Abstract
p63 belongs to a family of transcription factors, which, while demonstrating striking conservation of functional domains, regulate distinct biological functions. Its principal role is in the regulation of epithelial commitment, differentiation and maintenance programs, during embryogenesis and in adult tissues. The p63 gene has a complex transcriptional pattern, producing two subclasses of N-terminal isoforms (TA and ΔN) which are alternatively spliced at the C-terminus. Here, we report the identification of two new C-terminus p63 variants, we named p63 δ and ε, that increase from 6 to 10 the number of the p63 isoforms. Expression analysis of all p63 variants demonstrates a tissue/cell-type-specific nature of p63 alternative transcript expression, probably related to their different cellular functions. We demonstrate that the new p63 variants as ΔN isoforms are active as transcription factors as they have nuclear localization and can modulate the expression of p63 target genes. Moreover, we report that, like ΔNp63α, ΔNp63δ and ε sustain cellular proliferation and that their expression decreases during keratinocyte differentiation, suggesting their involvement in this process. Taken together, our results demonstrate the existence of novel p63 proteins whose expression should be considered in future studies on the roles of p63 in the regulation of cellular functions.
INTRODUCTION
p63 is a member of a family of transcription factors, also including the tumor suppressor p53 and p73, which show high level identity in their main functional domains: the transactivation domain (TA), the DNA-binding domain (DBD) and the oligomerization domain (OD).
The human p63 gene, like the p53 and p73 genes, produces multiple protein isoforms as a result of alternative promoter usage and alternative splicing events (1). The promoter upstream of exon 1 generates the class of the TAp63 isoforms containing the TA at the N-terminus, while an alternative promoter in intron 3 leads to the expression of the ΔNp63 isoforms lacking the N-terminal TA domain, although it has been shown that the ΔNp63 isoforms can act as regulators of transcription through different TAs present in the distinct N-terminus (2) and C-terminus regions (3). Within each subclass, C-terminal alternative splicing events confer additional complexity. To date, three variants, α, β and γ, which incorporate different portions of the C-terminus, have been described. The α proteins are the longest including all terminal exons and containing the C-terminal sterile alpha motif (SAM) domain—a protein–protein interaction domain, followed by an inhibitory domain (TI), which is able to auto-inhibit the transcriptional activity of the TA subclass isoforms (4). The β variants lack exon 13 and consequently the SAM and the TI domains. The γ variants lack the C-terminal exons 11, 12, 13, 14, but incorporate an additional sequence of unknown function from intron 10. Therefore, the p63 gene expresses at least six different p63 isoforms (TAp63 α, β, γ and ΔNp63 α, β, γ), with a complex array of similarities and differences in their structural domains and transcriptional activities.
Despite the structural conservation between members of the p53 family, they are not functionally redundant as p53-family transgenic knockout mice develop distinct phenotypes, indicating that each protein has specific biological functions. Several lines of evidence suggest that, while the main role of p53 is related to the inhibition of tumor progression, p73 and p63 appear to be more directly involved in development and differentiation (5–7).
Current data indicate that p63 is an essential mediator of embryonic development. p63−/− mice have no epidermis and other stratified epithelia and also show striking defects in limb development (6). TA and ΔNp63 isoforms are expressed during distinct stages of embryonic epidermal development. TAp63 isoforms are the first p63 isoforms to be expressed during embryonic development and they are necessary for the commitment to epithelial stratification while simultaneously blocking differentiation program. Therefore, a shift towards ΔNp63 isoforms during later stages would be required to counterbalance the activity of TAp63, thereby allowing cells to respond to terminal differentiation cues (8,9). In adults, the ΔNp63α isoform is predominantly expressed in the basal layers of stratified epithelial tissues, suggesting that it may contribute to maintain the proliferative potential of basal cells necessary for the epithelial stratification (10,11). In human p63, germline mutations have been reported in patients with ectodermal dysplasia syndromes, showing varying degrees of craniofacial, limb, skin and hair defects which resemble the phenotype of p63–/− mice (12).
The role of p63 in tumorigenesis is controversial. Initially, p63 was hypothesized to function as oncosuppressor based on its homology to p53. Mutations in the p63 gene are quite rare in human cancers and the gene maps in a region of chromosome 3 frequently amplified in squamous cell carcinoma. In primary head and neck squamous cell tumors and in other squamous epithelial cancers, ΔNp63α is over-expressed and it was suggested that this form is required to maintain a stem cell-like state, allowing continuous proliferation and promoting tumor growth (13). Recently, it has been reported that p63+/− mice show a predisposition towards squamous cell carcinoma and that loss of p63 can cooperate with loss of p53 in tumor development, as dual heterozygous p63+/− and p53+/− mice display higher tumor burden and metastasis, compared to p53+/− mice (14). On the contrary, another independent study reported that p63+/− mice show no evidence of enhanced tumor development and that dual heterozygous p63+/− and p53+/− mice display reduced tumor burden compared to p53+/− mice (15).
In order to investigate the expression of the p63 gene, we have applied a bioinformatics approach (16,17), which detects transcript variants through the multiple alignment of all available transcript and/or EST sequences of a gene to the corresponding genomic sequence. Here we describe the identification of two novel human p63 C-terminal variants, that we have named δ and ε; we describe the in vivo validation of these new p63 variants, and present results regarding their functional characterization.
MATERIALS AND METHODS
Bioinformatics
Novel p63 variants were identified using ASPicDB (10) (http://www.caspur.it/ASPicDB/index.php), which provided access to reliable annotations of the alternative transcriptional pattern of all human genes. AspicDB was used to obtain sequence data of every transcript and protein isoform predicted, and also to identify intron/exon boundaries of the genomic sequence corresponding to each variant, essential for the design of isoform-specific primer sets. Sequence data were aligned using Clustalw2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html).
Cell cultures, transfection and in vitro keratinocyte differentiation
H1299, MCF-7, 293 and HaCat cell lines were maintained in Dulbecco's modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum (FCS), l-glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C, 5% CO2. Transfections were carried out with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Eight micrograms of either empty pcDNA3 vector or vector containing the different ΔNp63 variants (α, β, γ, δ and ϵ) were used for a 60 mm plate of H1299 and MCF-7 cells (90–95% confluency). Cells were cultured in DMEM plus serum without antibiotics for 24 h. Then the medium was replaced with fresh complete medium and the cells were cultured for additional 24 h.
Normal human epidermal Keratinocytes were isolated from neonatal foreskin specimens derived from a normal human Caucasian (PromoCell). Cells were maintained in StemlineTM Keratinocyte Medium II (Sigma) supplemented with 1× Keratinocyte medium supplement, BPE free, l-glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C, 5% CO2. Keratinocytes were differentiated by adding CaCl2 (2 mM final concentration) and reducing the supplement, BPE free, to 0.1× for 24 and 48 h.
