Abstract
Stm1p is a Saccharomyces cerevisiae protein that is primarily associated with cytosolic 80S ribosomes and polysomes. Several lines of evidence suggest that Stm1p plays a role in translation under nutrient stress conditions, although its mechanism of action is not yet known. In this study, we show that yeast lacking Stm1p (stm1Δ) are hypersensitive to the translation inhibitor anisomycin, which affects the peptidyl transferase reaction in translation elongation, but show little hypersensitivity to other translation inhibitors such as paromomycin and hygromycin B, which affect translation fidelity. Ribosomes isolated from stm1Δ yeast have intrinsically elevated levels of eukaryotic elongation factor 3 (eEF3) associated with them. Overexpression of eEF3 in cells lacking Stm1p results in a growth defect phenotype and increased anisomycin sensitivity. In addition, ribosomes with increased levels of Stm1p exhibit decreased association with eEF3. Taken together, our data indicate that Stm1p plays a complementary role to eEF3 in translation.
INTRODUCTION
The Saccharomyces cerevisiae gene STM1 and its encoded protein Stm1p have been implicated in myriad biological processes, including apoptosis, cell-cycle regulation, telomere biosynthesis, cell-life span regulation, messenger RNA (mRNA) degradation and nutritional stress responses (1–11). However, it is not inherently obvious how a single protein is able to influence so many different biological processes. One possible explanation is that Stm1p is involved in a general process, such as protein synthesis, that, in turn, has an impact upon a whole host of cellular functions. While Stm1p was originally identified as a G*G multiplex nucleic-acid-binding protein (12,13), we showed that Stm1p is primarily a cytoplasmic protein that preferentially associates with 80S ribosomes and polysomes, which are the ‘engines’ of protein synthesis (8). We also found that Stm1p significantly affected rates of protein synthesis under nutrient stress conditions (9). Thus, a role of Stm1p in protein synthesis could help explain the variety of biological processes affected by it.
Translation consists of three steps: initiation, elongation and termination (14). Each step is facilitated by a variety of auxiliary proteins known as initiation, elongation and termination factors, many of which are functionally conserved among all organisms. Two elongation factors are key for the peptide chain elongation reaction, and both are highly conserved between prokaryotes and eukaryotes. Elongation factor 1 (eEF1A in eukaryotes and EF-Tu in prokaryotes) is responsible for binding cognate aminoacyl-tRNAs to the ribosomal A-site; elongation factor 2 (eEF2 in eukaryotes and EF-G in prokaryotes) is involved in translocating both mRNA and tRNA from the A-site to the P-site following peptidyl transfer. However, yeast and certain other fungi possess an additional elongation factor, eukaryotic elongation factor 3 (eEF3), which is indispensable for translation elongation (15–17). In S. cerevisiae, the YEF3 gene encodes eEF3, which is essential for viability (18,19). Yeast eEF3 is a 116 000-kDa protein that possesses ribosome-stimulated adenosine triphosphate (ATP)ase activity (19–21). eEF3 interacts with both ribosomal subunits and facilitates eEF1A-mediated cognate aminoacyl-tRNA binding to the ribosomal A-site (22–27). While a great deal is known about the role of eEF3 in translation elongation, details about its regulatory role in this process remain unknown.
Although we have previously shown that Stm1p affected protein synthesis under nutrient deprivation conditions and polysome profiles in the presence of rapamycin, we do not yet know the exact role that Stm1p plays in translation. Using Stm1p mutants and both in vivo and in vitro methods, we found that Stm1p perturbs the normal association of eEF3 with ribosomes and affects translation elongation.
MATERIALS AND METHODS
Yeast strains, media and microbiological methods
Yeast strains investigated include K699 (MATa ade2-1 can1-100 his3-11,-15 leu2-3,-112 ssd1-Δ trp1-1 ura3) BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and their respective stm1Δ mutants, D28 and 4107. Gene replacements in K699 were performed with a HIS3 selectable marker, while those in BY4741 were done using a kanMX selection module. Strains BY4741 and its isogenic stm1Δ counterpart transformed with the high-copy-number plasmid YLR249W, a pBG1805 derivative (ampR, ura3) containing the YEF3 gene, were used in experiments requiring galactose-inducible overexpression of eEF3. In addition, yeast strain AVL78 (MATa leu2 trp1 ura3-52 prb prc pep4-3), possessing defective proteasome activity (28), and transformed with the high-copy-number plasmid YLR150W, a pBG1805 derivative (ampR, ura3) containing the STM1 gene, was used in experiments requiring galactose-inducible overexpression of Stm1p. Yeast were routinely propagated in YPD medium (1% yeast extract, 2% peptone and 1% dextrose) at 30°C. For particular experiments, yeast were propagated in either synthetic minimal (SD) or synthetic complete (SC) medium supplemented with or lacking the appropriate amino acids and nucleic acid bases for the strains under investigation (29). For example, strain K669 and its stm1Δ derivative were propagated in SD medium supplemented with adenine sulfate (20 mg/l), l-histidine-HCl (20 mg/l), l-isoleucine (30 mg/l), l-leucine (30 mg/l), l-tryptophan (20 mg/l) and uracil (20 mg/l), while strain BY4741 and its derivatives and strain AVL78 were propagated in SC medium without uracil for the selection of transforming pBG1805-derived plasmids. The primary carbon source in SD and SC medium was glucose (2%) by default unless substituted with galactose (2%), where indicated, for protein induction experiments.
