Abstract
Selenocysteine (Sec) is the 21st amino acid in translation. Sec tRNA (tRNASec) has an anticodon complementary to the UGA codon. We solved the crystal structure of human tRNASec. tRNASec has a 9-bp acceptor stem and a 4-bp T stem, in contrast with the 7-bp acceptor stem and the 5-bp T stem in the canonical tRNAs. The acceptor stem is kinked between the U6:U67 and G7:C66 base pairs, leading to a bent acceptor-T stem helix. tRNASec has a 6-bp D stem and a 4-nt D loop. The long D stem includes unique A14:U21 and G15:C20a pairs. The D-loop:T-loop interactions include the base pairs G18:U55 and U16:U59, and a unique base triple, U20:G19:C56. The extra arm comprises of a 6-bp stem and a 4-nt loop. Remarkably, the D stem and the extra arm do not form tertiary interactions in tRNASec. Instead, tRNASec has an open cavity, in place of the tertiary core of a canonical tRNA. The linker residues, A8 and U9, connecting the acceptor and D stems, are not involved in tertiary base pairing. Instead, U9 is stacked on the first base pair of the extra arm. These features might allow tRNASec to be the target of the Sec synthesis/incorporation machineries.
INTRODUCTION
Selenocysteine (Sec) is known as the 21st amino acid used in translation (1,2). Its structure is similar to that of cysteine (Cys), and the selenium atom in Sec corresponds to the sulfur atom in Cys. The selenol group in Sec is more nucleophilic than the thiol group in Cys, with pKa values of ∼5.2 and ∼8.5, respectively (3). Therefore, the Sec residue is primarily used at the active center for oxidation-reduction reactions (4–6). Sec and its biosynthesis/insertion machineries are widely distributed from bacteria to humans, and are essential for their viability. Humans have 25 selenoproteins (7).
In contrast to the other amino acids, Sec is synthesized on the Sec-specific tRNA species (tRNASec) through a multistep process. tRNASec is first aminoacylated with serine by seryl-tRNA synthetase (SerRS) (8). In eukarya and archaea, the hydroxyl group of the seryl moiety is further phosphorylated by O-phosphoseryl-tRNA kinase (PSTK) (9). The phosphate group is then converted to the selenol group by Sep-tRNA:Sec-tRNA synthase (SepSecS), to produce selenocysteinyl-tRNASec (Sec-tRNASec) (10,11). In bacteria, Ser-tRNASec is directly converted to Sec-tRNASec by Sec synthase (SecS or SelA) (12). The selenophosphate generated by selenophosphate synthetase (SPS) is utilized as a reactive selenium donor for Sec synthesis in both eukarya/archaea and bacteria (13,14).
tRNASec has the anticodon complementary to the stop codon UGA (8), and translates it in a selenoprotein messenger RNA (mRNA) to Sec, in response to a Sec-insertion sequence (SECIS) (15,16). Sec-specific translation elongation factor (EF-Sec, SelB) brings Sec-tRNASec to the ribosome in response to the SECIS (17–20), and Sec is then incorporated into a nascent, growing polypeptide, by decoding the UGA codon. Thus, tRNASec plays a vital role in both the Sec biosynthesis and its insertion into selenoproteins. Bacterial SelB directly recognizes SECIS (18,21,22), while eukaryal/archaeal EF-Sec needs an additional factor for SECIS recognition (23,24). SECIS binding protein 2 (SBP2) recognizes SECIS in eukarya (23,25), but the additional factor has not been identified yet in archaea. Another factor, SECp43, is involved in the Sec synthesis and insertion in eukarya (26,27), but the precise function of SECp43 is not known.
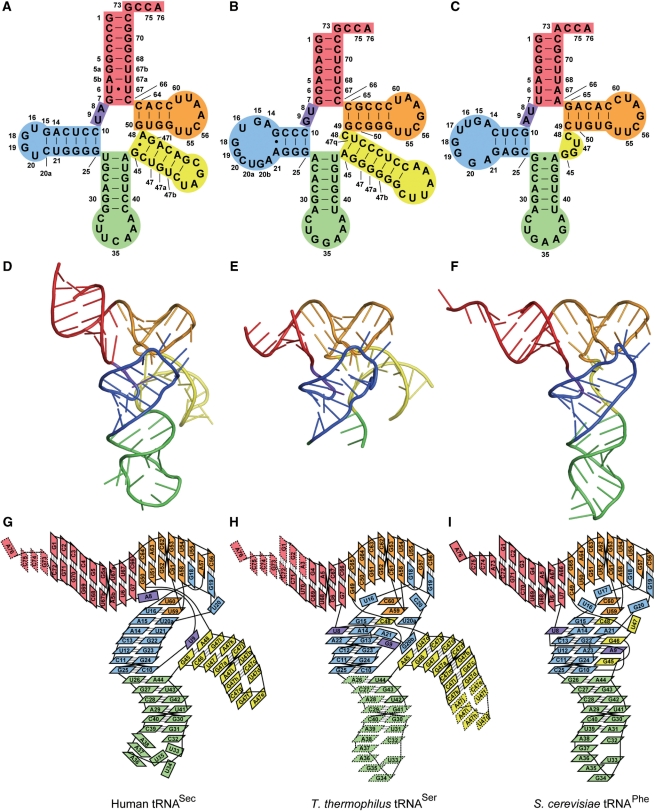
tRNASec is the largest among known tRNA species; it consists of 90–100-nt residues, in contrast to most other tRNAs, with ∼76-nt residues (28) (Figure 1A–C). Biochemical analyses using enzymatic and chemical probes revealed the unique structure of tRNASec (29,30). It has a long extra arm, similar to those of the serine, leucine and tyrosine tRNAs. However, eukaryal/archaeal tRNASec has a 9-bp acceptor stem and a 4-bp T stem (9/4 secondary structure), and bacterial tRNASec has an 8-bp acceptor stem and a 5-bp T stem (8/5 secondary structure). In both the eukaryal/archaeal and bacterial tRNASecs, the acceptor and T stems have 13 bp in total. Conversely, the canonical tRNAs have a 7-bp acceptor stem and a 5-bp T stem (7/5 secondary structure) (Figure 1A–C). tRNASec also has a 6-bp D-stem and a 4-nt D-loop, in contrast to the 4-bp D-stem and approximately 8-nt D-loop in the canonical tRNAs. The unique structure presumably allows tRNASec, like tRNASer, to function as a substrate of SerRS, and, in contrast, as the exclusive target of PSTK, SepSecS (or SelA), and EF-Sec. Despite its significance and distinctive features, the 3D structure of tRNASec has not yet been solved.