Cell proliferation assay by BrdU incorporation
MCF-7 cells were transfected with 100 ng of either empty pcDNA3 vector (control) or containing p53 or the different ΔNp63 variants (α, β, γ, δ and ϵ). Seventy-two hours later, bromodeoxyuridine (BrdU) incorporation, after 3 h pulses was determined by using Cell Proliferation ELISA BrdU (Roche) as described by the manufacturer.
Reverse transcriptase polymerase chain reaction and quantitative RT–PCR analyses
Total RNA from HaCat, H1299, MCF-7, 293 and primary keratinocyte cell lines was extracted using RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's instructions. Total RNA from human muscle and brain was purchased from commercial sources (Ambion). cDNAs were synthesized from 1 μg of total RNA using QuantiTect® Reverse Transcription kit (Qiagen).
For Reverse transcriptase polymerase chain reaction (RT–PCR) experiments, 1 μl of cDNA was used as template for amplifications carried out with specific primer sets. The sequences of all primers are available upon request. PCR was run in the exponential region to allow semi-quantitative comparisons among cDNAs developed from identical reactions.
For quantitative RT–PCR, 1 μl of cDNA was used as template in real-time PCR assays, performed in triplicate on ABI PRISM 7900HT (Applied Biosystems). Each p63 variant was analyzed using the QuantiTect SYBR Green PCR Master Mix (Qiagen) with validated specific primers pairs. The PCR conditions were designed as follows: hot start at 95°C for 15 min; 45 cycles of amplification (94°C for 15 s, 62°C for 30 s, 72°C for 30 s); dissociation curve step (95°C for 15 s, 60°C for 15 s, 95°C for 15 s). For the quantification of ada, fasn, redd1, bax, p21, k14 and k1 transcripts, real-time PCR assays were performed using the TaqMan Universal PCR Master Mix (Applied Biosystems) with specific TaqMan Gene Expression Assays (Applied Biosystems) as described by the manufacturer.
Results were analyzed with the SDS 2.2.1 software. The comparative threshold (Ct) method was used to determine the relative ratio of transcripts. The relative quantification (RQ) was expressed as 2–ΔΔCt, where ΔCt was calculated as the average Ct for target variant minus the average Ct of endogenous gapdh in each sample, and ΔΔCt was calculated as the ΔCt of the sample minus the ΔCt of the calibrator. The data shown are the average from at least three independent experiments.
Construction of recombinant vectors
The full-length coding region of the human ΔNp63δ and ΔNp63ϵ variants was amplified from HaCat cDNAs using the Platinum® Taq DNA Polymerase High Fidelity (Invitrogen) and cloned into SacII/ApaI sites of a pcDNA3 expression vector containing the laminin A 5′UTR downstream the CMV promoter. The resulting vectors were termed pcDNA3-ΔNp63δ and pcDNA3-ΔNp63ϵ.
Western blot analysis
For protein analysis, cells were washed twice in cold PBS and then lysed in RIPA buffer [50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholat, 0.1% SDS, protease inhibitors cocktail tablets (Roche)] for 1 h on ice. Lysates were then cleared by centrifugation at 10 000 g for 15 min at 4°C, aliquoted and stored at –20°C. Sixty micrograms of the total proteins, in 4× SDS–PAGE sample buffer, were heated at 100°C for 5 min, separated on 4–12% SDS polyacrylamide gels and transferred to nitrocellulose membranes (Hybond-ECL, Amersham Biosciences). Membranes were then blocked for 1 h in a PBS solution containing 3% non-fat milk powder and 0.1% Tween-20, and then probed at room temperature with Abp63 (4A4, Santa Cruz) for 3 h, in 3% milk, 0.1% Tween-20 PBS. For the analysis of keratinocyte differentiation, membranes were probed also with the following primary antibodies: Abp53 (DO-1, Santa Cruz) for 3 h, Abp21 (Calbiochem) for 3 h, Ab-actin (Calbiochem) for 1 h. After two 10 min washes in 0.1% Tween-20 PBS and one 10 min wash in PBS, proteins were visualized using ECL western blotting detection reagents (Amersham Pharmacia Biotech).
Luciferase assays
H1299 cells were plated in 35 mm tissue culture dishes (8 × 104 cells/dish) 24 h before transfection. Each well was then co-transfected using TransIT-LT1 Transfection Reagent (Mirus Bio), according to the manufacturer's instructions, with either empty pcDNA3 vector or containing the different ΔNp63 variants (150 ng), the reporter vector (1 μg) along with Renilla pRL-SV40 vector (Promega) (10 ng).
Thirty-six hours after transfection, H1299 were lysed in Passive Lysis buffer (Promega) and the luciferase assay was performed using the Dual Luciferase assay system (Promega), according to the manufacturer's instructions. Data were normalized to the Renilla reporter signal. The results reported represent the average of at least three independent experiments and are shown with the standard deviations.
Immunofluorescence
H1299 cells were plated on a glass coverslip polylisine treated in six-well plates (4 × 104 cells/well). Twenty-four hours later, cells were transfected using TransIT-LT1 Transfection Reagent (Mirus Bio), with either empty pcDNA3 vector or containing the different ΔNp63 variants (1 μg). Thirty-six hours after transfection, cells were fixed and permeabilized for 30 min with cold 50% methanol–50% acetone and then incubated at room temperature for 90 min with the Abp63 (4A4, Santa Cruz) at 1 : 50 dilution in DMEM plus serum. For cells transfected with empty pcDNA3 vector, only DMEM plus serum was added. After extensive washing, cells were incubated for 1 h at room temperature in the dark with FITC-conjugated secondary Ab against mouse Ig (Jackson) in DMEM plus serum (1 : 100). Nuclei were stained with DAPI (0.2 μg/ml). Fluorescence was analyzed with Axioplan 2 Imaging microscope (Zeiss).
RESULTS
In silico identification of two novel human p63 variants
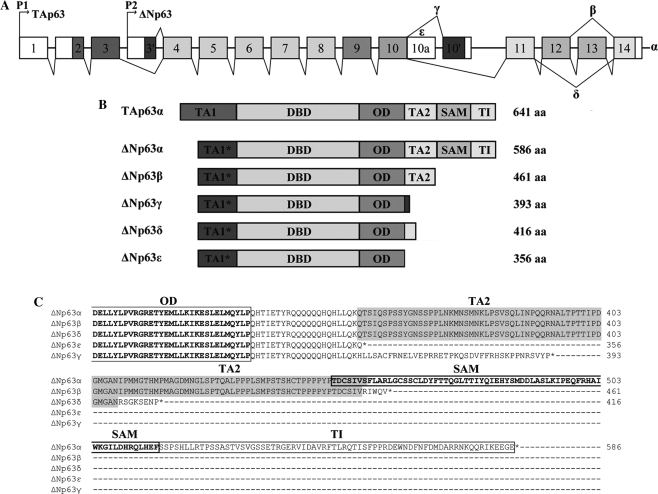
Through the application of ASPIC, an algorithm for the prediction of alternative transcripts (16,17), we investigated the transcriptional pattern of the human p63 gene. Among the p63 transcripts predicted by the software, which included known isoforms, we detected two previously unidentified C-terminus p63 variants. The first variant, herein referred to as δ, derives from the skipping of exons 12 and 13; the second variant, referred to as ε, is generated by a premature transcriptional termination in intron 10, retaining the 5′ portion of intron 10, which immediately presents a stop codon (Figure 1A). The nucleotide sequences of the ΔNp63 δ and ε transcripts were deposited in GenBank, with the accession numbers GQ202690 and GQ202689, respectively.
