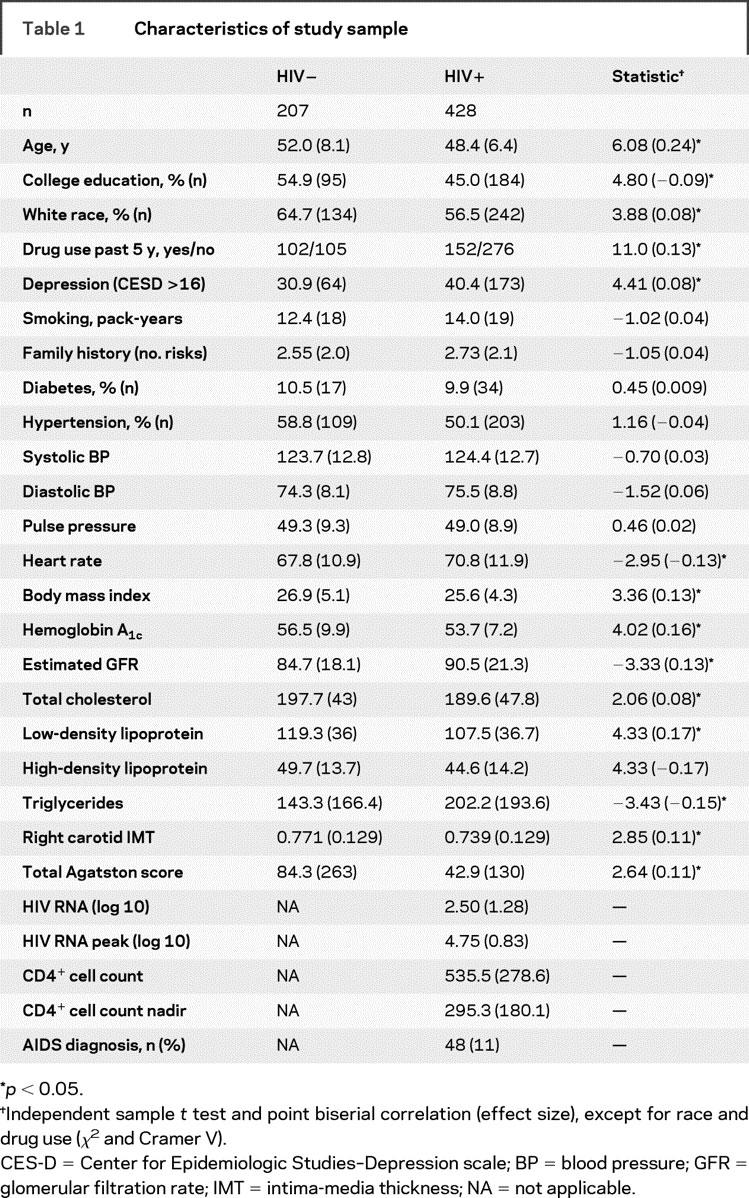
We compared the subjects who were included in this analysis to the 310 subjects who were not included (table e-1) on a variety of variables related to vascular disease. Family history of hypercholesterolemia was significantly lower among the subjects included in the study (χ2 = 8.91, df = 1, p = 0.012) and the estimated glomerular filtration rate (GFR) was higher [t(923) = 4.38, p < 0.001, r = 0.14]; otherwise, there were no significant differences between the subjects included in the study and those who were not.
Neuropsychological evaluation.
The neuropsychological battery uses conventional and computerized tests to assess a range of cognitive functions, including motor skills, cognitive flexibility and abstraction, verbal and nonverbal memory, and visuoconstructional skills. These include the Rey Auditory Verbal Learning Test (RAVLT), the Rey-Osterrieth Complex Figure, the Stroop Color-Interference Task, the Grooved Pegboard test, the Trail Making Test, the Symbol-Digit Substitution Task, and the California Computerized Assessment Package (CalCAP) Simple and Choice Reaction Time program (table e-2).
Cardiovascular disease evaluation.