Figure 1.
Structure of human tRNASec, and its comparison with other tRNAs. Human tRNASec is shown as a cloverleaf model (A), a ribbon model (D) and a diagram representing tertiary interactions (G). For comparison, T. thermophilus tRNASer [PDB ID: 1SER (38)] (B, E and H) and S. cerevisiae tRNAPhe [PDB ID: 4TNA (47)] (C, F and I) are shown. The acceptor arm, AD linker, D arm, anticodon arm, extra arm and T arm are colored red, purple, blue, green, yellow and orange, respectively.
Here, we report the crystal structure of human tRNASec. The structure revealed the unusual secondary structures of the acceptor, T and D stems, as well as unique tertiary interactions. In contrast with the usual tRNAs, the long D stem of tRNASec does not interact with the extra arm, and thus, tRNASec has an open cavity in place of the hydrophobic core.
MATERIALS AND METHODS
Preparation of human tRNASec
To synthesize the template DNA for the transcription of human tRNASec, the following oligonucleotides were used: 5′-GGTGGTggatccTAATACGACTCACTATAGCCCGGATGATCCTCAGTG GTCTGGGGTGCAGGCTTCAAACCT-3′ and 5′-GGTGGTgcatgcCCTGGCGCCCGAAAGGTGGAATTGAACCACTCTGTCGCTAG ACAGCTACAGGTTTGAAGCCT-3′. The underlined 3′ regions are complementary to each other for annealing. After annealing, the 3′-ends were both extended by overlap extension polymerase chain reaction (OE-PCR). The synthesized DNA includes the consensus sequence of T7 RNA polymerase promoters, just upstream of the tRNASec gene (indicated as bold letters). BamHI and SphI sites were placed at the upstream and downstream ends, respectively, for cloning. The synthesized fragment was cloned between the BamHI and SphI sites of the pUC18 vector (Roche).
Using this plasmid as a template, the DNA region containing the tRNASec gene was amplified by PCR and used as the template for in vitro transcription. The M13-reverse primer and a primer complementary to the 3′ end of the target tRNA (5′-TGGCGCCCGAAAGGTGGAATTG-3′) were used for PCR.
Human tRNASec was transcribed in vitro with T7 RNA polymerase, as described previously (31). The transcribed tRNASec was purified by anion-exchange chromatography using a ResourceQ (GE-Healthcare Bio-Sciences) column. The tRNASec was dissolved in 10 mM Tris–HCl buffer (pH 8.0) containing 10 mM MgCl2, and was heated at 65°C for 5 min for refolding.
Preparation of Methanocaldococcus jannaschii SepSecS
Methanocaldococcus jannaschii SepSecS gene was cloned into pET22b (Merck). The protein was overexpressed in Escherichia coli Rosetta 2(DE3) (Merck) transformed by the vector. The cells were sonicated, heat-treated at 85°C and purified by successive chromatography steps on a Phenyl-Toyopearl (Tosoh) column with a gradient of 1.8–0.0 M ammonium sulfate, and a SP-Sepharose FF (GE-Healthcare) column with a gradient of 0.05–0.6 M NaCl. SepSecS-containing fractions were pooled and dialyzed against 20 mM Tris–HCl buffer (pH 7.5), containing 300 mM NaCl and 10 mM 2-mercaptoethanol (2-ME).
Crystallization and X-ray diffraction data collection
tRNASec was dissolved in 20 mM Tris–HCl buffer (pH 7.5), containing 300 mM NaCl, 10 mM MgCl2 and 10 mM 2-ME, and the concentration was adjusted to 120 μM. This sample solution also contained 80 μM M. jannaschii SepSecS. The mixture was heated at 65°C for 10 min and gradually cooled to room temperature. Crystal Screen 2, Natrix (Hampton Research), and Wizard I and II (Emerald BioSystems) kits were used for the initial screening of crystallization conditions. Crystals were obtained with Crystal Screen 2 Reagent 13, and the conditions were further optimized. Crystals suitable for data collection were obtained by mixing 0.75-μl sample solution and 0.75-μl reservoir solution, containing 100 mM sodium acetate-HCl buffer (pH 4.1), 32% (w/v) polyethylene glycol (PEG) 1500 and 140 mM lithium sulfate, and by equilibrating the mixture against 200-μl reservoir solution at 20°C by the sitting-drop vapor-diffusion method. We confirmed that the crystals contained only tRNASec by SDS–PAGE and urea–PAGE analyses (data not shown). No crystal was obtained when sample solutions without SepSecS were used.
Prior to data collection, the crystals were soaked in a cryo-protective solution, containing 100 mM sodium acetate–HCl (pH 4.6), 35% (w/v) PEG 1000, 25 mM ammonium sulfate and 10 mM MgCl2, and were flash-cooled with liquid nitrogen. A 3.1-Å X-ray diffraction data set was obtained by using the synchrotron radiation at BL41XU of SPring-8 (Hyogo, Japan). The data were indexed, integrated and scaled with the HKL2000 program (32) (Table 1). The crystals belong to the space group C2, with unit cell parameters a = 78.9, b = 51.6, c = 81.4 Å, β= 101.6°.
Table 1.
Data collection and refinement statistics
| PDB ID | 3A3A |
| Data collection | |
| Wave length (Å) | 1.000 |
| Space group | C2 |
| Cell dimensions | a = 78.9, b = 51.6, c = 81.4 Å, β = 101.6° |
| Resolution (Å) | 50.0–3.10 (3.21–3.10) |
| Unique reflections | 5833 |
| Completeness (%) | 96.7 (85.9) |
| Redundancy | 5.4 (5.4) |
| Rsyma | 0.105 (0.390) |
| Mean I/σ(I) | 18.0 (3.53) |
| Structure refinement | |
| Working set reflections | 5523 |
| Test set reflections | 258 |
| Resolution (Å) | 50.0–3.10 |
| No. of RNA residues | 86 |
| No. of RNA atoms | 1829 |
| Rwork/Rfreeb | 0.254/0.314 |
| Average B factor (Å2) | 115.0 |
| RMSD values of bond lengths (Å) | 0.0046 |
| RMSD values of bond angles (°) | 0.92 |
aRsym = ∑hkl∑i [|Ii(hkl ) – <I(hkl)>|]/∑hkl∑i [Ii(hkl )], where Ii(hkl ) is the intensity of the i-th measurement of hkl and <I(hkl )> is the average value of Ii (hkl ) for all i measurements.
bRwork = ∑hkl (||Fobs| – k|Fcalc||)/∑hkl (|Fobs|), Rfree = ∑hklTest(||Fobs|–k|Fcalc||)/∑hklTest (|Fobs|), where hkl Test is the test set reflections that were not used during refinement (4.5% of the data set).