Protein synthesis inhibitor sensitivity assays
Yeast cultures were diluted in YPD to an optical density at 600 nm (OD600) of 0.4. Ten microliters of these cultures and 10-fold serial dilutions were spotted onto agar plates containing either YPD or synthetic defined (SD*) medium supplemented with essential amino acids and nucleic acid bases and inhibitory concentrations of the protein synthesis inhibitors anisomycin (20 μg/ml), cycloheximide (50 μg/ml), hygromycin B (0.1 μg/ml) or paromomycin (200 μg/ml), as indicated. Plates were incubated at 30°C for 3–7 days, and colony growth was determined by visual inspection.
Yeast extract preparation and ribosome fractionation
Yeast cells were grown to mid-logarithmic phase (OD600 = 0.5) in 200 ml of YPD medium at 30°C and then chilled on ice immediately before harvesting by centrifugation (3000g for 5 min at 4°C). Pelleted yeast cells were washed with ice-cold lysis buffer [50 mM Tris–HCl (pH 7.5), 100 mM NaCl, 7 mM MgCl2, 1 mM DTT, 1 mM PMSF, 1 μM leupeptin, 1 μM pepstatin and 2.5 μg/ml antipain] and then resuspended in 0.5 ml of lysis buffer together with a quarter-volume of acid-washed 0.5-mm Glasperlen glass beads (B. Braun Biotech, Allentown, PA, USA). Cells were disrupted by vortexing for 20 s and then cooling on ice for 30 s, for a total of 10 cycles. Unbroken cells and large debris were removed by low-speed centrifugation (800g for 10 min at 4°C), thereby yielding yeast whole-cell extract.
For polyribosome analysis, five OD260 units (∼150 μl) of whole-cell extracts were layered onto a 12-ml linear sucrose gradient (10–50%) containing 50 mM Tris-acetate (pH 7.0), 50 mM NH4Cl, 3 mM MgCl2 and 1 mM DTT. These gradients were centrifuged in an SW-40 rotor (Beckman Coulter) at 100 000g for 18 h, and 0.4-ml fractions of the gradients were recovered using an Auto Densi-Flow IIC gradient fractionator (Labconco, Kansas City, MO, USA). During fraction collection, the OD254 was recorded using a UA-5 absorbance/fluorescence detector (Isco, Lincoln, NE, USA). Polysome to 80S ratios were calculated by comparing the area under the 80S peak and the combined area under the polysome peaks.
Immunoblotting
Protein samples isolated by differential centrifugation or sucrose gradient fractionation were resuspended in Laemmli sample buffer, denatured by heating to 95°C for 5 min and loaded onto SDS-12% polyacrylamide gels using standard methods. After electrophoretic separation, the proteins were electroblotted onto Hybond-C Extra nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA). Membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline containing 0.2% Triton X-100 and then probed with anti-Stm1p (1 : 5000 dilution) (8) or anti-EF3 (1 : 5000 dilution) (25) rabbit polyclonal antibodies or with an anti-L3 mouse monoclonal antibody (1 : 5000 dilution) (30), followed by 1 : 6000 dilutions of sheep antirabbit or antimouse IgG horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences). Antibodies were visualized using a SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL, USA) following the manufacturer's instructions.
Immunoprecipitation
Whole-cell lysates were incubated with anti-Stm1p rabbit polyclonal antibodies at 4°C for 12 h with continuous rotation in a high-salt buffer [50 mM Tris–HCl (pH 7.5), 500 mM NaCl, 30 mM MgCl2, 1 mM DTT, 1 mM PMSF, 1 μM leupeptin, 1 μM pepstatin and 2.5 μg/ml antipain] to disrupt the interaction of Stm1p with ribosomes. The resulting antigen–antibody complexes were bound to protein G-agarose, washed thrice with high-salt buffer plus 0.1% Triton X-100, extracted with 2× Laemmli sample buffer, resolved by SDS–PAGE and western blotted using anti-ubiquitin or anti-HA epitope tag antibodies.