Figure 1.
Human p63 gene. (A) Schematic representation of human p63 gene structure: alternative promoters (P1 and P2), previously identified alternative splicing events (α, β and γ) and novel events (δ and ϵ) are indicated. (B) ΔNp63 protein isoforms are reported with their molecular size. TA1*: TA of ΔN isoforms; DBD: DNA-binding domain; OD: oligomerization domain; TA2: C-terminal TA; SAM: sterile alpha motif; TI: inhibitory domain. (C) Amino acid alignment among the C-terminal regions of δ and ε p63 proteins and the known α, β and γ.
The δ and ε transcripts produce proteins with different C-terminal regions (Figure 1B). In particular, the δ proteins contain 53/101 residues of the second TA (TA2), followed by eight unique amino acids after the oligomerization domain (OD). The ε proteins are the shortest isoforms, lack the TA2 domain and terminate, after the OD, with 22 amino acids shared with α, β and δ isoforms; the γ proteins also lack the TA2 domain, maintain 21 common residues and terminate with 38 unique amino acids. The alignment among the C-terminal regions of δ and ε p63 proteins and the known α, β and γ forms is reported in Figure 1C.
In vivo identification of δ and ε p63 isoforms in keratinocytes
To confirm the in vivo expression of the new δ and ε p63 isoforms, we used two human keratinocyte cell lines, HaCat and Nhek, as it is known that ΔN isoforms of p63 are highly expressed in these cells (1). We performed RT–PCR experiments and western blotting analysis in order to validate both the new p63 transcripts and proteins.
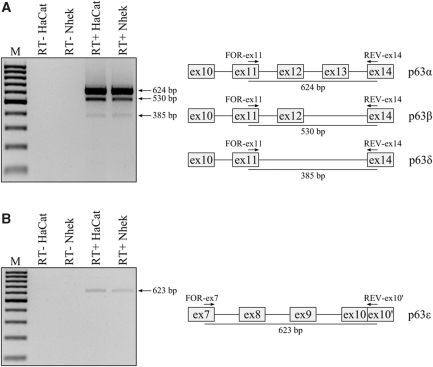
RT–PCR experiments were carried out on total RNA using primer pairs specifically designed for the amplification of each variant, as shown in Figure 2. Amplification with primers For-ex11 and Rev-ex14 produced a 385 bp amplicon corresponding to that expected for the new δ transcript as well as 624 and 530 bp amplicons expected for the α and β transcripts, respectively (Figure 2A). To identify the ϵ transcripts we used primers For-ex7 and Rev-ex10′ producing only one amplicon, whose size of about 620 bp corresponded to that expected for the ϵ transcripts (Figure 2B). All amplicons were subcloned into a PCR cloning vector and sequenced. Alignment of the δ and ϵ amplicon sequences to the p63 gene sequence confirmed the in silico prediction of the new p63 transcripts exactly (data not shown).
Figure 2.
In vivo identification of novel p63 δ and ϵ transcripts. (A) RT–PCR analysis of HaCat and Nhek RNAs which identifies the α, β and δ transcripts; on the right, a schematic representation of the portion of p63 transcripts amplified with the expected size. (B) RT–PCR analysis from HaCat and Nhek RNAs which identifies the ε transcripts; on the right, a schematic representation of the portion of p63 transcripts amplified with the expected size. Primers used are indicated by arrows.
Because the ΔNp63 isoforms are the most abundant class in keratinocytes, we also amplified and sequenced the entire coding region of ΔNp63δ and ΔNp63ϵ transcripts from HaCat and Nhek cDNAs. Analysis of sequences confirmed the presence of these new ΔNp63 transcripts.
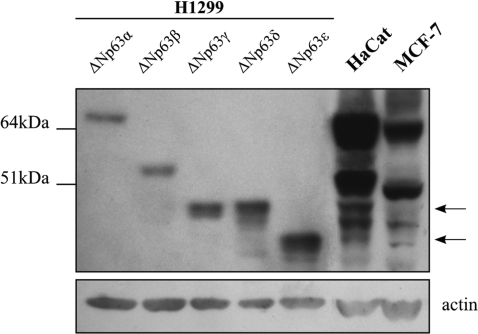
Next, we analyzed expression of ΔNp63 proteins in human keratinocytes and in a human breast carcinoma cell line. Lysates from HaCat and MCF-7 cells were resolved on a 4–12% SDS/PAGE gel together with size standard controls for each ΔNp63 isoform (ΔNp63α, β, γ, δ and ϵ). These standards were prepared by transfecting H1299 cells (which don’t express detectable p63 protein) with pcDNA3 vectors expressing the cDNAs of each ΔNp63 isoform. Western analysis was performed using a pan-p63 antibody that recognizes the core DBD of p63. As shown in Figure 3, after a long exposure, HaCat and MCF-7 cell lysates revealed two bands corresponding to the ΔNp63δ and ϵ isoforms in addition to the known ΔNp63 proteins. Moreover, differential expression of the ΔNp63 isoforms was observed. Indeed, in HaCat cells ΔNp63γ, δ and ε present lower levels of expression than ΔNp63β and in particular ΔNp63α, which is the predominant isoform, while in MCF-7 cells, ΔNp63δ seems to be the least expressed p63 isoform.
Figure 3.
Identification of the novel ΔNp63δ and ϵ proteins. Endogenous p63 protein expression was evaluated by western analysis in HaCat and MCF-7 cells. Five micrograms of lysate of H1299 over-expressing ΔNp63α, β, γ, δ and ϵ proteins were used as molecular marker to assess an identity to each ΔNp63 isoform in 60 μg of Hacat and MCF-7 lysates. The pan-p63 4A4 antibody was used to detect ΔNp63 isoforms. Ectopically expressed ΔNp63α, β and γ show a higher molecular mass than expected as they express the myc-tag in their N-terminus. Arrows on the right indicate the position of ΔNp63δ and ϵ isoforms in HaCat and MCF-7 lysate. The molecular weights of protein markers are shown on the left.
Expression of p63 transcripts in normal tissues and cell lines
In order to quantify the relative amounts of all p63 transcripts in different human tissues and cell lines and to determine their tissue-specificity, we performed real time RT–PCR assays.