Structure determination and refinement
The human tRNASec crystal structure was solved by the molecular replacement method with the Phaser program (33). Partial structures from the tRNAAsp and tRNAPhe coordinates [PDB ID: 2TRA (34) and 6TNA (35)] were used as the search models. The nucleotide sequences of the tRNAAsp and tRNAPhe structures were replaced by that of human tRNASec. The search model used for the first round of molecular replacement was composed of the acceptor stem (residues 1–7 and 66–72), the T arm (residues 49–65) and part of the D loop (residues 18 and 19) of the sequence-modified tRNAAsp. The search solution (solution I) was obtained just for the corresponding part of the tRNASec. Solution I was refined against the diffraction data by positional energy minimization refinement with the CNS program (36). The second round was done using the refined solution I as a fixed model. The search model used for the second round was the anticodon-arm part (residues 26–44) of the sequence-modified tRNAPhe structure. The second search solution (solution II) identified the extra-arm stem of the human tRNASec. Solution II was also refined and used as the fixed model for the final round. The search model used for the final round was the anticodon stem (residues 26–31 and 39–44) of the sequence-modified tRNAAsp structure. The final search solution yielded the anticodon stem of the tRNASec. The D-arm portion of tRNASec was not found by the molecular replacement, and its coordinates were built in an |Fo-Fc| electron density map.
The structure was refined against the diffraction data by iterative cycles of simulated-annealing, positional and temperature-factor refinements with the CNS program (36), and manual model building and revision with the Coot program (37). Multiple rounds of refinement generated the convergence of the Rwork and Rfree to 0.254 and 0.314, respectively (Table 1). The Rwork and Rfree are relatively higher than those of the reported crystal structures of RNAs, possibly due to the missing tRNA terminus and the partially disordered anticodon loop.
Docking model construction
The docking model of SerRS and tRNASec was created based on the structure of the Thermus thermophilus SerRS·tRNASer complex [PDB ID: 1SER (38)]. We used the structure of SerRS from the archaeon Pyrococcus horikoshii [PDB ID: 2DQ0 (39)], as a model of a eukaryal SerRS. SerRS in T. thermophilus SerRS·tRNASer was replaced by P. horikoshii SerRS, as described previously (39). The T. thermophilus tRNASer structure was then replaced by human tRNASec. The extra stem and the T arm were used for the superposition (residues 45–47c, 47h–48 and 50–64 in tRNASec versus residues 45–47c, 47l–47q and 50–64 in tRNASer).
The present tRNASec structure was docked onto the complex structure of Thermus aquaticus EF-Tu and Saccharomyces cerevisiae tRNAPhe [PDB ID: 1TTT (40)]. tRNAPhe in the EF-Tu·tRNAPhe complex was replaced by human tRNASec, to create the EF-Tu·tRNASec docking model. The 12 bp of the acceptor-T stem of human tRNASec (residues 1–7, 50–52 and 62–72) and those of tRNAPhe (residues 1–7, 49–53 and 61–72) were used as the corresponding region for the superposition. Furthermore, EF-Tu in the EF-Tu·tRNASec docking model was replaced by Methanococcus maripaludis SelB/EF-Sec [PDB ID: 1WB3 (41)], to create the EF-Sec·tRNASec docking model. Homologous domains I–III were used for the superposition.
RESULTS
Structure determination
Human tRNASec was prepared by in vitro transcription with T7 RNA polymerase. Single crystals of tRNASec were obtained under conditions containing M. jannaschii SepSecS. The crystal structure was solved by molecular replacement, using the RNA helix or hairpin portions of reported tRNA structures [tRNAAsp (34) and tRNAPhe (35)] as the search models. The resolution is 3.1 Å and the final refinement R factors, Rwork and Rfree, are 0.254 and 0.314, respectively (Table 1). The crystals contained one tRNASec molecule per asymmetric unit. Human tRNASec is composed of 90-nt residues (numbered as residues 1–76 in the conventional manner), and the final model includes 86 residues (residues 1–72). The four 3′-end residues, the discriminator G73 and the CCA end (residues 74–76), were disordered.
The tRNASec structure revealed that it consists of the 9-bp acceptor stem (residues 1–5, 5a, 5b, 6, 7, 66, 67, 67a, 67b and 68–72), the linker between the acceptor stem and the D stem (‘AD linker’, residues 8 and 9), the D arm (a 6-bp stem and a 4-nt loop, residues 10–16, 18–20, 20a and 21–25), the anticodon arm (a 6-bp stem and a 7-nt loop, residues 26–44), the extra arm (a 6-bp stem and a 4-nt loop, residues 45–47, 47a–47l and 48) and the T arm (a 4-bp stem and a 7-nt loop, residues 50–64) (Figure 1A, D and G). The nucleotide numbering is based on Sturchler et al. (29)
Several modified nucleosides have been reported in vertebrate tRNASecs, such as 5-methylcarboxymethyluridine (mcm5U) or O2′-methylated mcm5U (mcm5Um) at position 34, N6-isopentyladenosine (i6A) at position 37, pseudouridine (Ψ) at position 55 and 1-methyladenosine (m1A) at position 58 (42,43). The human tRNASec used for crystallization was synthesized by in vitro transcription, and thus lacks modified nucleosides.
