Abstract
α globin expression must be regulated properly to prevent the occurrence of α-thalassemias, yet many questions remain unanswered regarding the mechanism of transcriptional activation. Identifying factors that regulate chromatin structure of the endogenous α globin locus in developing erythroblasts will provide important mechanistic insight. Here, we demonstrate that the BRG1 catalytic subunit of SWI/SNF-related complexes co-immunoprecipitates with GATA-1 and EKLF in murine fetal liver cells in vivo and is recruited to the far-upstream major-regulatory element (MRE) and α2 promoter. Furthermore, based on our analysis of Brg1null/ENU1 mutant mice, BRG1 regulates DNase I sensitivity, H3ac, and H3K4me2 but not CpG methylation at both sites. Most importantly, BRG1 is required for chromatin loop formation between the MRE and α2 promoter and for maximal RNA Polymerase II occupancy at the α2 promoter. Consequently, Brg1 mutants express α globin mRNA at only 5–10% of wild-type levels and die at mid-gestation. These data identify BRG1 as a chromatin-modifying factor required for nucleosome remodeling and transcriptional activation of the α globin locus. These data also demonstrate that chromatin looping between the MRE and α2 promoter is required as part of the transcriptional activation mechanism.
INTRODUCTION
The α and β globin must be expressed at similar levels in erythrocytes to produce functional hemoglobin consisting of α2–β2 tetramers; a failure to do so results in globin chain imbalances and the occurrence of thalassemias (1). To facilitate their coordinately regulated transcription, the α and β globin loci are structurally similar with a series of evolutionarily conserved regulatory elements that direct tissue-specific transcription of far-downstream globin genes (Figure 1A). Furthermore, the genes are arranged in the order of their developmental expression with primitive (embryonic) globin genes followed by definitive (fetal/adult) globin genes. To account for their similarity in genomic structure, the α and β loci arose by duplication of an ancestoral locus at an early stage of vertebrate evolution ∼500 million years ago and have been constrained by purifying selection (2,3).
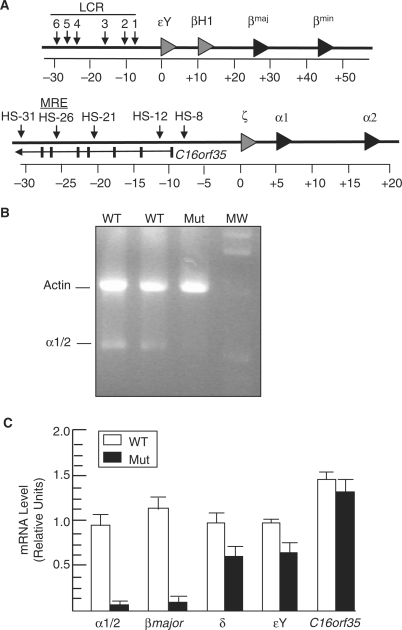
Figure 1.
BRG1 regulates α globin expression. (A) Schematic of the mouse β globin (top) and α globin (bottom) loci on chromosomes 7 and 11, respectively. DNase I hypersensitive sites are depicted by vertical arrowheads, while gray and black triangles represent embryonic and fetal/adult globin genes, respectively. Each globin gene has three exons, which are not shown, and the triangles point to the right to indicate transcriptional orientation. The α globin locus contains a gene (C16orf35) transcribed in the opposite orientation with the first seven exons shown as vertical bars. LCR, locus control region; MRE, major regulatory element; HS, hypersensitive site. Coordinates are shown in kb with the 0 position corresponding to the start of the first globin gene. (B) Expression of definitive α globin. Shown is an image of an ethidium bromide stained agarose gel containing Actin and α1/2 globin RT-PCR products amplified from wild-type (WT) and mutant (Mut) E12.5 FLs. MW, 1-kb molecular-weight standard (the 2.1-, 1.6-, 1.0- and 0.5-kb bands are visible). (C) Quantitative real-time RT–PCR analysis of α1/2, β major ζ, εY, and C16orf35 mRNA levels normalized to Gapdh levels in wild-type (WT, unfilled) and mutant (Mut, filled) E12.5 FLs. Each histogram shows the relative expression of a particular gene (mean ± SE for three independent experiments).
The β globin locus has been characterized in considerable detail and serves as a model for studying transcriptional regulatory mechanisms in general and the relationship between sequence-specific transcription factors and chromatin-modifying factors in particular (4). For example, the zinc-finger transcription factors GATA-1 and EKLF bind to their consensus sequences [(A/T)GATA(A/G) and CACCC, respectively] in the locus control region (LCR) as well as the β major and β minor promoters and recruit several co-regulators including the CBP/P300 histone acetyltransfereases (HATs) and the BRG1 catalytic subunit of SWI/SNF-related complexes (5–7). BRG1 occupancy is presumably reinforced by a bromodomain that binds acetylated histones (8), and it confers ATPase activity that alters the conformation and position of nucleosomes (9–11). As a result, DNase I hypersensitive sites (HSs) indicative of open chromatin are created in the LCR and the β major promoter. BRG1 also mediates chromatin looping such that GATA-1 and EKLF bound sites in HS2 of the LCR and β major promoter are brought into close physical proximity while the intervening ∼40-kb DNA segment is looped out (12). This step culminates in maximal RNA Polymerase II recruitment at the promoter and transcriptional activation.