Since p63 transcripts differ only in the 3′ end of their coding region, we were not able to construct primers/probe sets for each variant. For this reason, we used the SYBR Green chemistry, designing specific primer pairs, mapping in the limited different sequence, to distinctly amplify each p63 variant (α, β, γ, δ and ϵ). In addition, to distinguish between the TA and ΔNp63 transcript subclasses, we designed primer pairs able to amplify only the 5′ end of TA and ΔN transcripts.
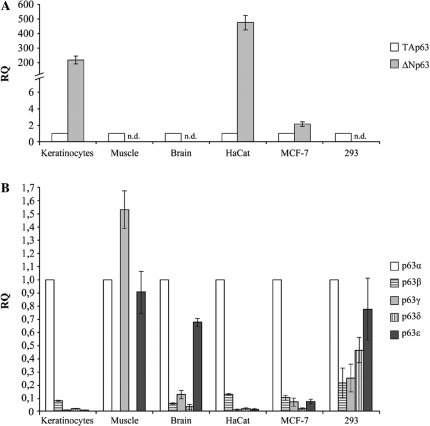
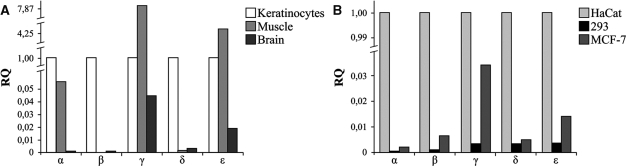
Total RNA from primary muscle, brain and keratinocyte samples, embryonic kidney 293, breast cancer MCF-7 and HaCat cell lines was analyzed. First, we compared the relative levels of the TA and ΔN p63 transcripts in each sample, using TA transcripts as calibrators (Figure 4A). Among tissues studied, only primary keratinocytes express high levels of ΔN transcripts (∼200-fold more than TAs), while muscle and brain show no detectable levels of ΔN transcripts. We could thus consider the TA class as the predominant one in these samples. Among cell lines, HaCat cells exhibit the highest levels of ΔN transcripts (∼400-fold more than TAs); in the MCF-7 cells, ΔN transcripts are ∼2-fold more expressed than TA, while no detectable levels of ΔNp63 mRNAs were observed in 293 cell line.
Figure 4.
Expression profile of human p63 transcripts in different normal tissues and cell lines. (A) TA and ΔN p63 transcripts levels were quantified by qRT–PCR. Values are expressed as fold change respect to TA transcripts, used as calibrator, after internal normalization for gapdh expression (see ‘Material and Methods’ section). (B) p63 α, β, γ, δ and ϵ mRNA levels were quantified by qRT–PCR. Values are expressed as fold change respect to α variant, used as calibrator, after internal normalization to gapdh expression.
Next, we assessed the relative levels of each p63 variant (α, β, γ, δ and ϵ) in each sample examined, using the α variant, which is generally the most expressed variant, as calibrator (Figure 4B). The profile of the TA and ΔN p63 transcripts in these samples allowed us to associate the expression values obtained for each variant, to either TA or ΔN subclasses. Results obtained, indicated that the new δ and ϵ p63 variants are expressed in all samples examined and, overall, all p63 variants show differences in their expression pattern. In fact, among tissues, the α variant is the most expressed in all samples, with the exception of muscle, in which the γ variant showed the highest expression level. Interestingly, we found that in muscle, apart from the γ and α variants the ϵ variant is the most highly expressed, while β and δ variants are almost undetectable. In brain, we found that, after the α variant, the ϵ variant is more highly expressed than the γ, β and δ variants, the latter being the least expressed in this tissue. Among cell lines, the α variant, is also the most highly expressed in all samples. HaCat cells showed an expression pattern similar to that of primary keratinocytes, with γ, δ and ε variants relatively less expressed than α and β variants. In 293 cells, ϵ and δ variants showed a higher expression level than β and γ variants, while in MCF-7 cells, after the β, the γ and ϵ variants showed higher expression levels than the δ variant, in accord with observed protein expression levels (see Figure 3).
Finally, we compared the relative levels of each p63 variant among the tissues and cell lines examined (Figure 5). Among tissues, we observed that α, β and δ variants are more expressed in keratinocytes than in muscle and brain, while, interestingly, the γ and ϵ variants present the highest levels in muscle tissue (respectively, 8- and 4-fold more expressed than in keratinocytes). Very low levels of all p63 variants are present in brain respect to the other tissues (Figure 5A). Among cell lines, HaCat cells show much higher expression levels of all five variants than MCF-7 and especially 293 cells (>9-fold expression drop) (Figure 5B).
Figure 5.
Differential expression of human p63 transcripts among different normal tissues (A) and cell lines (B). mRNA levels of p63 variants (α, β, γ, δ and ϵ) were quantified by qRT–PCR. Values are expressed as fold change respect to keratinocytes and HaCat cells, used as calibrators, respectively for tissues and cell lines, after internal normalization to gapdh expression.
Nuclear localization of ΔNp63δ and ΔNp63ε isoforms
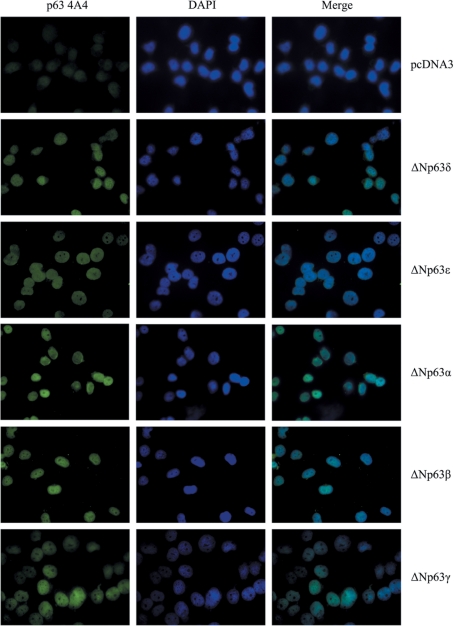
For the functional characterization of the new p63δ and ε variants, we focused our attention on the ΔN isoforms. Given that the p63 gene encodes transcription factors, we investigated whether ΔNp63δ and ΔNp63ε proteins show nuclear localization. The ΔNp63δ or ΔNp63ε complete coding region was cloned in the pcDNA3 expression vector and the recombinant vectors were transfected in H1299 cells which were immunostained using the p63 4A4 antibody and a FITC-conjugated secondary antibody. Empty pcDNA3 vector was used as negative control, while cells transfected with vectors expressing ΔNp63α, ΔNp63β and ΔNp63γ were used as positive controls. The subcellular distribution of all ΔNp63 isoforms was assessed by fluorescence microscopy. As shown in Figure 6, we found that the ectopic expression of ΔNp63δ and ΔNp63ε isoforms led to an exclusively nuclear localization of fluorescence, suggesting that these variants, showing nuclear localization, could act as transcription factors and modulate the expression of p63 target genes. Identical cellular localization was, as expected, observed for the ΔNp63α, ΔNp63β and ΔNp63γ proteins, used as controls.