The acceptor and T arms
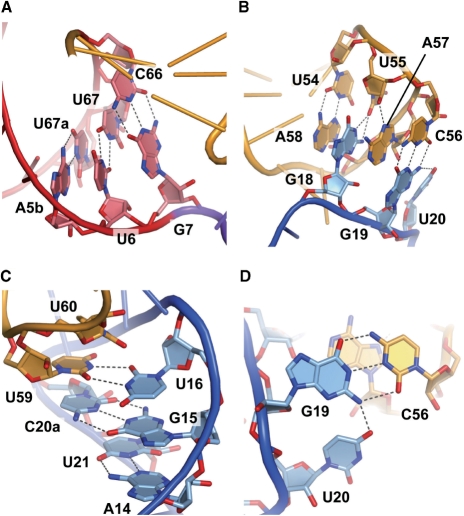
tRNASec has a 9-bp acceptor stem and a 4-bp T stem (9/4 secondary structure), in contrast to the canonical tRNAs (7/5 secondary structure) (Figure 1). The T loop is composed of seven residues, and its conformation is similar to those of the canonical tRNAs (38,44–47). U54 forms a reversed Hoogsteen base pair with A58. As in the canonical tRNAs, U55 and C56 form tertiary interactions with G18 and G19, respectively, in the D loop (Figure 2B). Therefore, the lack of the Ψ55 and m1A58 modifications does not appear to affect the tertiary structure due the T-loop:D-loop interaction.
Figure 2.
tRNASec-specific base pairs and base triples. (A) The U6:U67 base pair in the acceptor stem. (B) The interface of the D and T loops at the tRNA elbow. (C) The A14:U21, G15:C20a and U16:U59 base pairs in the D arm. (D) The U20:G19:C56 base triple. The structural models are colored as in Figure 1.
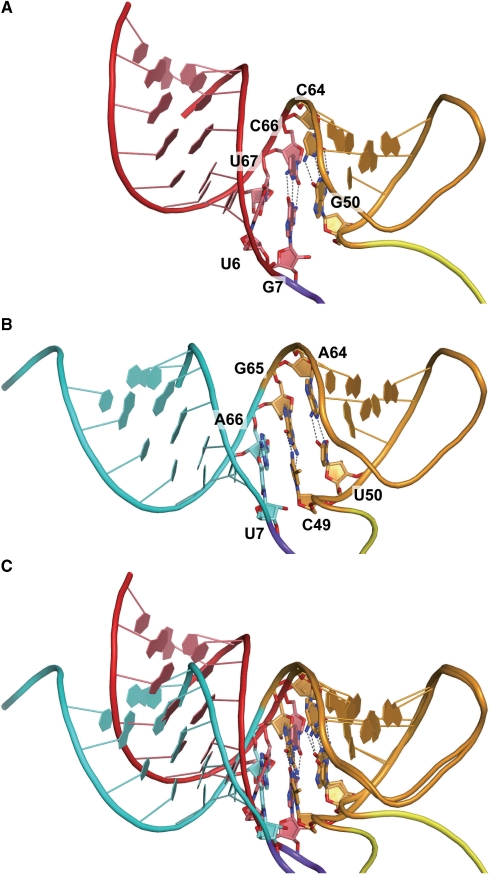
U6 and U67 in the acceptor stem are highly conserved among the eukaryal tRNASecs (Supplementary Figure 1), and they form a non-Watson–Crick-type (non-WC-type) base pair (Figure 2A). The acceptor stem is kinked between the U6:U67 and G7:C66 base pairs, resulting in a bent acceptor-T helix (Figure 3A and C). This is in contrast with the continuous acceptor-T helix observed in many tRNA structures (44–47) (Figure 3B and C). The G1:C72 base pair shares a crystal contact with the G19:C56 base pair in the other molecule in the crystal. We speculate that the acceptor-T helix is bent due to the crystal contact, and therefore is probably straight in solution. Nevertheless, the bent acceptor-T helix may mimic a conformational state required for interactions with other factors, e.g. EF-Sec, as discussed below.
Figure 3.
The bent acceptor-T stem helix. (A) The acceptor and T arms of human tRNASec, colored as in Figure 1. (B) The acceptor and T arms of S. cerevisiae tRNAPhe for comparison. The acceptor arm of S. cerevisiae tRNAPhe is colored cyan. (C) The acceptor and T arms of tRNASec are superposed on those of tRNAPhe. The phosphate atoms of the T arm residues (residues 50–64) were used as the corresponding atoms for the superposition.
The D arm and tertiary interactions
The D arm (residues 10–16, 18–20, 20a and 21–25) is the hairpin structure composed of a 6-bp stem (D stem) and a 4-nt loop (D loop). The D loop of tRNASec comprises only 4-nt residues, U16, G18, G19 and U20, in contrast to those of the canonical tRNAs (7–11 residues). In spite of the small D loop, the D-loop:T-loop interactions (the G18:U55 and G19:C56 base pairs) are conserved (Figure 2B). U16 forms a non-WC-type interaction with U59 (Figure 2C), while other types of the 16:59 pair are also found in a few canonical tRNAs (40,48). The U16:U59 pair is sandwiched between the sixth base pair of the D stem (the G15:C20a pair) and U60 in the T loop (Figure 2C). The last D-loop residue, U20, interacts with G19 to form a base triple (U20:G19:C56) (Figure 2B and D).
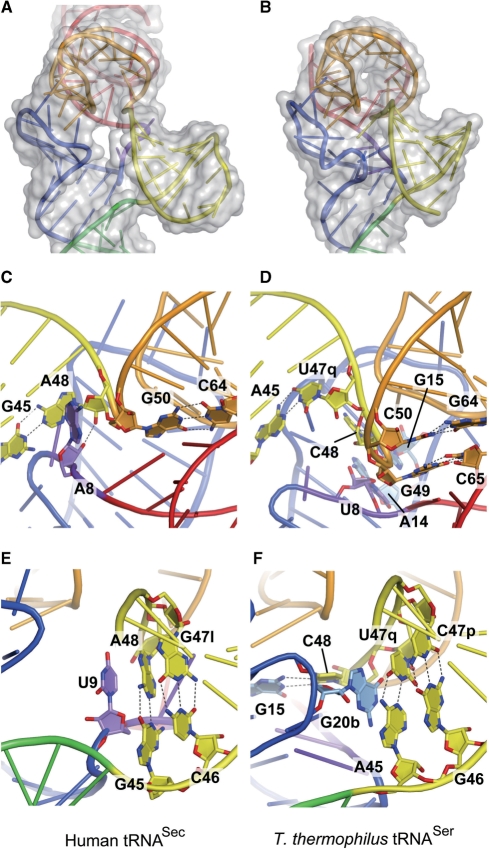
The D stem has 6 bp, as it includes the A14:U21 and G15:C20a pairs, which are found only in tRNASec (Figure 2C). Remarkably, none of the D-stem residues participates in tertiary interactions. The tertiary interactions are limited to those between the D and T loops. This is in a sharp contrast with the fact that the D stem interacts with the AD linker (residues 8 and 9) and the extra arm (residues 45–48) to form a core of tertiary interactions in the canonical tRNAs. These include the conserved pairs U8:A14 and R15:Y48, where R and Y denote G/A and C/U, respectively, and some nonconserved pairs, 9:23, 9:13, 10:45 and 22:46 (38,44,45). Probably due to the lack of these interactions, the D stem of tRNASec is shifted outward from the axis running along the anticodon stem to the T loop (Figure 4A). In other words, tRNASec has an open cavity instead of the conventional tertiary core.