Subclinical CVD was assessed using electron beam tomography or multidetector CT to measure coronary artery calcium. Ultrasound examination of the carotid artery was used to quantify intima-media thickness (IMT). Standardized blood pressure (BP) and heart rate measures were also determined, as well as total cholesterol, low-density (LDL) and high-density (HDL) lipoproteins, and glycosylated hemoglobin. GFR was estimated using a standard protocol.10 CVD variables are shown in table 1.
Data reduction.
Composite indices of psychomotor speed and memory were constructed based on performance from individual tests included in the comprehensive neuropsychological battery. These cognitive functions were of a priori interest. Psychomotor slowing is considered a diagnostic hallmark of HAND; memory deficits have become increasingly common with the advent of HAART.9,11 The Trail Making Test (Part A), Symbol-Digit Substitution Task, Grooved Pegboard (Non-Dominant Hand), Stroop Color Naming, and CalCAP (median simple reaction time) were used to generate the composite measure of Psychomotor Speed. Immediate and delayed recall scores from the RAVLT and the Rey-Osterrieth Complex Figure were used to construct the composite Memory measure. Composite scores were derived by transforming the individual test data into z scores based on the mean and SD of the HIV-seronegative participants and then calculating the arithmetic mean of the relevant z scores with the signs adjusted such that a positive score indicates better performance (table 1). The data were further reduced by creating dichotomous variables; scores less than 0.0 were coded as 1 (impaired), and scores greater than 0.0 were coded as 0 (normal). The binary outcomes were then the dependent variables in the subsequent logistic regression analyses.
The CVD variables were reduced to categorical variables (present/absent, normal/abnormal) based on standard criteria or on the median value of the uninfected men (in the case of estimated GFR). These included the presence of hypertension (self-reported diagnosis and use of antihypertensive medications, or diastolic BP >85 or systolic BP >130) or diabetes (self-reported diagnosis and use of diabetic medications, or fasting glucose ≥126 or hemoglobin [Hb] A1c ≥7%), elevated total cholesterol (>240), elevated LDL (>100), decreased HDL (<40), elevated HbA1c (>7.0), low GFR (<73.4), or elevated body mass index (>30). Carotid IMT was classified into 2 groups (±0.81), as was the total Agatston score for coronary calcification (±400). Men scoring above 16 on the CES-D were classified as depressed for this analysis. Education was classified as less than or greater than a college degree. Race was classified as white or nonwhite. Any abnormal scores, the presence of disorder, lower education, nonwhite race, and depression were all coded as 1 (vs 0).
Data analyses proceeded in 3 steps. Initially, the unadjusted risk of poorer performance on the 2 composite scores for the critical independent variables was calculated. A multivariate model for both Psychomotor Speed and Memory measures was then implemented. There were 3 models in this binary logistic regression analysis. In the first, education, race, and depression were entered into the model (forced entry). In the second model, CVD variables were entered in a forward stepwise fashion (Wald criteria12). In the third model, HIV serostatus was added. This process was also repeated for the HIV-infected participants, using CD4+ cell counts (±200), the presence of detectable plasma HIV RNA (>50 copies), and AIDS (ever had an AIDS indicator condition) in the place of serostatus.
RESULTS
Tables 2–5 present the odds ratios (ORs) for the unadjusted and adjusted models from the logistic regression analyses of the Psychomotor Speed and Memory functions. The ORs and associated significance testing are taken from the final model (i.e., after all variables had been selected for entry). In 3 cases, the multivariate ORs are from the third and final model; in the case of Psychomotor Speed among the HIV+ participants, the multivariate ORs are from the second model.