Protein synthesis efficiency determination
Total protein synthesis efficiency was determined by [35S]methionine incorporation, essentially as described previously (31). Briefly, yeast were grown in the appropriate liquid culture, as indicated, at 30°C until the OD600 reached 0.1. At that point, 10 μCi [35S]methionine in a final concentration of 130 μM methionine was added to the cultures. Aliquots (0.3 ml) were removed periodically and methionine incorporation determined by cold trichloroacetic acid precipitation and scintillation counting.
RESULTS
Yeast lacking Stm1p are more sensitive to inhibitors of translation elongation
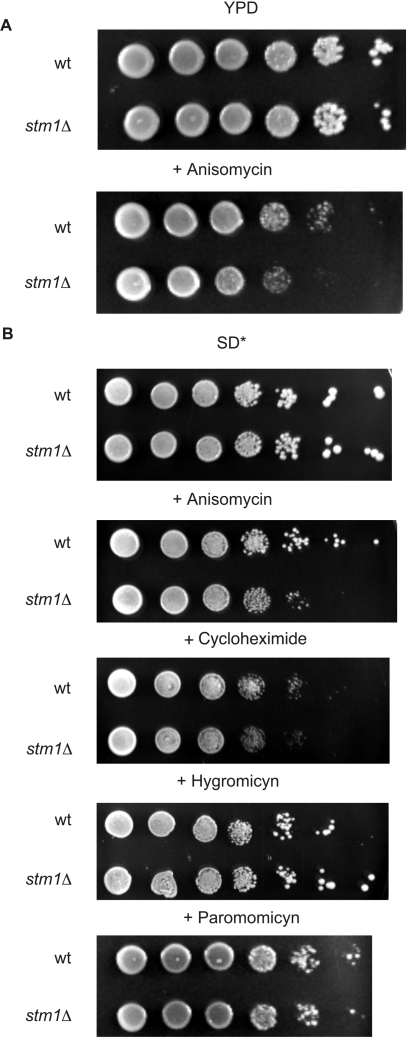
To determine how Stm1p might affect translation, we grew wild-type or stm1Δ mutant yeast on medium containing inhibitors that affect different steps in protein synthesis and are known to be effective in vivo. For example, anisomycin competes with the binding of the 3′ end of aminoacyl-tRNA to the peptidyltransferase center, thereby inhibiting translation elongation (32). Cycloheximide, in addition to its inhibition of translation initiation at low concentrations, can also inhibit polypeptide chain translocation when present at high concentrations (33). The aminoglycoside antibiotics hygromycin B and paromomycin affect translation through dual mechanisms, both by distorting the ribosomal A site, which causes the misreading of aminoacyl-tRNAs, and by inhibiting ribosomal translocation. (34,35). We observed no differences in colony size or number of yeast cultures grown on rich medium containing inhibitory concentrations of cycloheximide (0.1 µg/ml), paromomycin (200 μg/ml) or hygromycin B (50 µg/ml) (data not shown). We found that only anisomycin (20 µg/ml) had an appreciable but small inhibitory effect on stm1Δ yeast growth (Figure 1A). Because we had previously observed that the greatest phenotypic differences with stm1Δ occurred under nutrient deprivation conditions (9), we investigated the antibiotic sensitivity of stm1Δ yeast grown on SD medium supplemented with a minimal set of required amino acids and nucleic acid bases. Under these conditions, we found that anisomycin strongly inhibited the growth of stm1Δ yeast, whereas cycloheximide only slightly reduced growth (Figure 1B). Moreover, we found that neither hygromycin B nor paromomycin had any appreciable effect on the growth of either wild-type or stm1Δ yeast. Taken together, these data indicate that certain protein synthesis inhibitors that affect translation elongation have an inhibitory effect on yeast growth in the absence of Stm1p, especially under supplemented minimal medium conditions.
Figure 1.
Yeast lacking Stm1p are more sensitive to certain protein synthesis inhibitors. (A) Ten-fold serial dilutions of wild-type yeast strain K699 (wt) or its isogenic counterpart lacking Stm1p (stm1Δ) were plated on rich medium (YPD) containing 20 μg/ml anisomycin, as indicated. Colony growth after 3 days of incubation at 30°C is shown. (B) Same as in (A), except that yeast were plated on minimal synthetic defined medium supplemented with essential amino acids and nucleic acid bases (SD*) and containing 20 μg/ml anisomycin, 0.1 μg/ml cycloheximide, 50 μg/ml hygromycin B or 200 μg/ml paromomycin, as indicated.