The α globin locus also contains GATA-1 and EKLF binding sites in upstream regulatory elements as well as the α1 and α2 promoters. The regulatory elements share several properties with the β globin LCR including the presence of acetylated histones and HSs in erythroid cells but not other cell types (13–17). The most highly conserved of these regulatory elements, which corresponds to human HS -40 or mouse HS -26, confers robust, erythroid-specific enhancer activity on its own and is referred to as the major regulatory element (MRE) (18–22). The MRE is different from the LCR, however, because it is embedded in an intron of the ubiquitously expressed C16orf35 gene (Figure 1A) (23). Considering that the MRE does not impose erythroid-specific transcription on the C16orf35 promoter and also lacks a CTCF insulator, it is believed that the mechanism of transcriptional regulation is fundamentally different for the α and β globin loci.
Chromosome conformation capture (3C) experiments have recently demonstrated that the MRE and α2 promoter are brought into close physical proximity in erythroid cells and the ∼40 kb of intervening DNA is looped out in a manner analogous to the LCR and β major promoter (24–26). This finding suggests α globin and C16orf35 are recruited to distinct higher order chromatin environments and are regulated differently. Interestingly, EKLF is dispensable for chromatin looping at the α globin locus but required at the β globin locus, which highlights another functional difference in how these two loci are regulated (24). Most importantly, unlike the β globin locus, it is not known which chromatin-modifying factors are recruited to the α globin locus to mediate histone acetylation, nucleosome remodeling, and chromatin looping. These factors must be identified to elucidate the mechanism of α globin transcriptional activation.
We previously generated and characterized an ENU-induced hypomorphic mutation in the mouse brahma-related gene 1 (Brg1, also known as Smarca4) gene that results in a single amino-acid substitution (E1083G) in the ATPase domain (27). The mutant protein is stable and assembles into SWI/SNF-related complexes, but Brg1null/ENU1 mutants have markedly reduced nucleosome remodeling, chromatin looping, and transcription of the β globin locus and die between embryonic day (E) 11.5 and 14.5 due to severe anemia (12,27,28). Based on additional analysis of these mutants, we demonstrate here that BRG1 also remodels nucleosomes, mediates chromatin looping, and activates transcription of the α globin locus. These findings indicate that α and β globin transcription are regulated by a common, BRG1-dependent mechanism.
MATERIALS AND METHODS
RT–PCR
RNA was prepared using Trizol reagent (Invitrogen) and reverse transcribed using random hexamers and SuperScript II RT (Invitrogen) according to standard procedures. For end-stage PCR, the Actin primers were 5′-CAAGGTGTGATGGTGGGAAT-3′ and 5′-GGTGTAAAACGCAGCTCAGT-3′ and the α globin primers were 5′-CTGATTCTGACAGACTCAGG-3′ and 5′-ACCAAGAGGTACAGGTGCAA-3′. The α globin primers did not distinguish between α1 and α2 because the sequences are nearly identical. Multiplexed amplification of actin and α globin was performed at 1 mM MgCl2 and 55°C annealing temperature and yielded 1020-bp (Actin) and 525-bp (α1/2 globin) products. For quantitative PCR (qPCR), validated TaqMan assays (Applied Biosystems) were used with TaqMan gene expression master mix (Applied Biosystems) on an ABI 7300 instrument under default cycling conditions (95°C 15 s followed by 60°C 1 min for 45 cycles). Relative expression levels were determined from a standard curve of serial dilutions of wild-type cDNA samples and were normalized to Gapdh expression levels. Control reactions lacking RT yielded little or no signal.
ChIP assays
For each experiment, 2 × 106 fetal liver cells were crosslinked in 0.4% or 1.0% formaldehyde and sonicated with four 10-s pulses at 30% of maximum power. IP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris at pH 8.1, 167 mM NaCl, protease inhibitors) was added, 5% of the volume was removed and used as input while the remainder was incubated overnight at 4°C with the appropriate antibody: BRG1 (J1 antibody from G. Crabtree and W. Wang), total H3 (Abcam), pan-acetyl H3 (H3ac, Millipore/Upstate), H3K4me2 (Millipore/Upstate), GATA-1 (Santa Cruz), Pol II (Santa Cruz N-20) and P-Ser5 Pol II (Covance). Protein A/G agarose beads (Santa Cruz) were added and incubated for at least 2 h at 4°C, and then washed and eluted according to standard procedures.
qPCR was performed using Power SYBR Green Master Mix (Applied Biosystems (Applied Biosystems) on an ABI 7300 instrument under default cycling conditions (95°C 15 s followed by 60°C 1 min for 45°C cycles). The MRE primers were 5′-CCTACACTAACTAGGTCAAAG’3′ and 5′-TGCCCAGGTTTTGCTTCTCTT-3′, while the α2 promoter primers were 5′-TGAGGCCAGAAGCAGGTTGTG-3′ and 5′-GATCAAGGTCCTGTTCTCACC-3′. Dissociation curves and agarose gels demonstrated a single PCR product in each case without primer dimers. Relative enrichment was determined from a standard curve of serial dilutions of input samples.
Co-IPs and western blots
Immunoprecipitations were performed as previously described (27) with the following modifications. Single-cell suspensions of E12.5 fetal liver cells were incubated in the presence of 30 mM dithiobissuccimdyl propionate (DSP) (Pierce) at room temperature for 15 min and utilized anti-GATA-1 (Santa Cruz) or anti-EKLF (AEK-1 provided by Yu-Chiau Shyu and Che-Kun James Shen) (29). Western blots were performed with a BRG1 antibody (Santa Cruz G-7) as described previously.
DNase I sensitivity assays
Real-time, quantitative DNase I sensitivity assays were performed as previously described (30). The MRE and α2 promoter primers and cycling conditions were the same as for the ChIP assays (see above). The D9Mit59 control primers were 5′-CAGCCAGAGGCAGTGTTTTA-3′ and 5′-TAGGCTTCAGCTGCAACTCA-3′.