Table 2 Predictors of poor performance on tests of psychomotor speed among all study participants
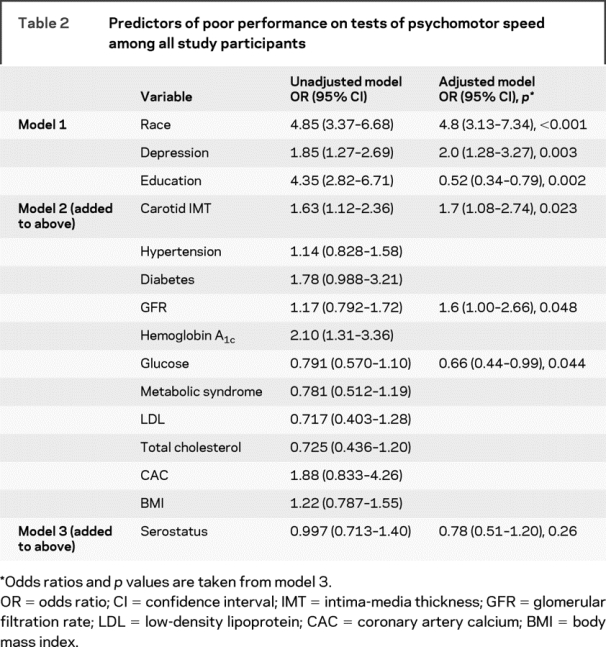
Table 3 Predictors of poor performance on tests of psychomotor speed among HIV-infected individuals only

Table 4 Predictors of poor performance on delayed verbal and nonverbal memory among all study participants
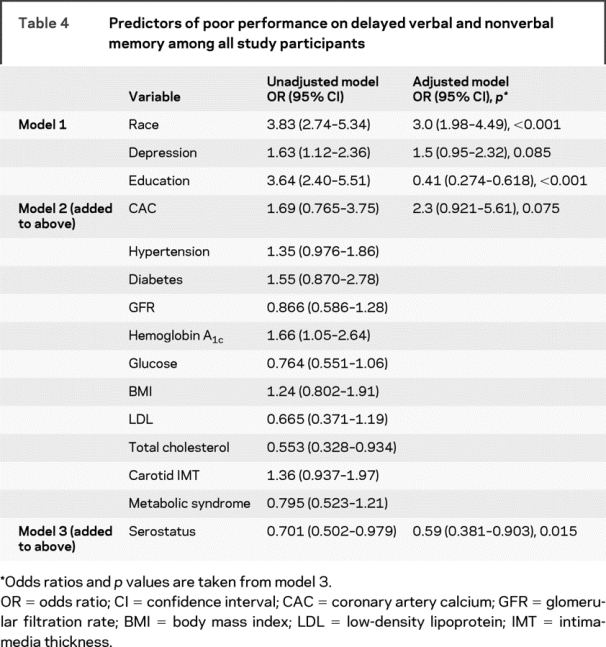
Table 5 Predictors of poor performance on delayed verbal and nonverbal memory among HIV-infected participants only

With regard to Psychomotor Speed, carotid IMT and estimated GFR were significantly associated with poorer performance (table 2). However, HIV serostatus was not associated with the Psychomotor Speed variable after the remaining variables had been entered into the model. Among the HIV-infected individuals, there was no association between the presence of detectable HIV RNA, CD4+ cell counts, or AIDS and test performance, although abnormal levels of coronary artery calcification increased risk of impaired (slower) performance (table 3).
For the Memory composite, the extent of coronary calcium was marginally associated with Memory functions (table 4). Unlike the results for the Psychomotor Speed variable, HIV serostatus was significantly associated with Memory performance by protecting against lower test scores. Among the HIV- infected individuals only, the presence of detectable HIV RNA in plasma significantly increased risk of poorer memory performance (table 5). An examination of the bivariate associations between GFR and the Memory scores reveals a difference in the size and direction of the associations when the entire cohort was studied (table 4) and when only the HIV-infected participants were analyzed (table 5). The apparent protective effect of abnormal GFR seen in the entire cohort, compared with the apparent increase in risk for impairment with abnormal GFR among the HIV+ participants, suggests the possibility of an interaction between GFR and serostatus. Further analysis indicated that GFR was a risk factor for impaired Memory performance among the HIV+ participants, but the effect was not significant (OR = 2.09).
DISCUSSION
These findings suggest that HIV infection may not be the most important predictor of cognitive functions among older gay and bisexual men in the post-HAART era, at least among those with access to medical care and to appropriate medications. Medical factors associated with normal aging, specifically those related to CVD and to metabolic disorders, are significantly associated with performance on neuropsychological tests. Good clinical management of these factors both in HIV-infected individuals and those at risk for infection may have beneficial effects in the short term and could reduce the risk of subsequent cognitive decline (e.g., references 13 and 14).