Figure 4.
Unique features in tRNASec. (A and B) The D, T and extra arms of human tRNASec (A) and T. thermophilus tRNASer (B) are shown with their surface models. There is an open cavity in tRNASec. (C and E) Close-up views showing the interactions between the AD linker residues and the first base pair of the extra stem. (D and F) The corresponding regions in T. thermophilus tRNASer are shown for comparison. (C) A8 in human tRNASec. (D) U8 in T. thermophilus tRNASer. (E) U9 and the first extra-stem base pair (G45:A48) in human tRNASec. (F) G20b and the first extra-stem base pair in T. thermophilus tRNASer.
The AD linker in tRNASec (A8 and U9) occupies a completely different position from those of the canonical tRNAs. A8 is not involved in tertiary base pairs. Instead, the adenine ring is surrounded by A48 and G50, and the O2′ atom hydrogen bonds with that of A48 (Figure 4C). In the canonical tRNAs, the conserved U8 has a reversed Hoogsteen interaction with A14 in the D arm (the U8:A14 pair) (38,44,45). U9 in tRNASec is stacked on the first base pair of the extra-arm stem (‘extra stem’) (Figure 4E). In the canonical tRNAs, the residue at position 9 interacts with a D-stem residue to form a base triple, such as the 12:23:9 or 9:13:22 pair (38,44,45).
The anticodon arm
The structure of the tRNASec anticodon arm (residues 26–44) is similar to those of the canonical tRNAs. Although the anticodon loop has an irregular conformation (Figure 1D), this is probably due to the crystal contact and/or lack of modification, and may not reflect the unique features of tRNASec. Several nucleotide modifications have been reported for eukaryal tRNASec, and some of them are located in the anticodon loop, e.g. mcm5U or mcm5Um at position 34, and i6A at position 37 (42,43). These modified nucleotides may reinforce the anticodon loop conformation, and contribute toward stabilizing the codon:anticodon pairing on the ribosome.
The extra arm
The extra arm (residues 45–47, 47a–47l and 48) forms a hairpin structure composed of a 6-bp stem and a 4-nt loop. G45 and A48 form a non-WC G45:A48 base pair, which is the first base pair of the extra stem (Figure 4E). None of the residues in the extra arm is involved in tertiary base pairing with the other arms. In the canonical tRNAs, Y48 interacts with R15 in the D arm, to form the Levitt pair (38,44,45). In the reported structure of tRNASer from the bacterium T. thermophilus, the linker between the extra stem and the T stem is C48 (Figure 1B and H). C48 is base paired with G15, and is connected to G49 in the T stem (Figure 4D and F) (38). tRNASec lacks such a linker residue, as A48 base pairs with G45, and the G45:A48 pair occupies the position corresponding to the A45:U47q pair in T. thermophilus tRNASer (Figures 1A and B; 4E and F). A48 is directly connected to G50, thus shortcutting the Levitt pair and the 49:65 pair (Figure 4C and E).
In human tRNASec, U9 is stacked on the first base pair of the extra stem (the G45:A48 pair) (Figure 4E). In T. thermophilus tRNASer, G20b in the D loop occupies the corresponding position (Figure 4F) (38). Although the secondary structures and tertiary interactions are different between human tRNASec and T. thermophilus tRNASer, the orientations of their extra stems against their T arms are nearly identical (Figure 4A and B). This partially explains the fact that both tRNASer and tRNASec are aminoacylated with serine by the same enzyme, SerRS.
DISCUSSION
The tRNASec structure
We solved the crystal structure of human tRNASec. The structure revealed that the acceptor stem of a eukaryal tRNASec has 9 bp, and the T stem has 4 bp (the 9/4 secondary structure). Conversely, bacterial tRNASecs have the 8/5 secondary structure and the canonical tRNAs have the 7/5 secondary structure (28). In the present structure, the acceptor-T stem is bent between the U6:U67 and G7:C66 base pairs (Figure 3A and C), although it is unclear whether this bending reflects a physiological tRNASec conformation. The D arm is composed of a 6-bp stem and a 4-nt loop. The interaction between the D and T loops is normal, but the D arm lacks tertiary interaction with the AD linker and the extra arm. As revealed by the hole between the D and extra arms, the tertiary interactions in tRNASec are less complicated than those in the canonical tRNAs (Figure 4A). This unique global feature of the tertiary structure with respect to the unusually long D stem of tRNASec should not be a consequence of the absence of modified nucleosides in the present tRNA transcript, as the natural tRNASec lacks modified nucleosides in the D stem. Conversely, mcm5U34 or mcm5Um34, i6A37, Ψ55 and m1A58 exist in the vertebrate tRNASecs (42,43). Positions 34 and 37 are in the anticodon loop, which does not participate in the tertiary interaction. The T-loop conformation involving positions 55 and 58 of tRNASec superimposes well on those of the canonical tRNA structures with the nucleoside modifications (38,44–47); the base pairs G18:U55 and U54:A58 are formed as the canonical tertiary base pairs G18:Ψ55 and T54:m1A58. Therefore, it is unlikely that the lack of these modifications either affects the D-loop:T-loop interaction or furthermore remotely alters the D stem structure.