Ribosomes lacking Stm1p exhibit increased eEF3 association
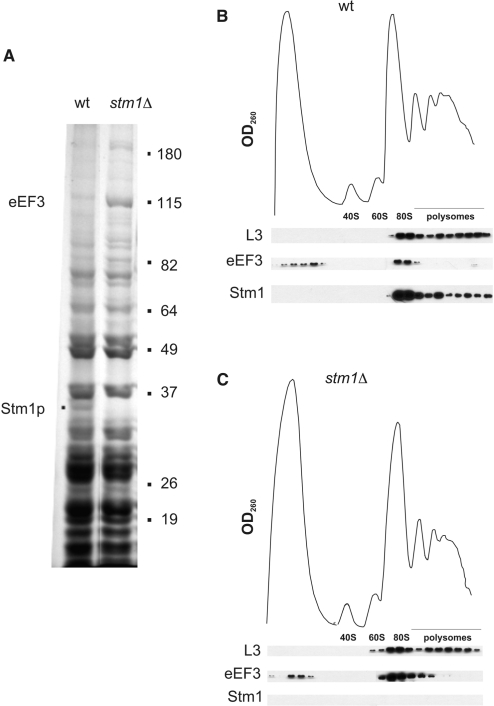
Translation elongation is a multistep process involving multiple factors (14). To better understand whether Stm1p affects any translation elongation factors, we purified 80S ribosomes from wild-type and stm1Δ yeast by sucrose gradient ultracentrifugation and analyzed the proteins present in these samples by SDS–PAGE (Figure 2A). Although most proteins associated with 80S ribosomes were found in similar amounts in either wild-type or stm1Δ yeast strains, there were a few candidates that were present in greater amounts in ribosomes isolated from one or the other yeast strain. One protein, with an apparent molecular mass of 35 kDa, was identified as Stm1p and was present in wild type but not stm1Δ yeast extracts. Another differentially observed protein was a 115-kDa species that was substantially more abundant in 80S ribosomes from stm1Δ yeast than in those from wild-type yeast. This protein was identified as yeast eEF3 (Yef3p) by peptide fingerprinting (data not shown). Thus, these biochemical data indicate that Stm1p affects the equilibrium binding of eEF3 to ribosomes.
Figure 2.
Increased association of eEF3 with ribosomes in the absence of Stm1p. (A) Whole-cell extracts from wild-type K699 (wt) or its isogenic counterpart lacking Stm1p (stm1Δ) were resolved by ultracentrifugation through a 10–50% sucrose gradient and fractionated. Shown is an SDS–PAGE analysis of proteins present in the 80S fractions stained with Coomassie brilliant blue G-250. (B) UV absorbance trace from the sucrose gradient fractionation of wild-type K699 yeast extract (top) and western blots of ribosomal protein L3, eEF3 and Stm1p present in each fraction (bottom). (C) Same as in (B) except the cell extract was from stm1Δ yeast.
To further understand the interplay between Stm1p and eEF3 and their association with ribosomes, we used sucrose gradient ultracentrifugation to separate the different ribosomal species present in wild-type and stm1Δ yeast whole-cell extracts followed by western blotting to determine the distribution of Stm1p and eEF3 in these species. Note that these ribosomal species were prepared in the absence of cycloheximide, which is usually added to prevent continued translation elongation and loss of ribosomes associating with mRNA. The ribosomal protein L3, which is normally present in the 60S large ribosomal subunit, 80S monosomes and polysomes, was used as an internal control. As we previously observed, there were no appreciable differences in the distribution or sedimentation properties of the different ribosomal species obtained from wild-type and stm1Δ yeast strains (Figure 2B and C) (9). This result was further confirmed by the similar L3 protein distribution in both gradients. Stm1p was predominantly distributed in 80S monosomes and to a lesser extent in polysomes, which is also consistent with our previous findings (9). However, we noted a striking difference in the eEF3 protein distribution in ribosomes either possessing or lacking Stm1p. When Stm1p was present, eEF3 was approximately equally distributed between a low-sedimentation species, thought to be ‘free’ eEF3 protein, and eEF3 that co-sedimented with 80S monosomes (Figure 2B). Very little eEF3 was detected in the polysome fractions. However, when Stm1p was absent (Figure 2C), the levels of eEF3 associated with 80S monosomes and lighter polysomes were substantially higher than eEF3 levels when Stm1p was present. Taken together, these data suggest that Stm1p negatively influences the equilibrium binding of eEF3 to ribosomes, which is consistent with our observation that increased amounts of eEF3 associate with ribosomes lacking Stm1p.