These findings support other data from the MACS indicating that in individuals with well-controlled viral replication and immune competence, there is little progression of clinical brain disease.15 In that analysis, the measures were the same ones that went into our Psychomotor Speed composite variable, but memory functions were not examined. Were we to track our current variables over time (i.e., both Psychomotor Speed and Memory), we would predict that performance on memory tests would also show little change in the face of viral suppression and good immunologic status.
The relationship between psychomotor speed and CVD measures is consistent with models of cognitive aging (e.g., reference 16). Individual measures comprising this composite score are highly sensitive to cognitive dysfunction but nonspecific as to underlying cause.17 Therefore, it is surprising that there was no significant association with HIV serostatus. The fact that detection of HIV RNA in plasma was a significant risk for poorer Memory scores is consistent with other findings indicating more prominent memory impairment in the HAART era (e.g., reference 9). Perhaps there are other HIV-associated factors not assessed in this study that are better markers of cognitive dysfunction than CD4+ cell counts and HIV RNA levels. For example, the level of proviral HIV DNA in peripheral blood mononuclear cells may have a stronger association with HIV-associated dementia18 and performance on individual neuropsychological tests19 than either CD4+ cell counts or viral load in plasma.
Chronological age is often entered as a covariate in the analysis of neuropsychological test data. However, in many of these studies, and this one in particular, the use of age as a covariate would be inappropriate. We agree with others (e.g., reference 20) that there is little effect of age per se on performance, but that it is the medical concomitants of “normal” aging that account for many of the changes in cognition and in brain structure and function. Consequently, controlling for chronological age (which is highly correlated with the presence of CVD) would severely attenuate (or remove) any effects related to the medical factors that are the subject of this study. Similarly, using “age-adjusted” standard scores from published test normative databases would have the same effect, because those standardized scores would be adjusted for age-related changes in performance. Were age to be included in our analyses as a covariate, the result would be that the variables of interest (i.e., IMT) would actually be testing an interaction: the effect of IMT on performance must be greater than that expected for an individual of that age (which already accounts for increases in IMT).
One important predictor of CVD is hypertension, which is a pathway to cognitive loss and (later) dementia.21 Hypertension has been linked to HIV serostatus and to treatment.22 Chronic high BP increases the risk of damage to target organs and is associated with cerebrovascular disease (strokes, white matter hyperintensities, and cortical atrophy),23,24 subclinical renal dysfunction,25 left ventricular hypertrophy,26 increased IMT (as a marker of atherosclerosis),25 and retinal arteriolar narrowing.27 BP measures28 and pulse pressure and pulse wave velocity, as markers of arterial stiffness, have been associated with decreased cognitive function.29 Markers of kidney function are associated with the long-term effects of hypertension on the kidney and on the brain.30 Thus, markers of renal (e.g., GFR) and cardiac (e.g., coronary artery calcium) integrity provide evidence of the deleterious effects of CVD on the brain.
There are some important points to consider in interpreting the findings of this investigation. First, these are older subjects (i.e., aged >40 years), who tended to be from the original MACS cohort and may have introduced a significant survivorship bias. That is, these are the individuals who (in the case of those with HIV infection) successfully survived for 15 to 25 years with the virus. It is likely that these individuals have a different biologic substrate than those individuals who succumbed to the infection. Second, among the older participants, both infected and uninfected, age-related conditions may have started to take their selective toll on the cohort. Data from the Veterans Administration Cohort Study show that the majority of deaths among HIV-infected veterans are not from HIV-related causes.1 Thus, stroke, heart disease, (non–AIDS-related) cancer, and other causes of death are selectively reducing the number of living participants who are able to provide both CVD and neuropsychological data to our study. In addition, there seems to be an increase in stroke in HIV-infected individuals,31 which would also result in a sample bias for this study (in terms of both physical survival and study inclusion criteria). Third, there is survivor bias among the seronegative participants. Although their risk of mortality may be lower than that of their HIV+ counterparts, they nevertheless remained active participants in the cohort and maintained a willingness to undergo additional research testing even though not infected with HIV. Fourth, the rates of many of the CVD risk factors were actually lower among the HIV-infected participants, in keeping with what has been reported previously by the MACS.32 We interpret this observation as another example of bias in the sample; because the majority of our HIV-infected men were receiving medical care and because they all receive regular medical testing from the MACS clinics, the treating physicians may be more aggressive in the care of cardiovascular risk factors. This is reflected in a weak association between HIV infection status and a higher rate of using lipid-lowering agents (χ2 = 2.09, p = 0.09 [1-tailed], OR = 1.33 [0.903–1.96]).