Figure 6.
Nuclear localization of ΔNp63δ and ΔNp63ε isoforms. H1299 cells were transiently transfected for 24 h with individual vectors expressing the novel ΔNp63δ and ϵ isoforms and ΔNp63α, β and γ isoforms as controls, and immunostained using the p63 4A4 antibody and a FITC-conjugated secondary antibody. Nuclei were counterstained with DAPI.
Transactivation activity of ΔNp63δ and ΔNp63ε isoforms towards some p53 family target genes
In order to demonstrate that ΔNp63δ and ΔNp63ε proteins have transcriptional activity, we performed luciferase assays, to assess whether they are able to directly activate some p53 family responsive promoters ( fasn, ada, bax and p21) (18–21). To this end, different luciferase constructs—pGL3promoter-fasn, pGL3promoter-ada, pGL3basic-bax and pGL3basic-p21—were co-transfected into p53 null H1299 cells either with a pcDNA3 empty vector as control or with the pcDNA3 vectors expressing ΔNp63δ and ΔNp63ε and the known ΔNp63 isoforms (α, β, γ), to compare the activity of all ΔNp63 isoforms. In parallel, for each assay, the intracellular level of transfected proteins was assessed by western blot.
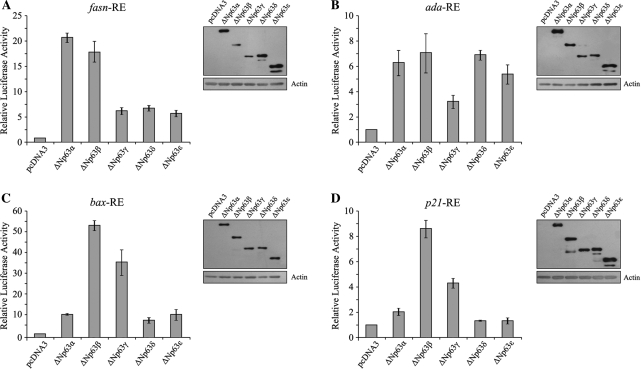
As shown in Figure 7A, we found that ΔNp63δ and ΔNp63ε have significant transcriptional activity towards fasn RE, with 7- and 6-fold activation, respectively, compared with the control, although ΔNp63α and β are the strongest transactivating variants on this element, with 21- and 18-fold activation of the target, respectively. The remaining variant, ΔNp63γ, shows similar levels of target activation to ΔNp63ϵ. Considering that ΔNp63β and γ show the lowest expression levels, the transactivation activity of these two isoforms could be even greater than that evaluated.
Figure 7.
ΔNp63δ and ΔNp63ε isoforms have transactivation activity. H1299 cells were transiently co-transfected with the pcDNA3 empty vector or expressing ΔNp63 α, β, γ, δ and ϵ and the reporter constructs pGL3-fasnRE (A), pGL3-adaRE (B), pGL3-baxRE (C) and pGL3-p21RE (D). The fold increase in relative luciferase activity was calculated using the empty pcDNA3 vector as control. The results represent the average of at least three independent experiments and are shown with the standard deviations. For each reporter construct, the expression level of the ΔNp63 isoforms was checked by western blotting.
Regarding the ada RE (Figure 7B), we found that ΔNp63δ and ε show transactivation activity similar to that of ΔNp63α and β, with an increase of the luciferase activity of about 7-fold for ΔNp63δ and of about 5.5-fold for ΔNp63ε, compared with the control. Weaker transactivation activity is showed by ΔNp63γ, which induced only 3-fold activation of the target. As ΔNp63α and ΔNp63ϵ transfected proteins have higher expression levels than other isoforms, their transactivation activity could be slightly lower than that estimated.
The activation of the bax RE is also differentially modulated by the ΔNp63 isoforms (Figure 7C). We found that ΔNp63δ and ΔNp63ε activate the bax RE, with an increase of the luciferase activity of about 8-fold for ΔNp63δ and of about 10-fold for ΔNp63ε, like ΔNp63α, while ΔNp63β and ΔNp63γ transactivate this element more efficiently, with an increase of about 53-fold and 39-fold, respectively, with respect to the control.
Finally, Figure 7D shows the transcriptional activity of the ΔNp63 isoforms on the p21 RE. ΔNp63δ and ΔNp63ϵ, do not show relevant transcriptional activity on the p21 promoter, as already observed for ΔNp63α (2). In agreement with other groups (2,3), we found that ΔNp63β and ΔNp63γ are able to activate the p21 RE, with an increase of about 9- and 4-fold activation of the target, respectively.
Taken together, these results indicated that the new ΔNp63 isoforms, ΔNp63δ and ΔNp63ϵ, show a significant transactivation activity towards the p63 REs examined, except for p21 and that the ΔNp63 isoforms differ in their regulatory activity on the p63 REs.
Effect of ΔNp63δ and ΔNp63ε isoforms over-expression on p63 target gene expression and cellular proliferation
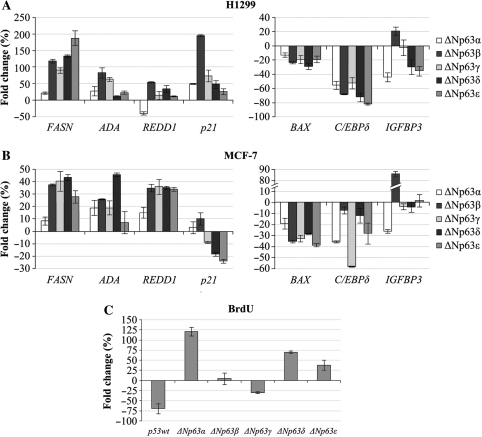
To determine whether the new ΔNp63 variants are able to modulate the endogenous expression of p63 target genes, we performed quantitative RT–PCR to detect the mRNA levels of some ΔNp63 positive (fasn, ada, redd1 and p21) and negative (bax, C/EBPdelta and igfbp-3) genes (18–24) in the presence of over-expression of the five ΔNp63 isoforms in H1299 and MCF-7 cell lines (Figure 8A and B). The levels of exogenously expressed ΔNp63 isoforms in H1299 and MCF-7 cells were monitored by western blot analysis (Figure S1). We found that, in both cell lines, fasn, ada and redd1 genes appear to be upregulated, to varying degrees, by ΔNp63δ and ΔNp63ϵ as well as by the other isoforms (with the exception of redd1 which is downregulated by ΔNp63α in H1299 cells). In particular, in H1299 cells, ΔNp63 ϵ increases the levels of fasn mRNA more than the other isoforms, and in MCF-7 cells ΔNp63δ determines the highest increase of ada mRNA levels. The p21 expression is upregulated by ΔNp63β and slightly by ΔNp63δ and ΔNp63ε in H1299 cells, in accordance with luciferase assay results, while no p21 activation by all isoforms is detected in MCF-7, as previously reported for ΔNp63α and ΔNp63γ (2). In both cell lines, bax expression is downregulated by all ΔNp63 isoforms, suggesting that, in conditions of over-expression, the ΔNp63 isoforms repress the expression of the pro-apoptotic bax gene in accordance with their known antiapoptotic role (25). This result is in contrast with the luciferase results, but could be due to the artificial nature of the luciferase construct. In both cell lines, the other two negative target genes C/EBPdelta and igfbp-3 are downregulated by ΔNp63δ and ΔNp63ϵ while, interestingly, igfbp-3 appears to be upregulated by ΔNp63β. These results demonstrate that ΔNp63δ and ΔNp63ϵ, like the other ΔNp63 isoforms, are able to modulate in vivo the expression of ΔNp63 target genes and that this modulation varies in the two cellular contexts.