Overexpression of eEF3 exacerbates the reduced cell growth phenotype, decreased protein synthesis, increased polysomes and increased eEF3 : ribosome association observed in stm1Δ yeast strains
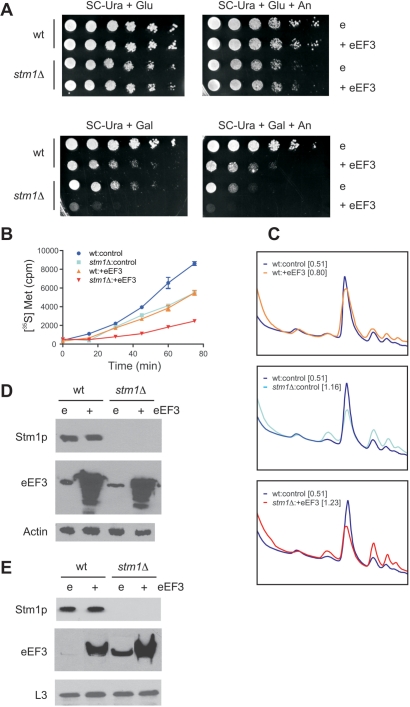
Based on the above results linking the function of eEF3 to Stm1p, we used a genetic approach to test the effects of eEF3 overexpression on cell viability in the presence or absence of Stm1p. Yeast strain BY4741 or its isogenic counterpart stm1Δ was transformed with plasmid YLR249W, which allowed galactose-inducible overexpression of the yeast eEF3 protein, or the control vector pBG1805. Overexpression of eEF3 in cells possessing Stm1p conferred a reduced cell growth phenotype compared to that observed with cells possessing endogenous levels of eEF3 (Figure 3A). Reduced cell growth was also observed with an isogenic stm1Δ strain possessing endogenous levels of eEF3 when propagated in a galactose-containing medium. Notably, when eEF3 was overexpressed in a strain lacking Stm1p, a dramatic growth inhibition was observed. Addition of anisomycin exacerbated the reduced growth phenotypes observed when eEF3 was overexpressed or when Stm1p was absent and caused increased anisomycin sensitivity in stm1Δ yeast overexpressing eEF3. Thus, under growth conditions with minimal medium, the absence of Stm1p can exacerbate the slow growth phenotypes resulting from eEF3 overexpression and anisomycin treatment.
Figure 3.
In the absence of Stm1p, eEF3 overexpression exacerbates the cell grow defect, reduces translational efficiency and increases eEF3 : ribosome association. (A) Ten-fold serial dilutions of wild-type yeast strain BY4741 (wt) or its isogenic counterpart lacking Stm1p (stm1Δ), transformed with either a galactose-inducible eEF3 expression plasmid (+eEF3) or a control plasmid (e), were plated on synthetic complete medium lacking uracil (SC-Ura), with either glucose (Glu) or galactose (Gal) and 20 μg/ml anisomycin (An), as indicated. Colony growth after 3 days of incubation at 30°C is shown. (B) Wt and stm1Δ BY4741 yeast, transformed with either a galactose-inducible eEF3 expression plasmid (+eEF3) or a control plasmid, were propagated in synthetic complete medium lacking uracil and containing galactose and 10 μCi [35S]methionine for the durations indicated. Aliquots were removed and trichloroacetic-acid-precipitable radioactivity determined by scintillation counting. (C) UV absorbance trace from the sucrose gradient fractionation of whole-cell extract from wild-type BY4741 yeast transformed with an eEF3 expression plasmid (top, orange trace), stm1Δ BY4741 yeast transformed with a control plasmid (middle, cyan trace) and stm1Δ BY4741 yeast transformed with an eEF3 expression plasmid (bottom, red trace), each compared with the UV absorbance trace from the sucrose gradient fractionation of whole-cell extract from wild-type BY4741 yeast transformed with a control plasmid (blue traces). For each, their polysome to 80S ratio is indicated in brackets. All yeast strains were propagated for 4 h in SC-Ura medium plus galactose. (D) Proteins from whole-cell extracts from the yeast described in (C) were separated by SDS–PAGE and analyzed by western blotting using antibodies against Stm1p, eEF3 or actin, as indicated. In this experiment, actin served as a loading control. (E) Ribosomes from the aforementioned yeast were purified by sucrose gradient ultracentrifugation and proteins from sucrose gradient fractions corresponding to the 80S peak were separated by SDS–PAGE and analyzed by western blotting using antibodies against Stm1p, eEF3 or L3, as indicated. In this experiment, L3 protein served as a loading control.
Yeast eEF3 has a well-established function in translation, where it is thought to facilitate elongation (15–19). Likewise, there exists some evidence that the observed phenotypes resulting from STM1 deletion are the result of its role in translation (9). To investigate whether the complementary effects of these two proteins on cell growth could be the result of changes in translation efficiency, bulk protein synthesis in wild-type and stm1Δ BY4741 yeast strains, either with endogenous eEF3 levels or overexpressing eEF3, was investigated using a [35S]methionine-incorporation assay (31). Under the conditions tested, both STM1 deletion and eEF3 overexpression had similar effects on protein synthesis, with a 36% reduction of [35S]methionine incorporation into proteins after 60 min (Figure 3B). However, when both STM1 was deleted and eEF3 was overexpressed, [35S]methionine incorporation was suppressed by 72%. These data demonstrate that the absence of Stm1p and overexpression of yeast eEF3 have additive effects on the process of translation.