The structure of tRNASec had been predicted based on biochemical analyses using enzymatic and chemical probes (29,30). Structural models of both the eukaryal and bacterial tRNASecs were reported. The study of Xenopus laevis tRNASec predicted the 9/4 secondary structure of the acceptor-T stem, consistent with our crystal structure, as well as several tertiary base pairs (29). Among them, G18:U55, G19:U56 and U16:U59 were confirmed by the present structure (Figure 2B and C), but the A8:A14:U21 base triple was not present in the structure. In the crystal structure, A8 does not participate in base pairing. The U20:G19:C56 base triple and the base stacking of U9 on the G45:A48 pair were not predicted by the biochemical analyses, but they exist in the crystal structure.
Interaction with SerRS
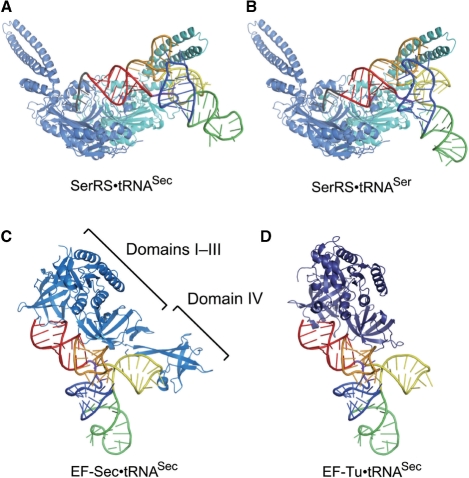
SerRS possesses a long coiled-coil that is responsible for the tRNA binding (38). In the T. thermophilus SerRS·tRNASer structure, the coiled-coil interacts with the tRNA elbow (G19:C56 pair) and the extra stem (38), and these interactions are the major determinants for the tRNA aminoacylation (49). The SerRS·tRNASec docking model suggests that these interactions are also conserved in tRNASec (Figure 5A and B). Since the extra-stem orientations are similar between tRNASer and tRNASec (Figure 4A and B), SerRS could interact with tRNASec and tRNASer in similar manners.
Figure 5.
The docking models. (A) A docking model of SerRS and tRNASec. The two subunits of the P. horikoshii SerRS dimer are colored sky blue and cyan, respectively. Human tRNASec is colored as in Figure 1. (B) A docking model of SerRS and tRNASer. (C) A docking model of EF-Sec and tRNASec. Methanococcus maripaludis SelB/EF-Sec is shown as a ribbon diagram, colored marine blue. (D) A docking model of EF-Tu and tRNASec. Thermus aquaticus EF-Tu is colored deep blue.
In addition to these interactions, SerRS should contact the tRNASec acceptor arm, to ligate serine to the 3′ end. In the SerRS·tRNASec docking model, the distance between the O3′ atom of C72 and the SerRS catalytic site is ∼26 Å, which is comparable to the distance of ∼24 Å in the tRNASer complex. Although the acceptor-T stem of tRNASec is longer than that of tRNASer, the docking model suggests that the tRNASec acceptor stem can place the CCA end in the SerRS catalytic site.
Interactions with PSTK and SepSecS
In eukarya, the phosphorylation of Ser-tRNASec depends mainly on the larger number of base pairs in the D stem, rather than the long acceptor stem or extra arm (50,51). This suggests that PSTK recognizes the D stem structure as the main discrimination site. The D stem of tRNASec is 2 bp longer than those of the canonical tRNAs, and is quite independent, lacking tertiary interactions with either the extra arm or the AD linker. These properties are responsible for the unique D-arm conformation. The D stem is shifted outward from the axis running along the anticodon stem to the T loop, resulting in a hole in place of the canonical tertiary core (Figure 4A). In contrast, the D stem of tRNASer is tightly packed in the tertiary core. It seems reasonable that PSTK discriminates tRNASec from tRNASer by relying on the characteristic D-arm conformation of tRNASec. In archaea, the second base pair of the acceptor stem is the major determinant for the tRNASec discrimination by PSTK (52). This base pair is conserved as G2:C71 and C2:G71 in tRNASec and tRNASer, respectively. Conversely, in eukarya, the second base pair is conserved as C2:G71 in tRNASec, but is nonconserved with some C2:G71 pairs in tRNASer (28). Therefore, the second base pair of the acceptor stem should not be important for eukaryal PSTK.
The Sec-tRNASec formation also depends on the long D stem, rather than the long extra arm (51). The length of the acceptor stem also affects the Sec-tRNASec synthesis. The reduction of the length of the acceptor stem by 1 bp decreased the Sec-tRNASec synthesis by 3–5-fold (51,53). These results suggest that SepSecS and/or PSTK recognize the unique D and acceptor stems of tRNASec as the discrimination sites. In the bacterial system, the long acceptor stem of tRNASec is important for the Sec-tRNASec synthesis by SelA (54). The long extra arm is not important, and the involvement of the D arm in the tRNASec discrimination has not been reported.
Interaction with EF-Sec
Eukaryal/archaeal EF-Sec/SelB comprises four domains: the three N-terminal domains (domains I–III) homologous to EF-Tu (or EF1A) and the unique C-terminal domain (domain IV) (41). The docking model of EF-Sec and tRNASec suggests that EF-Sec can interact with the tRNASec acceptor stem, as in the EF-Tu·tRNAPhe complex. The EF-Sec domain III is close to the boundary of the acceptor and T stems, and could recognize the parts characteristic of tRNASec (Figure 5C). The EF-Sec-specific domain IV is located near the tRNASec extra arm, and might also participate in the tRNASec recognition (Figure 5C). In the reported structures of EF-Tu·tRNA complexes, the tRNA acceptor-T stem is curved outward, presumably by an induced fit to EF-Tu (40,48). The bent acceptor-T stem in the present structure may mimic the EF-Sec-bound conformation of tRNASec.
The docking model of EF-Tu and human tRNASec suggests that EF-Tu can interact with the tRNASec acceptor stem in a similar manner as in EF-Tu·tRNAPhe (Figure 5D). There are no severe steric clashes between EF-Tu and tRNASec. The dissociation constant (Kd) between EF-Tu and Ser-tRNASec was reportedly 50 nM (55). The high affinity seems to be consistent with our model, although this value is about 70-fold larger than that between EF-Tu and Ser-tRNASer (Kd = 0.7 nM) (55). The structures of the EF-Tu·tRNA complexes suggest that the interaction depends on the conformational adaptability of the acceptor-T stem backbone to EF-Tu (40,48). The unique bending point of the human tRNASec acceptor stem might be related to the lower affinity of EF-Tu for tRNASec in comparison to tRNASer.