Figure 8.
Ectopic expression of ΔNp63δ and ΔNp63ε isoforms affects the in vivo expression of p63 target genes and promotes cellular proliferation. H1299 (A) and MCF-7 (B) cell lines were transfected with the pcDNA3 vector or vector expressing ΔNp63 α, β, γ, δ and ϵ and harvested after 48 h. mRNA levels of fasn, ada, redd1, p21, bax, C/EBPdelta and igfbp-3 genes were quantified by qRT–PCR. Values are expressed as percentage of fold change respect to the control (pcDNA3), after internal normalization to gapdh expression. (C) Bromodeoxyuridine (BrdU) incorporation after 3 h pulses by MCF-7 cells transfected with pcDNA3 control vector, or with the indicated expression vectors.
On the basis of previous data and in order to assess whether ΔNp63 isoforms affect cellular proliferation, we compared MCF-7 cells over-expressing ΔNp63 isoforms with wild-type cells, by BrdU incorporation (Figure 8C). The levels of exogenously expressed ΔNp63 isoforms in MCF7 cell line were monitored by western blot analysis (data not shown). Interestingly, the ectopic expression of the ΔNp63α, ΔNp63δ and ϵ isoforms, but not of p53 and ΔNp63β and γ isoforms, increased cell proliferation rate to different degrees: ΔNp63α showing a higher efficacy than ΔNp63δ and ϵ. These data demonstrate that ΔNp63δ and ϵ isoforms, like ΔNp63α, have effects distinct from those of ΔNp63β, ΔNp63γ and p53 on the cell cycle and that their over-expression increases cell proliferation rate.
Expression of ΔNp63δ and ΔNp63ϵ isoforms during keratinocyte differentiation
It has been reported that ΔNp63α expression is modulated during keratinocyte differentiation; being required to maintain the proliferative potential of basal cells of the epidermis but being down-regulated in terminally differentiated cells in more luminal strata (8). We investigated the expression of the new ΔNp63δ and ΔNp63ϵ isoforms during keratinocyte differentiation, by analyzing the expression pattern of all ΔNp63 isoforms in differentiating keratinocyte cells, used as a model of squamous epithelium. To induce differentiation of proliferating keratinocytes, we altered the extracellular Ca2+ concentration from 0 mM to 2 mM and the medium serum concentration from 1× to 0.1×. The expression of the ΔNp63 isoforms was analyzed at 24 and 48 h following the differentiation stimulus.
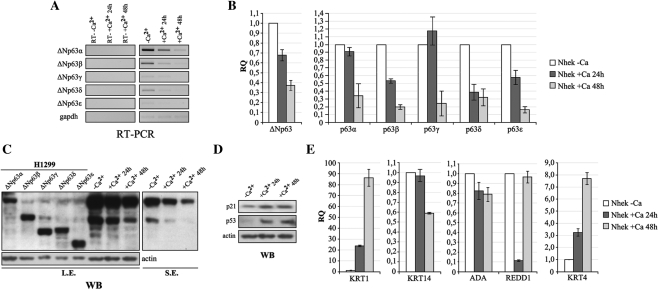
RT–PCR experiments (Figure 9A), performed using primer pair sets able to amplify the full-length ΔNp63 transcripts, and qRT–PCR analysis (Figure 9B) of ΔNp63 variants showed a progressive reduction of the levels of all ΔNp63 transcripts, in particular, at 48 h after the differentiation stimulus. Figure 9C shows the ΔNp63 protein pattern during the differentiation process which is similar to that of the transcripts, as a reduction of all ΔNp63 isoforms is observed, such that, only ΔNp63α remains detectable 48 h after the differentiation stimulus.
Figure 9.
Expression of ΔNp63δ and ΔNp63ε isoforms is modulated during keratinocyte differentiation. Differentiation of proliferating keratinocytes was induced by varying the extracellular Ca2+ concentration from 0 to 2 mM and the medium serum concentration from 1× to 0.1× for 24 and 48 h. (A) RT–PCR analysis of full-length ΔNp63 mRNA levels in proliferating keratinocytes (–Ca2+) and at 24 and 48 h after Ca2+ addition. (B) qRT–PCR analyses of ΔNp63 variants in proliferating keratinocytes (–Ca2+) and at 24 h and 48 h after Ca2+ addition. Values are expressed as fold change respect to the control (–Ca2+) after internal normalization to gapdh expression. (C) Western blotting analysis of all ΔNp63 proteins in proliferating keratinocytes (–Ca2+) and at 24 and 48 h after Ca2+ addition. Ectopically expressed ΔNp63 proteins were used as molecular marker to assess an identity to each ΔNp63 isoform. The pan-p63 4A4 antibody was used to detect p63 isoforms. L.E. = long exposure, S.E. = short exposure. (D) Western blotting analysis of p21 and p53, used as controls of the differentiation induction. (E) qRT–PCR analysis of ada, redd1, keratin 4, keratin 14 and keratin 1 mRNA levels during keratinocyte differentiation. Values are expressed as fold induction respect to the control (–Ca2+), after internal normalization to gapdh expression.
As controls for the differentiation induction, we first monitored the levels of p21 and p53 proteins, involved in cell cycle block. As shown in Figure 9D, at 24 and 48 h after the differentiation stimulus, p21 levels and especially p53 levels increased, with respect to proliferating cells.
Moreover, we analyzed the transcript levels of keratin 14 and keratin 1, two keratinocyte differentiation markers, to confirm the proper differentiation status of Nhek cells (Figure 9E). We found a remarkable progressive increase of keratin 1 mRNA and a decrease, more evident at 48 h, of the transcript of keratin 14, a marker of the less differentiated layers of epidermis, which is also positively regulated by ΔNp63 (26). In addition, we examined the expression of other ΔNp63-regulated genes (ada, redd1, keratin 4) (Figure 9E). Consistent with the reduction of all ΔNp63 isoforms during differentiation, we also observed a relative decrease of transcripts of ada and redd1 target genes and an increase of keratin 4 expression, which was reported to be downregulated by ΔNp63 (27).