An effect of STM1 deletion on translation should be observed through changes in polysome profiles. For example, when translation elongation is inhibited one often observes an increase in polysomes relative to the 80S ribosome peak (36). We found that wild-type BY4741 yeast overexpressing eEF3 exhibited a slight increase in polysomes (Figure 3C). Comparing the area under the polysome peaks to that under the 80S peak, we measured a polysome to 80S ratio (P/80S) of 0.80, which was slightly greater than the polysomes : 80S ratio observed for wild-type BY4741 harboring a control plasmid (P/80S = 0.51). A more pronounced increase in polysomes was observed with stm1Δ BY4741 yeast (P/80S = 1.16), with the greatest increase being found for stm1Δ BY4741 yeast overexpressing eEF3 (P/80S = 1.23). Taken together, these data support the contention that STM1 deletion and/or eEF3 overexpression negatively impact translation elongation.
We also analyzed the amount of eEF3 protein from cell lysates by western blot analysis to determine whether Stm1p influenced cellular levels of the eEF3 protein. Basal levels of the eEF3 protein were similar in both wild-type and stm1Δ yeast strains (Figure 3D). Likewise, the extent to which eEF3 could be overexpressed was comparable in both strains. Thus, Stm1p apparently does not appreciably affect the homeostasis of eEF3. Similarly, levels of Stm1p were unchanged when eEF3 was overexpressed. Thus, eEF3 does not appreciably affect the homeostasis of Stm1p. Next, we determined whether the observed growth defect correlated with increased levels of eEF3 associated with ribosomes. Ribosomes from wild-type and stm1Δ yeast strains, both with and without eEF3 overexpression, were purified by centrifugation through sucrose gradients and the proteins associated with 80S ribosomes were analyzed by western blotting (Figure 3E). Regardless of whether endogenous eEF3 levels were present or eEF3 was overexpressed, the amount of eEF3 associated with 80S ribosomes was dramatically increased in ribosomal fractions from stm1Δ yeast strains as compared with isogenic wild-type strains. Notably, the amount of Stm1p found associating with ribosomes was not affected by the overexpression of eEF3. These data demonstrate a direct correlation between the observed cell-growth inhibition and the amount of eEF3 associated with ribosomes, suggesting a functional relationship between eEF3 and Stm1p in vivo.
Ribosomes with increased levels of Stm1p exhibit decreased eEF3 association
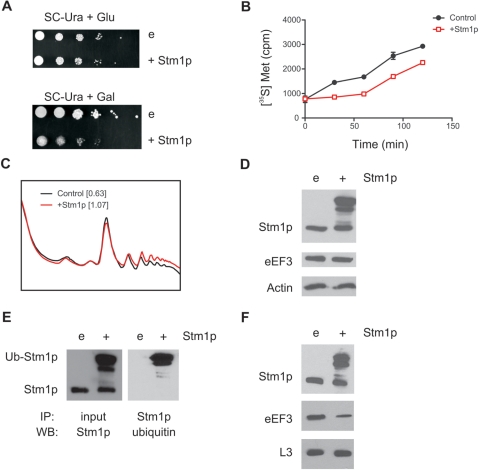
Because we found that ribosomes from stm1Δ yeast strains exhibited increased eEF3 association, we hypothesized that Stm1p negatively regulates the amount of eEF3 associated with ribosomes. We sought reciprocal support for this model by testing the effects of Stm1p overexpression on eEF3 association with ribosomes. Given that Stm1p has been shown to be a target for proteasomal degradation (5), we chose to pursue these studies using the proteasome defective strain AVL78 (28). Yeast strain AVL78 was transformed with plasmid YLR150W, which allows galactose-inducible overexpression of Stm1p, or the control vector pBG1805. Overexpression of Stm1p in these cells conferred a substantially reduced growth phenotype compared to yeast with endogenous levels of Stm1p (Figure 4A). This reduced growth was correlated with an initial reduction in total protein synthesis, although the rate of protein synthesis was similar at later time points (Figure 4B). This apparent delay in protein synthesis may be, in part, a result of the impaired proteasome function in these cells, which allows accumulation of otherwise relatively labile proteins. In addition, the observed reduction in protein synthesis correlated with an increase in heavy polysomes (Figure 4C, P/80S increased from 0.63 to 1.07), suggesting that Stm1p overexpression might inhibit translation elongation.
Figure 4.