Bisulfite mutagenesis
Bisulfite mutagenesis was performed as previously described (27), and the MRE was amplified using 5′-GTATATTTATATTAATTAGGTTAAAGTAG-3′ and 5′-ACAAATCTACCCAAATTTTACTTCTCTTT-3′ modified primers. Subcloning and sequencing were also performed as described previously. Over 95% of cytosines outside the context of CpG were converted to thymines indicating the sodium bisulfite mutagenesis step occurred efficiently.
3C assays
3C assays were performed as previously described (31). Single-cell suspensions were isolated from wild-type and Brg1 mutant E12.5 FLs. 3C products were normalized to a control interaction at Ercc3. Band intensities were quantified with ImageJ 1.38v software. 3C primer sequences are available upon request.
RESULTS
BRG1 directly regulates α globin expression
We previously identified reduced transcription of the α and β globin genes in flow-sorted erythroid cells from E12.5 Brg1null/ENU1 fetal livers (FLs) (12,27,28). We subsequently validated the β globin result and have studied the role of BRG1 in β globin regulation (12,27,28). To validate the α globin result, we performed RT–PCR and demonstrated that expression is markedly reduced in mutants (Figure 1B). RT–qPCR indicated that mutants express α globin at 5-10% of wild-type levels (Figure 1C). A similar reduction was observed for β major previously and in this study (Figure 1C). Transcription of the ζ and εY embryonic globin genes from yolk sac derived primitive erythrocytes still circulating at E12.5 was also reduced but to a lesser extent, ∼60% of wild-type levels (Figure 1C), which is similar to what was observed in Brg1Tie2-Cre conditional mutants (32). We have analyzed other genes whose expression is not significantly affected in Brg1 mutants, including Gata1, Fog1 and Eklf, which indicates BRG1 does not regulate the expression of erythroid transcription factors and is not a general transcriptional co-regulator (12,28). Here, we analyzed the mouse ortholog of C16orf35 (also known as major alpha globin regulatory element containing gene or Mare), which serves as an important control because intron 5 contains the MRE. C16orf35 mRNA levels are unaffected in mutants (Figure 1C), which can be explained by the aforementioned 3C experiments demonstrating chromatin looping at the α globin locus.
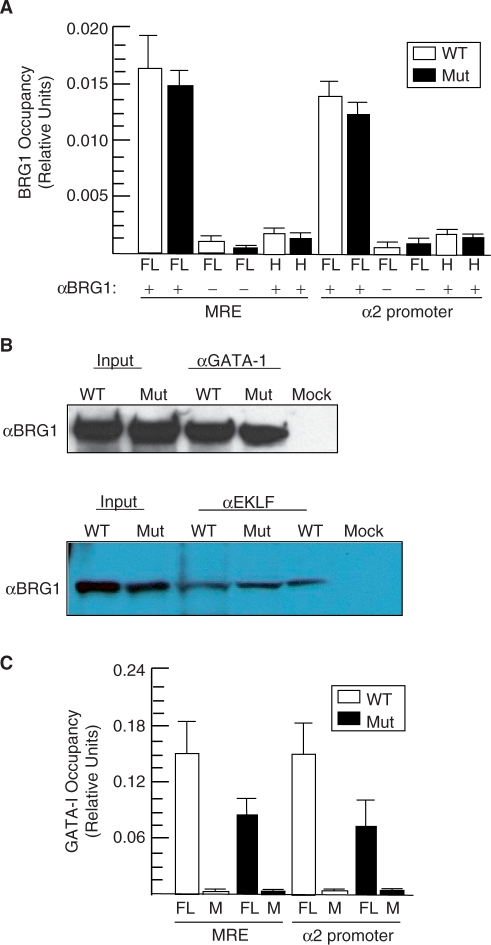
To determine whether BRG1 directly regulates the α globin locus, we performed ChIP assays and localized it to the MRE and α2 promoter in FLs (Figure 2A). BRG1 did not occupy either site in non-erythroid head tissue, which indicates that it binds to the α globin locus in a tissue-specific manner (Figure 2A). GATA-1 and EKLF are also known to bind these sites and presumably recruit BRG1 based on their ability to physically interact with BRG1 in vitro and recruit it and SWI/SNF-related complexes to the β globin locus (6,7,33–38). However, these protein-protein interactions have not been confirmed at physiological levels in vivo. Therefore, we performed co-immunoprecipitations (co-IPs) using E12.5 FLs, and western blot analyses indicated that endogenous GATA-1 and EKLF physically interact with endogenous BRG1 (Figure 2B). The ability of GATA-1 and EKLF to bind BRG1 and recruit it to the α globin locus was unaffected in mutants (Figure 2A, B). These findings are consistent with the fact that the E1083G substitution is in the ATPase domain located far away from the N-terminal domain of BRG1 that binds these zinc-finger transcription factors (7). BRG1 is also required for maximal GATA-1 occupancy at the MRE and α2 promoter (Figure 2C).
Figure 2.