DISCUSSION
The p63 gene, along with p53 and p73, belongs to the p53 gene family. Although their products show some common structural and functional features, each protein seems to have specific biological functions. In fact, while p53-deficient mice grow normally but undergo spontaneous tumor development, p73 and p63 knockout mice exhibit severe developmental and differentiation defects (6,7,28). A common feature of the three genes is to produce a great variety of isoforms, either derived from the use of an internal cryptic promoter or by alternative splicing, giving rise to a network of proteins whose fine modulation is fundamental in the regulation of biological activities, ranging from development and differentiation to growth arrest and apoptosis (29). The p63 gene generates the expression of two subclasses of isoforms, namely, those containing the TA, called TA isoforms, and those lacking this domain, called ΔN isoforms. Moreover, three alternative splicing events have been identified, producing the α, β and γ, variants, which incorporate various portions of the C-terminus, for both TA and ΔNp63 isoforms.
Therefore, until now six different p63 isoforms (TAp63 α, β, γ and ΔNp63 α, β, γ) have been identified, with a complex array of similarities and differences in their structural domains and transcriptional activities.
By using the ASPIC algorithm (16,17), a tool for alternative splicing prediction, based on multiple transcripts and/or EST sequence comparison and alignment against the gene sequence, we identified two new human p63 C-terminus variants, one we named δ, deriving from an alternative splicing event between exons 11 and 14, and the other, we defined ε, that keeps the 5′ portion of intron 10, which immediately presents a stop codon. Transcript isoforms homologous to the ε and δ variants were not detected for p73 gene. The ASPIC algorithm, unlike other alternative splicing prediction methods which perform independent single transcript alignments with the genomic region, carries out an optimized alignment of all the gene-related transcripts with the aim of minimizing the number of predicted splice sites and consequently of the relevant transcript variants. The reliability of the ASPIC algorithm along with the significant number of EST sequences supporting these new p63 variants, prompted us to investigate the presence of these new p63 proteins in vivo.
Here, first we identified these new p63 variants in keratinocytes and MCF-7 cells, indicating that the ASPIC algorithm could be considered to provide valid starting point in the search for transcriptional variants of genes of interest (Figures 2 and 3). In keratinocytes, known to express high levels of p63 as ΔN variants, we observed a differential expression of the novel variants with respect to the other known isoforms. In particular, ΔNp63δ is highly expressed respect to ΔNp63γ and ΔNp63ϵ and, as expected, the ΔNα and β variants are the predominant isoforms (Figure 4B).
We extended the study of the expression of all five p63 variants, by qRT–PCR methodology, to different normal human tissues and cell lines in order to determine whether it was tissue-specific (Figure 4). The results confirmed that the p63 variants are differentially expressed, either as TA and ΔN form, in tested samples and that the two new variants, δ and ϵ, show interesting expression profiles. In brain, where only the TA class was detected, the ϵ variant is the most expressed after the α variant, and in muscle tissue, the TAp63ϵ transcript shows levels only slightly lower than TAp63α. Among the cell lines analyzed, HaCat cells show a p63 expression profile similar to keratinocytes, while in MCF-7 cells, ϵ variants show similar expression level to β and γ variants, while δ variants show the lowest expression. Interestingly, in 293 cells, the ϵ and δ variants seem to be the predominant variants after α. We also compared the relative levels of each p63 variant among the tissues and cell lines (Figure 5), showing that α, β and δ variants are more expressed in keratinocytes, while γ and ϵ variants are present at higher levels in muscle tissue. Very low levels of all p63 variants are present in brain with respect to the other tissues analyzed. Among cell lines, very low expression of all variants were observed in MCF-7 and 293 cells with respect to HaCat cells.
After demonstrating the in vivo expression of the new δ and ϵ p63 variants, we have performed initial functional characterization. An indication that, like other isoforms, ΔNp63δ and ΔNp63ϵ act as transcription factors, was derived from the study of their cellular localization and transactivation activity. In fact, both ΔNp63δ and ΔNp63ϵ show a nuclear localization (Figure 6), have significant transactivation activity towards the p63 REs examined (Figure 7) and are able to regulate in vivo the expression of ΔNp63 positive and negative target genes although with different efficiency with respect to the other ΔNp63 isoforms (Figure 8A and B). The effect of the ectopic expression of the ΔNp63 isoforms on the expression of the target genes is variable in the two cellular contexts examined, suggesting that other cell-specific transcription factors may participate with ΔNp63 proteins in the regulation of these genes. Moreover, we show that the ectopic expression of the ΔNp63δ and ϵ isoforms, like ΔNp63α, increase the cell proliferation rate, while ΔNp63β, ΔNp63γ and p53 reduce proliferation rates (Figure 8C). Taken together, these data may suggest that ΔNp63δ and ϵ isoforms, as ΔNp63α, could have a role in sustaining cell proliferation as they activate genes involved in cell cycle progression (fasn and ada) but not the pro-apoptotic gene bax.
The δ and ϵ p63 proteins lack one of the two TAs (TA2), which was demonstrated to be required for the transcriptional activity of the ΔN isoforms (3). Overall, our results demonstrate that the ΔN forms of these variants are functionally active, suggesting that the TA (TA1*) (2) present at the N-terminus of all ΔN isoforms contributes significantly to their transcriptional activity.
Moreover, we studied the expression profile of the new ΔNp63 variants during keratinocyte differentiation. Many studies report a role for p63 in the differentiation of epidermis, a key process by which the epithelial cells acquire their proper morphological and functional properties (30). However, it remains unclear how exactly this takes place. The basal compartment of stratified epithelia is made of cells with high proliferative capacity that replenish the terminally differentiated populations in the more luminal strata. ΔNp63α is the isoform predominantly expressed in this basal compartment while TA isoforms are not detectable. It is suggested that ΔNp63α is required to maintain the proliferative potential of basal cells and to allow the initial induction of keratinocyte differentiation, while it must be downregulated to allow terminal differentiation (9).
Our results confirm that the differentiation process is associated with lower levels of all ΔNp63 isoforms (Figure 9) not only ΔNp63α, at both the transcripts and protein levels, although their relative abundance seems to be maintained. Moreover, during differentiation, we observed a reduction of the expression of some known ΔNp63 target genes, consistent with the reduction of the ΔNp63 variants, supporting the hypothesis that all isoforms, including ΔNp63δ and ΔNp63ϵ, could, along with ΔNp63α, play a pro-proliferative role in this physiological condition. Further studies to define the signals controlling the balance in the expression of all the p63 isoforms will help to better understand the contribution of p63 to proper epidermal homeostasis.