Overexpression of Stm1p in a proteasome-deficient strain (AVL78) led to decreased amounts of eEF3 associated with ribosomes. (A) Ten-fold serial dilutions of wild-type yeast strain AVL78 transformed with either a galactose-inducible Stm1p expression plasmid (+Stm1p) or a control plasmid (e), were plated on synthetic complete medium lacking uracil (SC-Ura) and containing either glucose (Glu) or galactose (Gal), as indicated. Colony growth after 3 days of incubation at 30°C is shown. (B) AVL78 yeast transformed with either a galactose-inducible Stm1p expression plasmid (+Stm1p) or a control plasmid were propagated in synthetic complete medium lacking uracil and supplemented with galactose and 10 μCi [35S]methionine for the durations indicated. Aliquots were removed and trichloroacetic-acid-precipitable radioactivity determined by scintillation counting. (C) UV absorbance traces from the sucrose gradient fractionation of whole-cell extracts from wild-type AVL78 yeast transformed with either an Stm1p expression plasmid (red trace) or a control plasmid (black trace). For each, their polysome to 80S ratio is indicated in brackets. All yeast strains were propagated for 4 h in SC-Ura medium plus galactose. (D) Proteins from whole-cell extracts of AVL78 were separated by SDS–PAGE and analyzed by western blotting using antibodies against Stm1p, eEF3 or actin, as indicated. (E) Stm1p from the above whole-cell extracts was immunoprecipitated with an anti-Stm1p antibody in the presence of 500 mM NaCl, proteins were resolved by SDS–PAGE and analyzed by western blotting using anti-ubiquitin antibodies (right). SDS–PAGE-resolved proteins from this experiment western blotted with anti-Stm1p antibodies are shown on the left. (F) Ribosomes from the above yeast extracts were purified by sucrose gradient ultracentrifugation and proteins from sucrose gradient fractions corresponding to the 80S peak were separated by SDS–PAGE and analyzed by western blotting using antibodies against Stm1p, eEF3 or L3, as indicated.
To verify the overexpression of Stm1p and its effects on cellular eEF3 levels, cell lysates were prepared from galactose-induced yeast, soluble proteins resolved by SDS–PAGE and specific proteins visualized by western blotting. We found that yeast overexpressing Stm1p demonstrated an accumulation of a more slowly migrating species that cross-reacted with anti-Stm1p antibodies (Figure 4D). Accumulation of this high-molecular-weight species had no effect on cellular eEF3 or actin levels. Given that Stm1p overexpression was performed in a proteasome-deficient yeast strain, we hypothesized that the high-molecular-weight species corresponded to ubiquitinated Stm1p. This hypothesis was verified with an immunoprecipitation experiment (Figure 4E). Curiously, no apparent increase in levels of normally migrating Stm1p was observed when Stm1p was overexpressed. This result suggests that Stm1p levels may be tightly regulated in cells to ensure that excess Stm1p does not accumulate.
To test the effects of Stm1p overexpression on the complement of proteins associating with ribosomes, we used ultracentrifugation of whole-cell extracts to obtain ribosomes and their constituent proteins from yeast both with or without overexpression of Stm1p. Proteins were resolved by SDS–PAGE and analyzed by western blotting. We found that overexpression of Stm1p led to an increased amount of ubiquitinated Stm1p associated with ribosomes (Figure 4F). Correspondingly, we found decreased amounts of eEF3 associated with ribosomes from cells overexpressing Stm1p. This experiment confirms our hypothesis that Stm1p negatively regulates the amount of eEF3 associated with ribosomes.
DISCUSSION
In this study, we demonstrated that yeast lacking the ribosome-associated protein Stm1p exhibited increased sensitivity to specific protein synthesis inhibitors, e.g. anisomycin and cycloheximide, especially when propagated in minimal medium. The effect of STM1 deletion on the sensitivity of the cells to anisomycin provides good in vivo evidence of a role for Stm1p in translational elongation. We also found that ribosomes lacking Stm1p had elevated levels of the yeast-specific elongation factor eEF3 associated with them as compared with wild-type ribosomes. In addition, we found that when ribosomes had elevated levels of associated Stm1p, they exhibited decreased levels of eEF3 association. Similarly, overexpression of eEF3 in cells lacking Stm1p resulted in an enhanced growth defect, which notably correlated strongly with elevated levels of eEF3 associated with ribosomes. Taken together, these genetic and biochemical data suggest that Stm1p is important for eEF3 function, presumably by affecting the proper association of eEF3 with 80S ribosomes.