After adjusting for education level, there is a significant effect of race on test performance. This is likely due to potential differences in available educational opportunities. One method for adjusting for this difference in educational achievement is to use a measure of reading skill, or other similar marker.33 Although the MACS administers the Shipley-Hartford Institute of Living Scale (and this test yields an IQ equivalent score), there were too many missing data to warrant inclusion in the analysis. However, an analysis of the subset of participants who did have Shipley Institute of Living Scale data (n = 451) revealed that this was significantly linked to memory (β = 0.467) and Psychomotor Speed (β = 0.372) performance (controlling for race and depression). In the case of the Memory score, the IQ score fully mediated the effect of race (β = −0.089 vs −0.253), whereas in the case of Psychomotor Speed, the mediation was only partial (β = −0.187 vs −0.309).
Findings from this cross-sectional observational study suggest that risk of cognitive impairment among gay and bisexual men is more strongly related to cardiovascular and metabolic disease profiles than to HIV serostatus. These findings do not demonstrate that HIV is no longer a risk factor for impaired cognition. Rather, under the conditions in which we evaluated the participants—ambulatory and without overt CVD—the effects of HIV on cognition are overshadowed by the effects of subclinical CVD and related metabolic abnormalities. Findings that HIV can infect arterial smooth muscle cells raise the possibility that such infection may exacerbate any vasculopathy found among HIV+ individuals.34 Longitudinal studies are needed to determine the independent and interactive contributions of HIV infection and CVD on brain structure and development of clinical cognitive disorder in infected individuals.
These findings add to evidence that control of viral replication and of immunologic competence may protect against the cognitive impairment that was so common before the introduction of HAART. However, infected individuals remain vulnerable to the consequences of a Western lifestyle and aging, and these factors influence cognitive decline. As risk factors for HAND continue to change35 and we develop a better understanding of the multiple factors that can affect brain structure and function, being able to place HAND in the context of normal age-related processes becomes increasingly important.
AUTHOR CONTRIBUTIONS
Dr. Becker completed the statistical analysis with assistance from L. Kingsley and J. Mullen.
ACKNOWLEDGMENT
The authors thank the participants and the staff of the MACS for the additional time and effort that they contributed toward the successful completion of this project. The authors are particularly grateful to Prof. Paul Satz for his leadership in the development, implementation, and analysis of the MACS neuropsychological study.
DISCLOSURE
Dr. Becker receives research support from the NIH [UO1-AI-35041 (Coinvestigator)]. Dr. Kingsley receives research support from the NIH [UO1-AI-35041 (Coinvestigator)]. Ms. Mullen reports no disclosures. Dr. Cohen has served as consultant and/or received speaker honoraria from Acorda Therapeutics, Astellas Pharma Inc., Bayer Schering Pharma (Berlex), Biogen Idec, EMD Serono, Inc., Novartis, and Teva Pharmaceutical Industries Ltd.; serves on speakers’ bureaus for Bayer Schering Pharma (Berlex), EMD Serono, Inc., and Teva Pharmaceutical Industries Ltd.; receives funding for an annual