ACCESSION NUMBER
The coordinates have been deposited in the Protein Data Bank (ID: 3A3A).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (to S.S.); JSPS Global Centers of Excellence Program (Integrative Life Science Based on the Study of Biosignaling Mechanisms); Ministry of Education, Culture, Sports, Science, and Technology Targeted Proteins Research Program; and Research Fellowships from JSPS (to Y.I.). Funding for open access charge: RIKEN
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staffs of the SPring-8 BL41XU (Hyogo, Japan) and the Photon Factory beam lines (Tsukuba, Japan) for assistance with our data collection. We also thank A. Ishii and T. Nakayama for assistance in the manuscript preparation.
Footnotes
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors.
REFERENCES
- 1.Zinoni F, Birkmann A, Leinfelder W, Böck A. Cotranslational insertion of selenocysteine into formate dehydrogenase from Escherichia coli directed by a UGA codon. Proc. Natl Acad. Sci. USA. 1987;84:3156–3160. doi: 10.1073/pnas.84.10.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böck A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol. Microbiol. 1991;5:515–520. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 3.Huber RE, Criddle RS. Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch. Biochem. Biophys. 1967;122:164–173. doi: 10.1016/0003-9861(67)90136-1. [DOI] [PubMed] [Google Scholar]
- 4.Cone JE, Del Rio RM, Davis JN, Stadtman TC. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc. Natl Acad. Sci. USA. 1976;73:2659–2663. doi: 10.1073/pnas.73.8.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers I, Frampton J, Goldfarb P, Affara N, McBain W, Harrison PR. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the ‘termination' codon, TGA. EMBO J. 1986;5:1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinoni F, Birkmann A, Stadtman TC, Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc. Natl Acad. Sci. USA. 1986;83:4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 8.Leinfelder W, Zehelein E, Mandrand-Berthelot MA, Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988;331:723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- 9.Carlson BA, Xu XM, Kryukov GV, Rao M, Berry MJ, Gladyshev VN, Hatfield DL. Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl Acad. Sci. USA. 2004;101:12848–12853. doi: 10.1073/pnas.0402636101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan J, Palioura S, Salazar JC, Su D, O'Donoghue P, Hohn MJ, Cardoso AM, Whitman WB, Söll D. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc. Natl Acad. Sci. USA. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2007;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forchhammer K, Böck A. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J. Biol. Chem. 1991;266:6324–6328. [PubMed] [Google Scholar]
- 13.Ehrenreich A, Forchhammer K, Tormay P, Veprek B, Böck A. Selenoprotein synthesis in E. coli. Purification and characterisation of the enzyme catalysing selenium activation. Eur. J. Biochem. 1992;206:767–773. doi: 10.1111/j.1432-1033.1992.tb16983.x. [DOI] [PubMed] [Google Scholar]
- 14.Low SC, Harney JW, Berry MJ. Cloning and functional characterization of human selenophosphate synthetase, an essential component of selenoprotein synthesis. J. Biol. Chem. 1995;270:21659–21664. doi: 10.1074/jbc.270.37.21659. [DOI] [PubMed] [Google Scholar]
- 15.Heider J, Baron C, Böck A. Coding from a distance: dissection of the mRNA determinants required for the incorporation of selenocysteine into protein. EMBO J. 1992;11:3759–3766. doi: 10.1002/j.1460-2075.1992.tb05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry MJ, Banu L, Harney JW, Larsen PR. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993;12:3315–3322. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forchhammer K, Leinfelder W, Böck A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature. 1989;342:453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- 18.Baron C, Heider J, Böck A. Interaction of translation factor SELB with the formate dehydrogenase H selenopolypeptide mRNA. Proc. Natl Acad. Sci. USA. 1993;90:4181–4185. doi: 10.1073/pnas.90.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada K, Mizutani T, Ejiri S, Totsuka T. A factor protecting mammalian [75Se]SeCys-tRNA is different from EF-1 alpha. FEBS Lett. 1994;347:137–142. doi: 10.1016/0014-5793(94)00523-0. [DOI] [PubMed] [Google Scholar]
- 20.Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 2000;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selmer M, Su XD. Crystal structure of an mRNA-binding fragment of Moorella thermoacetica elongation factor SelB. EMBO J. 2002;21:4145–4153. doi: 10.1093/emboj/cdf408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshizawa S, Rasubala L, Ose T, Kohda D, Fourmy D, Maenaka K. Structural basis for mRNA recognition by elongation factor SelB. Nat. Struct. Mol. Biol. 2005;12:198–203. doi: 10.1038/nsmb890. [DOI] [PubMed] [Google Scholar]
- 23.Lesoon A, Mehta A, Singh R, Chisolm GM, Driscoll DM. An RNA-binding protein recognizes a mammalian selenocysteine insertion sequence element required for cotranslational incorporation of selenocysteine. Mol. Cell Biol. 1997;17:1977–1985. doi: 10.1128/mcb.17.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rother M, Wilting R, Commans S, Böck A. Identification and characterisation of the selenocysteine-specific translation factor SelB from the archaeon Methanococcus jannaschii. J. Mol. Biol. 2000;299:351–358. doi: 10.1006/jmbi.2000.3756. [DOI] [PubMed] [Google Scholar]
- 25.Copeland PR, Fletcher JE, Carlson BA, Hatfield DL, Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding F, Grabowski PJ. Identification of a protein component of a mammalian tRNASec complex implicated in the decoding of UGA as selenocysteine. RNA. 1999;5:1561–1569. doi: 10.1017/s1355838299991598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu XM, Mix H, Carlson BA, Grabowski PJ, Gladyshev VN, Berry MJ, Hatfield DL. Evidence for direct roles of two additional factors, SECp43 and soluble liver antigen, in the selenoprotein synthesis machinery. J. Biol. Chem. 2005;280:41568–41575. doi: 10.1074/jbc.M506696200. [DOI] [PubMed] [Google Scholar]