BRG1 occupies the α globin locus and physically interacts with GATA-1 and EKLF in vivo. (A) Quantitative ChIP assays demonstrating that wild-type (WT) and mutant (Mut) BRG1 are localized to the MRE and α2 promoter. FL or head (H) samples were immunoprecipitated with BRG1 antibody (+) or pre-immune serum (−), which were otherwise processed identically and serve as negative controls. Histograms show the relative enrichment by comparing each ChIP sample to input by qPCR (mean ± SE for three independent experiments). (B) GATA-1 and EKLF co-IP with BRG1 in vivo. Shown are western blot panels probed with a BRG1 antibody (αBRG1). Samples include wild-type (WT) and mutant (Mut) E12.5 FLs (Input) and protein lysates immunoprecipitated with αGATA-1 (left) or αEKLF (right) from FLs. Mock, WT FLs immunoprecipitated in the absence of αGATA-1 or αEKLF but processed in an identical manner. (C) Quantitative ChIP assays of GATA-1 occupancy at the MRE and α2 promoter in wild-type (WT) and Brg1 mutants (Mut). FL samples were immunoprecipated with GATA-1 antibody (+) or pre-immune serum (−), which were otherwise processed identically and serve as negative controls. Histograms show the relative enrichment by comparing each ChIP sample to input by qPCR (mean ± SE for three independent experiments).
BRG1 confers DNase I sensitivity and mediates epigenetic modifications of the MRE and α2 promoter
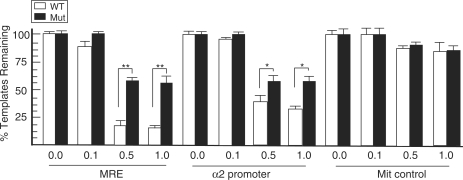
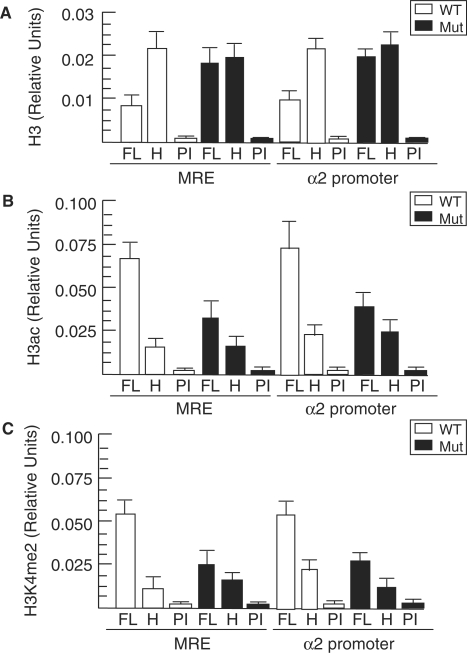
Considering that mutant BRG1 is recruited to the α globin locus, we reasoned that impaired chromatin remodeling underlies the reduced expression in mutants. DNase I sensitivity assays demonstrated that the MRE and α2 promoter are hypersensitive in wild-type FL nuclei, as expected, but are significantly less sensitive in mutant samples (Figure 3). An Mit microsattelite marker that is relatively resistant to DNase I digestion served as a control and is unaffected in mutant nuclei as expected (Figure 3). To determine whether the reduced DNase I sensitivity of mutant samples is correlated with higher nucleosome density, we performed ChIP assays for total histone 3 (H3) using an antibody that binds to the C terminus. Compared to wild-type samples, mutants have increased total H3 at the MRE and α2 promoter (Figure 4A). Therefore, decreased DNase I sensitivity is correlated with increased H3 in mutants, and these findings are consistent with the role of BRG1 directly altering the conformation and position of nucleosomes.
Figure 3.
DNase I sensitivity of the α globin locus is reduced in Brg1 mutants. Nuclei from wild-type (WT) and mutant (Mut) E12.5 FL cells were undigested (0.0) or digested with increasing amounts of DNase I (from left to right: 0.1, 0.50, 1.0 U). Genomic DNA was subsequently prepared, and the percentage of templates remaining for the MRE and α2 promoter was determined by comparing qPCR yields to a standard curve of DNA from undigested sample qPCR yields (mean ± SE for three independent experiments). To correct for small differences in the amount of DNA that might have been recovered from each sample, an Mit marker that is relatively resistant to DNase I was amplified as a control; *P < 0.01; **P < 0.001.
Figure 4.
Aberrant epigenetic modifications at the α globin locus in Brg1 mutants. (A–C) Quantitative ChIP assays showing total H3 (A), H3ac (B) and H3K4me2 (C) at the MRE and α2 promoter from wild-type (WT, unfilled) and mutant (Mut, filled) fetal liver (FL) and head (H) samples. FL samples were also immunoprecipitated with pre-immune (PI) serum, which were otherwise processed identically to ChIPs and serve as negative controls. Histograms show the relative enrichment by comparing each ChIP sample to input by qPCR (mean ± SE for three independent experiments).
To assess chromatin structure in more detail, we evaluated two covalent histone modifications associated with transcriptionally permissive promoters. In wild-type FLs, the MRE and α2 promoter are enriched for H3 acetylation (H3ac) and H3 lysine 4 dimethylation (H3K4me2) in erythroid cells of the FL several fold higher than non-erythroid cells of the head as expected (Figure 4B, C). In contrast, mutant FLs had low-level enrichment of H3ac comparable to head tissue at both sites (Figure 4B). Higher H3ac levels in wild-type compared to mutant is consistent with BRG1 having a bromodomain and functioning in concert with HATs. Mutant FLs also had lower levels of H3K4me2 similar in magnitude to H3ac (Figure 4C). The previous total H3 ChIP experiment (Figure 4A) serves as a control to indicate that decreased H3ac and H3K4me2 in mutants is not due to depletion of H3. The effect of BRG1 on H3ac and H3K4me is presumably indirect and could be a consequence of decreased transcription (39).