neurology resident education program sponsored through his department and institution from Teva Pharmaceutical Industries Ltd.; and has received research support from Biogen Idec, BioMS Medical, EMD Serono, Inc., Novartis, Sanofi-aventis, and the NIH [NINDS 1 U01n NS45719-01A (Clinical Investigator–Site PI), NIAID 1U01A169471 (Clinical Investigator), NICHD NCIRE 725-SW-001/R01HD04 (Coinvestigator), HIH RO1 MH080636-01A2 (Coinvestigator), and NIAID 5 U01 A035039–15/Mod 40 (Coinvestigator)]. Dr. Martin receives research support from the National Institute on Drug Abuse [R01DA12828 (PI) and R03DA02497 (PI)]. Dr. Miller is the author and distributor of the CalCAP reaction time program and has a financial interest in this software (1986–present); and has received research support from the NIH [NIAID AI-35040 (Coinvestigator)]. Dr. Ragin receives research support from the NIH [MH080636 (PI), HL088437 (Coinvestigator), MH083553 (Consultant), and HL092386 (Consultant)]. Dr. Sacktor receives research support from the NIH [NIAID AI-35042 (Coinvestigator), NIMH N01MH22005 (Coinvestigator), NIMH MH71150 (PI), NINDS U01-NS32228 (PI), NIMH P30-MH075673 (Core PI), NIMH MH058076 (Coinvestigator), and NIMH MH083465 (PI)]. Dr. Selnes receives research support from the NIH [NIMH RO1-MH0711500 (Coinvestigator), NIMH P30-MH075673 (Coinvestigator), NIAID U01-AI035042 (Coinvestigator), and NINCDS NS-35610 (Coinvestigator)]. Dr. Visscher serves on a scientific advisory board for the National NeuroAIDS Tissue Consortium.
APPENDIX
The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (Principal Investigator), Haroutune Armenian, Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, John Hylton, Lisette Johnson, Shenghan Lai, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio; Chicago: Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: John P. Phair (Principal Investigator), Joan S. Chmiel (Co–Principal Investigator), Sheila Badri, Bruce Cohen, Craig Conover, Maurice O’Gorman, David Ostrow, Frank Palella, Daina Variakojis, Steven M. Wolinsky; Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (Principal Investigator), Barbara R. Visscher (Co–Principal Investigator), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Thomas Coates, Rita Effros, John Fahey, Beth Jamieson, Otoniel Martínez-Maza, Eric N. Miller, John Oishi, Paul Satz, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (Principal Investigator), Lawrence Kingsley (Co–Principal Investigator), James T. Becker, Robert W. Evans, John Mellors, Sharon Riddler, Anthony Silvestre; Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (Principal Investigator), Alvaro Munoz (Co–Principal Investigator), Stephen R. Cole, Christopher Cox, Gypsyanber D’Souza, Stephen J. Gange, Janet Schollenberger, Eric C. Seaberg, Sol Su; NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez; National Heart, Lung, and Blood Institute: Cheryl McDonald; National Institute of Mental Health: Pim Brouwers.
Supplementary Material
Address correspondence and reprint requests to Dr. James T. Becker, Neuropsychology Research Program, Suite 830, 3501 Forbes Ave., Pittsburgh, PA 15213
beckerjt@upmc.edu
Supplemental data at www.neurology.org
*See appendix.
Supported in part by funds from the National Institute for Allergy and Infectious Diseases to the collaborating MACS sites: UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, and UO1-AI-35041.
Disclosure: Author disclosures are provided at the end of the article.
Received February 19, 2009. Accepted in final form July 16, 2009.