- 28.Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturchler C, Westhof E, Carbon P, Krol A. Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNASec. Nucleic Acids Res. 1993;21:1073–1079. doi: 10.1093/nar/21.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron C, Westhof E, Böck A, Giegé R. Solution structure of selenocysteine-inserting tRNASec from Escherichia coli. Comparison with canonical tRNASer. J. Mol. Biol. 1993;231:274–292. doi: 10.1006/jmbi.1993.1282. [DOI] [PubMed] [Google Scholar]
- 31.Sekine S, Nureki O, Sakamoto K, Niimi T, Tateno M, Go M, Kohno T, Brisson A, Lapointe J, Yokoyama S. Major identity determinants in the “augmented D helix” of tRNAGlu from Escherichia coli. J. Mol. Biol. 1996;256:685–700. doi: 10.1006/jmbi.1996.0118. [DOI] [PubMed] [Google Scholar]
- 32.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 33.Read RJ. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallogr. D. 2001;57:1373–1382. doi: 10.1107/s0907444901012471. [DOI] [PubMed] [Google Scholar]
- 34.Westhof E, Dumas P, Moras D. Restrained refinement of two crystalline forms of yeast aspartic acid and phenylalanine transfer RNA crystals. Acta Crystallogr. A. 1988;44(Pt 2):112–123. [PubMed] [Google Scholar]
- 35.Sussman JL, Holbrook SR, Warrant RW, Church GM, Kim SH. Crystal structure of yeast phenylalanine transfer RNA. I. Crystallographic refinement. J. Mol. Biol. 1978;123:607–630. doi: 10.1016/0022-2836(78)90209-7. [DOI] [PubMed] [Google Scholar]
- 36.Adams PD, Pannu NS, Read RJ, Brunger AT. Cross-validated maximum likelihood enhances crystallographic simulated annealing refinement. Proc. Natl Acad. Sci. USA. 1997;94:5018–5023. doi: 10.1073/pnas.94.10.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 38.Biou V, Yaremchuk A, Tukalo M, Cusack S. The 2.9 Å crystal structure of T. thermophilus seryl-tRNA synthetase complexed with tRNASer. Science. 1994;263:1404–1410. doi: 10.1126/science.8128220. [DOI] [PubMed] [Google Scholar]
- 39.Itoh Y, Sekine S, Kuroishi C, Terada T, Shirouzu M, Kuramitsu S, Yokoyama S. Crystallographic and mutational studies of seryl-tRNA synthetase from the archaeon Pyrococcus horikoshii. RNA Biol. 2008;5:169–177. doi: 10.4161/rna.5.3.6876. [DOI] [PubMed] [Google Scholar]
- 40.Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Reshetnikova L, Clark BF, Nyborg J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995;270:1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- 41.Leibundgut M, Frick C, Thanbichler M, Böck A, Ban N. Selenocysteine tRNA-specific elongation factor SelB is a structural chimaera of elongation and initiation factors. EMBO J. 2005;24:11–22. doi: 10.1038/sj.emboj.7600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diamond AM, Choi IS, Crain PF, Hashizume T, Pomerantz SC, Cruz R, Steer CJ, Hill KE, Burk RF, McCloskey JA, et al. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA[Ser]Sec. J. Biol. Chem. 1993;268:14215–14223. [PubMed] [Google Scholar]
- 43.Sturchler C, Lescure A, Keith G, Carbon P, Krol A. Base modification pattern at the wobble position of Xenopus selenocysteine tRNASec. Nucleic Acids Res. 1994;22:1354–1358. doi: 10.1093/nar/22.8.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robertus JD, Ladner JE, Finch JT, Rhodes D, Brown RS, Clark BF, Klug A. Structure of yeast phenylalanine tRNA at 3 Å resolution. Nature. 1974;250:546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- 45.Kim SH, Suddath FL, Quigley GJ, McPherson A, Sussman JL, Wang AH, Seeman NC, Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 46.Moras D, Comarmond MB, Fischer J, Weiss R, Thierry JC, Ebel JP, Giegé R. Crystal structure of yeast tRNAAsp. Nature. 1980;288:669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- 47.Hingerty B, Brown RS, Jack A. Further refinement of the structure of yeast tRNAPhe. J. Mol. Biol. 1978;124:523–534. doi: 10.1016/0022-2836(78)90185-7. [DOI] [PubMed] [Google Scholar]
- 48.Nissen P, Thirup S, Kjeldgaard M, Nyborg J. The crystal structure of Cys-tRNACys-EF-Tu-GDPNP reveals general and specific features in the ternary complex and in tRNA. Structure. 1999;7:143–156. doi: 10.1016/s0969-2126(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 49.Wu XQ, Gross HJ. The long extra arms of human tRNA(Ser)Sec and tRNASer function as major identify elements for serylation in an orientation-dependent, but not sequence-specific manner. Nucleic Acids Res. 1993;21:5589–5594. doi: 10.1093/nar/21.24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu XQ, Gross HJ. The length and the secondary structure of the D-stem of human selenocysteine tRNA are the major identity determinants for serine phosphorylation. EMBO J. 1994;13:241–248. doi: 10.1002/j.1460-2075.1994.tb06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amberg R, Mizutani T, Wu XQ, Gross HJ. Selenocysteine synthesis in mammalia: an identity switch from tRNASer to tRNASec. J. Mol. Biol. 1996;263:8–19. doi: 10.1006/jmbi.1996.0552. [DOI] [PubMed] [Google Scholar]
- 52.Sherrer RL, Ho JM, Söll D. Divergence of selenocysteine tRNA recognition by archaeal and eukaryotic O-phosphoseryl-tRNASec kinase. Nucleic Acids Res. 2008;36:1871–1880. doi: 10.1093/nar/gkn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sturchler-Pierrat C, Hubert N, Totsuka T, Mizutani T, Carbon P, Krol A. Selenocysteylation in eukaryotes necessitates the uniquely long aminoacyl acceptor stem of selenocysteine tRNASec. J. Biol. Chem. 1995;270:18570–18574. doi: 10.1074/jbc.270.31.18570. [DOI] [PubMed] [Google Scholar]
- 54.Baron C, Böck A. The length of the aminoacyl-acceptor stem of the selenocysteine-specific tRNASec of Escherichia coli is the determinant for binding to elongation factors SELB or Tu. J. Biol. Chem. 1991;266:20375–20379. [PubMed] [Google Scholar]
- 55.Förster C, Ott G, Forchhammer K, Sprinzl M. Interaction of a selenocysteine-incorporating tRNA with elongation factor Tu from E.coli. Nucleic Acids Res. 1990;18:487–491. doi: 10.1093/nar/18.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.