Sodium bisulfite mutagenesis assays were also performed to evaluate DNA methylation. Wild-type and mutant samples were hypomethylated to a similar degree (Supplementary Figure 1). These results are different than what we previously observed at the β globin locus where wild-type samples were hypomethylated to an even greater extent and mutants were hypermethylated (27). Previous studies have demonstrated that hypomethylation at the α globin locus occurs in erythroid and non-expressing tissues, whereas hypomethylation at the β globin locus occurs only in erythroid tissues (40–42). Therefore, CpG methylation appears to be less utilized as a regulatory mechanism at the α globin locus compared to the β globin locus.
BRG1 induces chromatin looping between the MRE and α2 promotor and facilitates maximal RNA Polymerase II occupancy at the α2 promotor
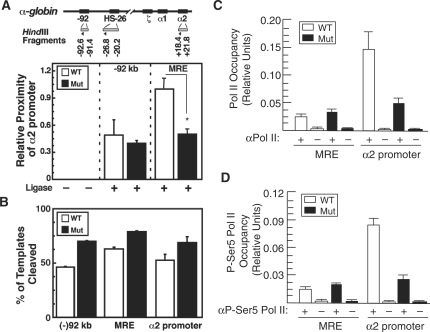
BRG1 has been shown to mediate chromatin looping between far-upstream regulatory elements and promoters at the CIITA and β globin loci (12,43). The MRE and α2 promoter are also known to undergo chromatin looping (24–26), so we hypothesized that BRG1 is required for this process to occur considering that it regulates nucleosome structure/position (manifest by reduced DNase I sensitivity and increased H3 in mutants) and covalent histone modifications at both sites. Therefore, we performed semi-quantitative 3C experiments and determined that the MRE and α2 promoter are in close physical proximity in wild-type FLs (Figure 5A), which is in accord with previously published studies. Importantly, however, this MRE-α2 promoter chromatin loop is undetectable in mutant samples (Figure 5A), despite having restriction enzyme cleavage efficiencies similar to wild-type samples (Figure 5B). BRG1 does not participate in chromatin-loop formation at several other loci including c-Kit and Gata2, which indicates that it does not play a general, non-specific role in chromatin-loop formation throughout the genome (12,43).
Figure 5.
BRG1 is required for chromatin looping and maximal Pol II promoter occupancy at the α globin promoter. (A) 3C strategy. HindIII fragments and primers are depicted as shaded rectangles and triangles, respectively. The graph depicts 3C results measuring the proximity of a HindIII fragment containing the α2 promoter relative to fragments containing either the −92 kb region (control) or the MRE (HS-26 kb region) in E12.5 FL cells from wild-type (WT) and Brg1 mutant (Mut) embryos (mean ± SE, two independent experiments). (B) Real-time PCR quantification of HindIII cleavage efficiencies for the WT and Mut samples. (C and D) Quantitative ChIP assays showing Pol II (C), and P-Ser5 Pol II (D) at the MRE and α2 promoter from wild-type (WT, unfilled) and mutant (Mut, filled) FL samples. Samples were immunoprecipitated with Pol II or P-Ser5 Pol II antibody (+) or pre-immune serum (−), which were otherwise processed identically and serve as negative controls. Histograms show the relative enrichment of Pol II occupancy by comparing each ChIP sample to input by qPCR (mean ± SE for three independent experiments); *P < 0.003.
Chromatin looping is thought to transfer RNA Polymerase II (Pol II) from upstream regulatory elements to the promoter to facilitate transcription (44). Consistent with this notion, the Brg1 mutation does not affect Pol II occupancy at the MRE but the loss of chromatin looping in Brg1 mutants is associated with loss of high-level occupancy of Pol II and P-Ser5 Pol II at the α2 promoter (Figure 5C, D). This finding is similar to Drosophila BRM promoting Pol II association with polytene chromosomes (39) and accounts for the observed decrease in α globin transcription in this study.
DISCUSSION
Molecular determinants of histone acetylation, nucleosome remodeling and chromatin looping at the α globin locus are unknown. Here, we demonstrate that the BRG1 catalytic subunit of SWI/SNF-related complexes is required for each of these inter-related processes and for α globin transcription. We also demonstrate that chromatin looping between the MRE2 and α2 promoter is an essential part of the mechanism of the transcriptional activation mechanism. In this regard, it is noteworthy that not all chromatin loops will be functionally important. For example, the H enhancer undergoes chromatin looping with numerous olfactory receptor genes but is required for the expression of only a relatively small subset that are most tightly linked (45).
Other transcription factors and chromatin-modifying factors are likely involved in chromatin-loop formation between the MRE and α2 promoter, and GATA-1 and FOG-1 are good candidates because they recruit BRG1 and are required for chromatin looping at the β globin locus (24,46). It should be noted, however, that EKLF also recruits BRG1 and is essential for chromatin looping at the β globin locus but is dispensable for chromatin looping at the α globin locus (24). CTCF is also required for chromatin looping at the β globin locus but is not a good candidate because it is not known to bind the α globin locus or regulate its expression (47). Conversely, ATRX is a good candidate because it is a putative chromatin-remodeling factor similar to BRG1 and is required for α globin expression (48,49).
Based on our previous work and data presented here, we propose that BRG1 performs a similar role at the α and β globin loci: 1, it binds to GATA-1, EKLF, and possibly other transcription factors such as p45/NF-E2 in erythroblasts (50), which leads to the recruitment of SWI/SNF-related complexes to the far-upstream regulatory elements (the αMRE and βLCR) and globin promoters; 2, it confers DNA-dependent ATPase activity and harnesses this energy to alter nucleosome conformation and position directly and promotes histone acetylation and H3K4me2 indirectly; 3, it is required for chromatin looping between the MRE-α2 promoter and the LCR-β major promoter, which is thought to transfer Pol II from the upstream regulatory elements to the promoters and initiate transcription (44).