Taken together, our results demonstrate the existence of new p63 variants, which are functionally active as transcription factors, increasing to 10 the number of the isoforms that are produced by the p63 gene.
The implications of this study open interesting avenues for future investigation. In particular, we have demonstrated tissue/cell type-specific expression of p63 alternative transcripts, suggesting possible relationships to their different cellular function. A better understanding of the regulation of expression of all p63 possible variants will thus be essential for meaningful p63 functional studies. Indeed, future studies on p63 function should take into account the possible presence of these new variants in the tissue or cell type examined, which if ignored would provide partial and/or potentially incorrect results.
Furthermore, the existence of new p63 variants increases the known complexity of the crosstalk among all the p53 family members and of their mutual regulation in the maintenance of the proper cellular growth of epithelial tissues.
ACCESSION NUMBERS
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Telethon (grant number GGP06158), Progetto Strategico Regione Puglia and Grant 2008 of the Bari University (ex 60%). Funding for open access charge: Associazione Italiana Ricerca sul Cancro (AIRC).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr D. Horner for suggestions and critical reading of the manuscript.
REFERENCES
- 1.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 2.Helton ES, Zhu J, Chen X. The unique NH2-terminally deleted (DeltaN) residues, the PXXP motif, and the PPXY motif are required for the transcriptional activity of the DeltaN variant of p63. J. Biol. Chem. 2006;281:2533–2542. doi: 10.1074/jbc.M507964200. [DOI] [PubMed] [Google Scholar]
- 3.Ghioni P, Bolognese F, Duijf PH, Van Bokhoven H, Mantovani R, Guerrini L. Complex transcriptional effects of p63 isoforms: identification of novel activation and repression domains. Mol. Cell Biol. 2002;22:8659–8668. doi: 10.1128/MCB.22.24.8659-8668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serber Z, Lai HC, Yang A, Ou HD, Sigal MS, Kelly AE, Darimont BD, Duijf PH, Van Bokhoven H, McKeon F, et al. A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol. Cell Biol. 2002;22:8601–8611. doi: 10.1128/MCB.22.24.8601-8611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 6.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 7.Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 8.King KE, Weinberg WC. p63: defining roles in morphogenesis, homeostasis, and neoplasia of the epidermis. Mol. Carcinogen. 2007;46:716–724. doi: 10.1002/mc.20337. [DOI] [PubMed] [Google Scholar]
- 9.Koster MI, Roop DR. The role of p63 in development and differentiation of the epidermis. J. Dermatol. Sci. 2004;34:3–9. doi: 10.1016/j.jdermsci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, De Laurenzi V, Spagnoli LG, Catani MV, Ramadan S, et al. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Diff. 2006;13:1037–1047. doi: 10.1038/sj.cdd.4401926. [DOI] [PubMed] [Google Scholar]
- 11.Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinne T, Brunner HG, van Bokhoven H. p63-associated disorders. Cell Cycle. 2007;6:262–268. doi: 10.4161/cc.6.3.3796. [DOI] [PubMed] [Google Scholar]
- 13.Deyoung MP, Ellisen LW. p63 and p73 in human cancer: defining the network. Oncogene. 2007;26:5169–5183. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 14.Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D, Yang A, McKeon F, Jacks T. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell. 2005;7:363–373. doi: 10.1016/j.ccr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Keyes WM, Vogel H, Koster MI, Guo X, Qi Y, Petherbridge KM, Roop DR, Bradley A, Mills AA. p63 heterozygous mutant mice are not prone to spontaneous or chemically induced tumors. Proc. Natl Acad. Sci. USA. 2006;103:8435–8440. doi: 10.1073/pnas.0602477103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castrignano T, Rizzi R, Talamo IG, De Meo PD, Anselmo A, Bonizzoni P, Pesole G. ASPIC: a web resource for alternative splicing prediction and transcript isoforms characterization. Nucleic Acids Res. 2006;34:W440–W443. doi: 10.1093/nar/gkl324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castrignano T, D'Antonio M, Anselmo A, Carrabino D, D'Onorio De Meo A, D'Erchia AM, Licciulli F, Mangiulli M, Mignone F, Pavesi G, et al. ASPicDB: a database resource for alternative splicing analysis. Bioinformatics. 2008;24:1300–1304. doi: 10.1093/bioinformatics/btn113. [DOI] [PubMed] [Google Scholar]
- 18.D'Erchia AM, Tullo A, Lefkimmiatis K, Saccone C, Sbisa E. The fatty acid synthase gene is a conserved p53 family target from worm to human. Cell Cycle. 2006;5:750–758. doi: 10.4161/cc.5.7.2622. [DOI] [PubMed] [Google Scholar]
- 19.Sbisa E, Mastropasqua G, Lefkimmiatis K, Caratozzolo MF, D'Erchia AM, Tullo A. Connecting p63 to cellular proliferation: the example of the adenosine deaminase target gene. Cell Cycle. 2006;5:205–212. doi: 10.4161/cc.5.2.2361. [DOI] [PubMed] [Google Scholar]
- 20.Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 21.Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol. Cell Biol. 2003;23:2264–2276. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, Haber DA. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 23.Borrelli S, Testoni B, Callari M, Alotto D, Castagnoli C, Romano RA, Sinha S, Vigano AM, Mantovani R. Reciprocal regulation of p63 by C/EBP delta in human keratinocytes. BMC Mol. Biol. 2007;8:85. doi: 10.1186/1471-2199-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbieri CE, Perez CA, Johnson KN, Ely KA, Billheimer D, Pietenpol JA. IGFBP-3 is a direct target of transcriptional regulation by DeltaNp63alpha in squamous epithelium. Cancer Res. 2005;65:2314–2320. doi: 10.1158/0008-5472.CAN-04-3449. [DOI] [PubMed] [Google Scholar]
- 25.Chu WK, Lee KC, Chow SE, Chen JK. Dual regulation of the DeltaNp63 transcriptional activity by DeltaNp63 in human nasopharyngeal carcinoma cell. Biochem. Biophys. Res. Commun. 2006;342:1356–1360. doi: 10.1016/j.bbrc.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 26.Romano RA, Birkaya B, Sinha S. A functional enhancer of keratin14 is a direct transcriptional target of deltaNp63. J. Invest. Dermatol. 2007;127:1175–1186. doi: 10.1038/sj.jid.5700652. [DOI] [PubMed] [Google Scholar]
- 27.Boldrup L, Coates PJ, Gu X, Nylander K. DeltaNp63 isoforms regulate CD44 and keratins 4, 6, 14 and 19 in squamous cell carcinoma of head and neck. J. Pathol. 2007;213:384–391. doi: 10.1002/path.2237. [DOI] [PubMed] [Google Scholar]
- 28.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 29.Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Diff. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 30.Barbieri CE, Pietenpol JA. p63 and epithelial biology. Exp. Cell Res. 2006;312:695–706. doi: 10.1016/j.yexcr.2005.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.