REFERENCES
- 1.Braithwaite RS, Justice AC, Chang CC, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med 2005;118:890–898. [DOI] [PubMed] [Google Scholar]
- 2.Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology 1992;42:1472–1476. [DOI] [PubMed] [Google Scholar]
- 3.Chiesi A, Vella S, Dally LG, et al. Epidemiology of AIDS dementia complex in Europe. J Acquir Immun Defic Syndr 1996;11:39–44. [DOI] [PubMed] [Google Scholar]
- 4.Stoff DM. Mental health research in HIV/AIDS and aging: problems and prospects. AIDS 2004;18(suppl 1):3–10. [PubMed] [Google Scholar]
- 5.Valcour V, Shikuma C, Shiramizu B, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaiian Aging with HIV Cohort. J Neuroimmunol 2004;157:197–202. [DOI] [PubMed] [Google Scholar]
- 6.Valcour VG, Shikuma CM, Shiramiza BT, et al. Diabetes, insulin resistance, and dementia among HIV-1 infected patients. J Acquir Immun Defic Syndr 2005;38:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005;19:407–411. [DOI] [PubMed] [Google Scholar]
- 8.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta-42 and tau levels correlate with AIDS dementia complex. Neurology 2005;65:1490–1492. [DOI] [PubMed] [Google Scholar]
- 9.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol 2004;10:350–357. [DOI] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 11.Sacktor N, Skolasky R, Selnes OA, et al. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol 2007;13:203–209. [DOI] [PubMed] [Google Scholar]
- 12.Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans Am Math Soc 1943;54:426–482. [Google Scholar]
- 13.Skoog I, Lernfeldt B, Landahl S. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 14.Guo Z, Viitanen M, Fratiglioni L. Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ 1996;312:805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole MA, Margolick JB, Cox C, et al. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology 2007;69:2213–2220. [DOI] [PubMed] [Google Scholar]
- 16.Salthouse TA. Speed mediation of adult age differences in cognition. Dev Psychol 1993;29:722–728. [Google Scholar]
- 17.Lezak MD. Neuropsychological Assessment, 2nd edition. New York: Oxford University Press; 1983. [Google Scholar]
- 18.Shiramizu B, Ratto-Kim S, Sithinamsuwan P, et al. HIV DNA and dementia in treatment-naive HIV-1-infected individuals in Bangkok, Thailand. Int J Med Sci 2007;4:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiramizu B, Paul R, Williams A, et al. HIV proviral DNA associated with decreased neuropsychological function. J Neuropsychiatry Clin Neurosci 2007;19:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabbitt P, Scott M, Thacker N, et al. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology 2006;20:549–557. [DOI] [PubMed] [Google Scholar]
- 21.Struijs J, van Genugten LL, Evers SMAA, Ament AJHA, Baan CA, van den Bos GAM. Modeling the future burden of stroke in the Netherlands: impact of aging, smoking, and hypertension. Stroke 2005;36:1648–1655. [DOI] [PubMed] [Google Scholar]
- 22.Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005;19:953–960. [DOI] [PubMed] [Google Scholar]
- 23.Longstreth WT, Bernick C, Fitzpatrick A, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology 2001;56:368–375. [DOI] [PubMed] [Google Scholar]
- 24.Bernick C, Kuller L, Dulberg C, et al. Silent MRI infarcts and the risk of future stroke: the Cardiovascular Health Study. Neurology 2001;57:1222–1229. [DOI] [PubMed] [Google Scholar]
- 25.Tatasciore A, Remda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 2007;50:325–332. [DOI] [PubMed] [Google Scholar]
- 26.Almuntaser I, Brown A, Murphy R, et al. Comparison of echocardiographic measures of left ventricular diastolic function in early hypertension. Am J Cardiol 2007;100:1771–1775. [DOI] [PubMed] [Google Scholar]
- 27.Tikellis G, Arnett DK, Skelton TN, et al. Retinal arteriolar narrowing and left ventricular hypertrophy in African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2008;21:352–359. [DOI] [PubMed] [Google Scholar]
- 28.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 2005;45:374–379. [DOI] [PubMed] [Google Scholar]
- 29.Waldstein SR, Rice SC, Thayer JF. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008;51:99–104. [DOI] [PubMed] [Google Scholar]
- 30.Knopman DS, Mosley TH Jr, Bailey KR, Jack CR Jr, Schwartz GL, Turner ST. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci 2008;271:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tipping B, de Villiers L, Candy S, Wainwright H. Stroke caused by human immunodeficiency virus-associated intracranial large-vessel aneurysmal vasculopathy. Arch Neurol 2006;63:1640–1642. [DOI] [PubMed] [Google Scholar]
- 32.Kingsley LA, Cuervo-Rojas J, Munoz A, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS 2008;22:1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manly JJ, Echemendia RJ. Race-specific norms: using the model of hypertension to understand issues of race, culture, and education in neuropsychology. Arch Clin Neuropsychol 2007;22:319–325. [DOI] [PubMed] [Google Scholar]
- 34.Eugenin EA, Morgello S, Klotman ME, et al. Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular disease. Am J Pathol 2008;172:1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol 2003;9:205–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