ΔLCR mutants phenocopy Brg1 mutants, which supports our model, but ΔMRE mutants have a smaller decrease in α globin transcription (reduced to 50% of normal) and exhibit a more subtle phenotype (51,52). To reconcile this difference, the LCR consists of six HSs that function in an additive manner such that deletion of any one HS results in a relatively mild phenotype. Similarly, deletion of the MRE plus the other four HSs at the mouse α globin locus, even though they confer less enhancer activity than the MRE when incorporated into transgenes, may result in a more severe phenotype similar to Brg1 mutants. There are also human–mouse differences with the human MRE playing a more important role based on transgenic mouse experiments and deletions in thalassemia patients (19,22). A gene-targeting experiment also has been performed that replaced 85 kb of the mouse α globin locus with the corresponding 120 kb of human sequence; although the human α globin genes knocked into the mouse were expressed in a tissue- and stage-appropriate manner, mRNA levels were 60% less than endogenous mouse transcripts (53). The MRE might function differently between the two species, despite being highly conserved, because of breaks in the conserved synteny within the locus that occurred after the rodent and primate lineages diverged (54).
The α and β globin loci are known to move from their chromosomal territories at the nuclear periphery, where they are transcribed at low levels, to transcription factories where multiple chromatin loops converge at Pol II foci and transcription is upregulated (25,55,56). The LCR is required for re-localization of β globin from its repressive compartment to transcription factories (56), but no other cis elements or trans-acting factors have been identified that are required for this process to occur at β globin or any other locus to our knowledge. BRG1 is an attractive candidate because it is a DNA-dependent ATPase that could provide the necessary energy and it participates in the formation of local chromatin loops, referred to as active chromatin hubs, at the α and β globin loci. Additionally, SWI/SNF-related complexes contain actin and actin-related subunits that could potentially move loci via the nuclear cytoskeleton/matrix to transcription factories (57). It must be determined whether BRG1 functions exclusively in the formation of local chromatin loops (where active chromatin hubs allow upstream enhancers to load Pol II onto promoters) or if it also transports these domains to transcription factories.
In summary, BRG1 plays a fundamental role in the mechanism of transcriptional activation at the α globin locus as well as the β globin locus. Our findings reveal a common regulatory mechanism, notwithstanding certain differences such as the role of EKLF and CpG methylation, which was not anticipated because the α globin locus contains a ubiquitously expressed gene (C16orf35) and lacks a CTCF insulator.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health (CA125237 to S.J.B.; DK50107 to E.H.B.); predoctoral fellowship from the American Heart Association (to S.I.K.). Funding for open access charge: CA125237.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. Crabtree and W. Wang for the BRG1 J1 antibody and Y.-C. Shyu and C.-K. Shen for EKLF antibody. We thank D. Donohoe, G. Rosson and K. Pandya for comments on this article.
REFERENCES
- 1.Weatherall D. The Thalassemias. 3rd edn. Philadelphia, PA: Saunders; 2001. [Google Scholar]
- 2.Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 2002;19:416–421. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- 3.Gillemans N, McMorrow T, Tewari R, Wai AW, Burgtorf C, Drabek D, Ventress N, Langeveld A, Higgs D, Tan-Un K, et al. Functional and comparative analysis of globin loci in pufferfish and humans. Blood. 2003;101:2842–2849. doi: 10.1182/blood-2002-09-2850. [DOI] [PubMed] [Google Scholar]
- 4.Kim SI, Bresnick EH. Transcriptional control of erythropoiesis: emerging mechanisms and principles. Oncogene. 2007;26:6777–6794. doi: 10.1038/sj.onc.1210761. [DOI] [PubMed] [Google Scholar]
- 5.Blobel GA, Nakajima T, Eckner R, Montminy M, Orkin SH. CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc. Natl Acad. Sci. USA. 1998;95:2061–2066. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Kadam S, Emerson BM, Bieker JJ. Site-specific acetylation by p300 or CREB binding protein regulates erythroid Kruppel-like factor transcriptional activity via its interaction with the SWI-SNF complex. Mol. Cell Biol. 2001;21:2413–2422. doi: 10.1128/MCB.21.7.2413-2422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadam S, Emerson BM. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell. 2003;11:377–389. doi: 10.1016/s1097-2765(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 8.Shen W, Xu C, Huang W, Zhang J, Carlson JE, Tu X, Wu J, Shi Y. Solution structure of human Brg1 bromodomain and its specific binding to acetylated histone tails. Biochemistry. 2007;46:2100–2110. doi: 10.1021/bi0611208. [DOI] [PubMed] [Google Scholar]
- 9.Becker PB, Horz W. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 2002;71:247–273. doi: 10.1146/annurev.biochem.71.110601.135400. [DOI] [PubMed] [Google Scholar]
- 10.Fan HY, He X, Kingston RE, Narlikar GJ. Distinct strategies to make nucleosomal DNA accessible. Mol. Cell. 2003;11:1311–1322. doi: 10.1016/s1097-2765(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 11.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SI, Bultman SJ, Kiefer CM, Dean A, Bresnick EH. BRG1 requirement for long-range interaction of a locus control region with a downstream promoter. Proc. Natl Acad. Sci. USA. 2009;106:2259–2264. doi: 10.1073/pnas.0806420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anguita E, Johnson CA, Wood WG, Turner BM, Higgs DR. Identification of a conserved erythroid specific domain of histone acetylation across the alpha-globin gene cluster. Proc. Natl Acad. Sci. USA. 2001;98:12114–12119. doi: 10.1073/pnas.201413098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anguita E, Hughes J, Heyworth C, Blobel GA, Wood WG, Higgs DR. Globin gene activation during haemopoiesis is driven by protein complexes nucleated by GATA-1 and GATA-2. EMBO J. 2004;23:2841–2852. doi: 10.1038/sj.emboj.7600274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu XH, Liu DP, Tang XB, Liu G, Lv X, Li YJ, Liang CC. A conserved, extended chromatin opening within alpha-globin locus during development. Exp. Cell Res. 2005;309:174–184. doi: 10.1016/j.yexcr.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 16.De Gobbi M, Anguita E, Hughes J, Sloane-Stanley JA, Sharpe JA, Koch CM, Dunham I, Gibbons RJ, Wood WG, Higgs DR. Tissue-specific histone modification and transcription factor binding in alpha globin gene expression. Blood. 2007;110:4503–4510. doi: 10.1182/blood-2007-06-097964. [DOI] [PubMed] [Google Scholar]
- 17.Fathallah H, Portnoy G, Atweh GF. Epigenetic analysis of the human alpha- and beta-globin gene clusters. Blood Cells Mol. Dis. 2008;40:166–173. doi: 10.1016/j.bcmd.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgs DR, Wood WG, Jarman AP, Sharpe J, Lida J, Pretorius IM, Ayyub H. A major positive regulatory region located far upstream of the human alpha-globin gene locus. Genes Dev. 1990;4:1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- 19.Sharpe JA, Chan-Thomas PS, Lida J, Ayyub H, Wood WG, Higgs DR. Analysis of the human alpha globin upstream regulatory element (HS-40) in transgenic mice. EMBO J. 1992;11:4565–4572. doi: 10.1002/j.1460-2075.1992.tb05558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernet A, Sabatier S, Picketts DJ, Ouazana R, Morle F, Higgs DR, Godet J. Targeted inactivation of the major positive regulatory element (HS-40) of the human alpha-globin gene locus. Blood. 1995;86:1202–1211. [PubMed] [Google Scholar]
- 21.Gourdon G, Sharpe JA, Higgs DR, Wood WG. The mouse alpha-globin locus regulatory element. Blood. 1995;86:766–775. [PubMed] [Google Scholar]
- 22.Viprakasit V, Harteveld CL, Ayyub H, Stanley JS, Giordano PC, Wood WG, Higgs DR. A novel deletion causing alpha thalassemia clarifies the importance of the major human alpha globin regulatory element. Blood. 2006;107:3811–3812. doi: 10.1182/blood-2005-12-4834. [DOI] [PubMed] [Google Scholar]
- 23.Vyas P, Vickers MA, Picketts DJ, Higgs DR. Conservation of position and sequence of a novel, widely expressed gene containing the major human alpha-globin regulatory element. Genomics. 1995;29:679–689. doi: 10.1006/geno.1995.9951. [DOI] [PubMed] [Google Scholar]
- 24.Drissen R, Palstra RJ, Gillemans N, Splinter E, Grosveld F, Philipsen S, de Laat W. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 2004;18:2485–2490. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou GL, Xin L, Song W, Di LJ, Liu G, Wu XS, Liu DP, Liang CC. Active chromatin hub of the mouse alpha-globin locus forms in a transcription factory of clustered housekeeping genes. Mol. Cell Biol. 2006;26:5096–5105. doi: 10.1128/MCB.02454-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vernimmen D, De Gobbi M, Sloane-Stanley JA, Wood WG, Higgs DR. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26:2041–2051. doi: 10.1038/sj.emboj.7601654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bultman SJ, Gebuhr TC, Magnuson T. A Brg1 mutation that uncouples ATPase activity from chromatin remodeling reveals an essential role for SWI/SNF-related complexes in beta-globin expression and erythroid development. Genes Dev. 2005;19:2849–2861. doi: 10.1101/gad.1364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SI, Bultman SJ, Jing H, Blobel GA, Bresnick EH. Dissecting molecular steps in chromatin domain activation during hematopoietic differentiation. Mol. Cell Biol. 2007;27:4551–4565. doi: 10.1128/MCB.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shyu YC, Wen SC, Lee TL, Chen X, Hsu CT, Chen H, Chen RL, Hwang JL, Shen CK. Chromatin-binding in vivo of the erythroid kruppel-like factor, EKLF, in the murine globin loci. Cell Res. 2006;16:347–355. doi: 10.1038/sj.cr.7310045. [DOI] [PubMed] [Google Scholar]
- 30.McArthur M, Gerum S, Stamatoyannopoulos G. Quantification of DNaseI-sensitivity by real-time PCR: quantitative analysis of DNaseI-hypersensitivity of the mouse beta-globin LCR. J. Mol. Biol. 2001;313:27–34. doi: 10.1006/jmbi.2001.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SI, Bultman SJ, Kiefer CM, Dean A, Bresnick EH. Building chromatin loops: expanding the repertoire of chromatin remodeler functions. Proc. Natl Acad. Sci. 2009;106:2259–2264. doi: 10.1073/pnas.0806420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin CT, Brennan J, Magnuson T. The chromatin-remodeling enzyme BRG1 plays an essential role in primitive erythropoiesis and vascular development. Development. 2008;135:493–500. doi: 10.1242/dev.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong JA, Bieker JJ, Emerson BM. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell. 1998;95:93–104. doi: 10.1016/s0092-8674(00)81785-7. [DOI] [PubMed] [Google Scholar]
- 34.Lee CH, Murphy MR, Lee JS, Chung JH. Targeting a SWI/SNF-related chromatin remodeling complex to the beta-globin promoter in erythroid cells. Proc. Natl Acad. Sci. USA. 1999;96:12311–12315. doi: 10.1073/pnas.96.22.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadam S, McAlpine GS, Phelan ML, Kingston RE, Jones KA, Emerson BM. Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev. 2000;14:2441–2451. doi: 10.1101/gad.828000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RC, Pattison S, van Ree J, Coghill E, Perkins A, Jane SM, Cunningham JM. Distinct domains of erythroid Kruppel-like factor modulate chromatin remodeling and transactivation at the endogenous beta-globin gene promoter. Mol. Cell Biol. 2002;22:161–170. doi: 10.1128/MCB.22.1.161-170.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bottardi S, Ross J, Pierre-Charles N, Blank V, Milot E. Lineage-specific activators affect beta-globin locus chromatin in multipotent hematopoietic progenitors. EMBO J. 2006;25:3586–3595. doi: 10.1038/sj.emboj.7601232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z, Meng X, Cai Y, Koury MJ, Brandt SJ. Recruitment of the SWI/SNF protein Brg1 by a multiprotein complex effects transcriptional repression in murine erythroid progenitors. Biochem. J. 2006;399:297–304. doi: 10.1042/BJ20060873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong JA, Papoulas O, Daubresse G, Sperling AS, Lis JT, Scott MP, Tamkun JW. The Drosophila BRM complex facilitates global transcription by RNA polymerase II. EMBO J. 2002;21:5245–5254. doi: 10.1093/emboj/cdf517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen CK, Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc. Natl Acad. Sci. USA. 1980;77:6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Ploeg LH, Flavell RA. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980;19:947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]
- 42.Bird AP, Taggart MH, Nicholls RD, Higgs DR. Non-methylated CpG-rich islands at the human alpha-globin locus: implications for evolution of the alpha-globin pseudogene. EMBO J. 1987;6:999–1004. doi: 10.1002/j.1460-2075.1987.tb04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni Z, Abou El Hassan M, Xu Z, Yu T, Bremner R. The chromatin-remodeling enzyme BRG1 coordinates CIITA induction through many interdependent distal enhancers. Nat. Immunol. 2008;9:785–793. doi: 10.1038/ni.1619. [DOI] [PubMed] [Google Scholar]
- 44.Johnson KD, Christensen HM, Zhao B, Bresnick EH. Distinct mechanisms control RNA polymerase II recruitment to a tissue-specific locus control region and a downstream promoter. Mol. Cell. 2001;8:465–471. doi: 10.1016/s1097-2765(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 45.Fuss SH, Omura M, Mombaerts P. Local and cis effects of the H element on expression of odorant receptor genes in mouse. Cell. 2007;130:373–384. doi: 10.1016/j.cell.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 46.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol. Cell. 2005;17:453–462. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 47.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006;20:2349–2354. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibbons RJ, Picketts DJ, Villard L, Higgs DR. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome) Cell. 1995;80:837–845. doi: 10.1016/0092-8674(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 49.Gibbons RJ, McDowell TL, Raman S, O'R;ourke DM, Garrick D, Ayyub H, Higgs DR. Mutations in ATRX, encoding a SWI/SNF-like protein, cause diverse changes in the pattern of DNA methylation. Nat. Genet. 2000;24:368–371. doi: 10.1038/74191. [DOI] [PubMed] [Google Scholar]
- 50.Brand M, Ranish JA, Kummer NT, Hamilton J, Igarashi K, Francastel C, Chi TH, Crabtree GR, Aebersold R, Groudine M. Dynamic changes in transcription factor complexes during erythroid differentiation revealed by quantitative proteomics. Nat. Struct. Mol. Biol. 2004;11:73–80. doi: 10.1038/nsmb713. [DOI] [PubMed] [Google Scholar]
- 51.Bender MA, Bulger M, Close J, Groudine M. Beta-globin gene switching and DNase I sensitivity of the endogenous beta-globin locus in mice do not require the locus control region. Mol. Cell. 2000;5:387–393. doi: 10.1016/s1097-2765(00)80433-5. [DOI] [PubMed] [Google Scholar]
- 52.Anguita E, Sharpe JA, Sloane-Stanley JA, Tufarelli C, Higgs DR, Wood WG. Deletion of the mouse alpha-globin regulatory element (HS -26) has an unexpectedly mild phenotype. Blood. 2002;100:3450–3456. doi: 10.1182/blood-2002-05-1409. [DOI] [PubMed] [Google Scholar]
- 53.Wallace HA, Marques-Kranc F, Richardson M, Luna-Crespo F, Sharpe JA, Hughes J, Wood WG, Higgs DR, Smith AJ. Manipulating the mouse genome to engineer precise functional syntenic replacements with human sequence. Cell. 2007;128:197–209. doi: 10.1016/j.cell.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 54.Tufarelli C, Hardison R, Miller W, Hughes J, Clark K, Ventress N, Frischauf AM, Higgs DR. Comparative analysis of the alpha-like globin clusters in mouse, rat, and human chromosomes indicates a mechanism underlying breaks in conserved synteny. Genome Res. 2004;14:623–630. doi: 10.1101/gr.2143604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat. Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- 56.Ragoczy T, Bender MA, Telling A, Byron R, Groudine M. The locus control region is required for association of the murine beta-globin locus with engaged transcription factories during erythroid maturation. Genes Dev. 2006;20:1447–1457. doi: 10.1101/gad.1419506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olave IA, Reck-Peterson SL, Crabtree GR. Nuclear actin and actin-related proteins in chromatin remodeling. Annu. Rev. Biochem. 2002;71:755–781. doi: 10.1146/annurev.biochem.71.110601.135507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.