One of the best ways of determining how Stm1p affects translation in vivo is to investigate Stm1p-dependent changes in ribosome distribution following sucrose gradient ultracentrifugation. We observed that the absence of Stm1p caused a notable increase in polysomes, which was exacerbated by eEF3 overexpression. Similarly, depletion or inactivation of the yeast translation factor eIF5A was shown to cause a pronounced increase in polysomes and inhibit translation elongation (37,38). Given that our methionine incorporation experiments indicated decreased protein synthesis in stm1Δ yeast that was exacerbated by eEF3 overexpression, these data strongly support the contention that the absence of Stm1p affects translation elongation in a cooperative fashion with eEF3. In addition, overexpression of Stm1p caused both an increase in heavy polysomes and a decrease in protein synthesis. Such is reminiscent of human ribosomal S5 protein expression in yeast, which demonstrated an increase in heavy polysomes and a decrease in eEF3-ribosome interactions, thereby affecting translation elongation (39). Thus, there may be an ideal range of Stm1p and eEF3 concentrations that permit optimal translation elongation.
Previously, we found that Stm1p exists in a 1:1 complex with 80S ribosomes and interacts with both the 40S and 60S ribosomal subunits (9). The interface between ribosomal subunits is well-known to be very important for ribosomal function, as it is the interaction site for aminoacyl-tRNA and the canonical elongation factors eEF1A and eEF2. As mutations or deletions of genes directly involved in translation elongation usually lead to a lethal phenotype or substantial alterations in protein synthesis (40–45), we postulate that Stm1p most likely does not have an overlapping interaction site with eEF1A and eEF2. Cryo-electron microscopy of the eEF3-80S ribosome complex has indicated that eEF3 interacts with both the large and small ribosomal subunits but at a different site than those recognized by eEF1A and eEF2 (27,46,47). The fact that eEF3 has a completely different binding site from the canonical elongation factors is consistent with the observation that in the absence of Stm1p more eEF3 associates with ribosomes. It is of interest to note that sequence alignments between S. cerevisiae eEF3 (Yef3p) and Stm1p show some degree of sequence similarity, especially in the C-terminal regions of each protein (Pickering,B., unpublished data). However, this sequence similarity is not conserved in homologs of these proteins found in other yeasts and lower fungi. In fact, the most significant sequence homology between these different eEF3 and Stm1p proteins maps to the C-terminal end of eEF3, which is rich in arginine and lysine residues, and basic patches within the C-terminal region of Stm1p. Thus, it is tempting to speculate that eEF3 and Stm1p share overlapping ribosome-binding sites recognized by these basic domains.
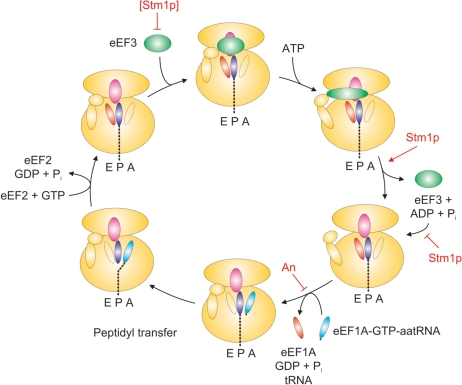
Translocation of tRNAs in yeast ribosomes during translation elongation requires two canonical elongation factors, eEF1A and eEF2 (14). eEF1A facilitates cognate aminoacyl-tRNA binding to the A-site in 80S ribosomes. Following the peptidyl transferase reaction, eEF2 facilitates the translocation of deacylated tRNA from the P- to the E-site and aminoacyl-tRNA from the A- to the P-site. Afterwards, eEF3, which is thought to interact with 80S ribosomes near the E-site, facilitating dissociation of deacyl-tRNA from the ribosome (23,24,27). These findings are consistent with the allosteric model of translation, whereby release of tRNA from the E-site in turn facilitates occupancy of the A-site with cognate aminoacyl-tRNA, thereby permitting efficient translation elongation. Andersen et al. (27) have proposed a detailed model for the specific role of eEF3 in the translation elongation cycle (Figure 5). This model involves the following steps: (i) weak binding of eEF3 to post-translocation ribosomes with the deacyl-tRNA ‘locked’ in the ribosome E-site by an ‘in’-position L1 stalk and the 40S head; (ii) a conformational change in eEF3, which is caused by ATP binding, which results in higher-affinity ribosome binding and the repositioning of the L1 stalk to an ‘out’ position; and (iii) eEF3 conformation-promoted ATP hydrolysis, resulting in eEF3 dissociation from the ribosome, E-site opening and unlocking of the 40S head. This last step allows the eEF1A–GTP–aminoacyl-tRNA complex to bind to the ribosomal A-site and deacyl-tRNA to be released from the E-site, allowing a subsequent round of elongation to ensue. Our data are consistent with a model in which ribosome-bound Stm1p tempers binding of eEF3 to 80S ribosomes. This could occur through Stm1p directly competing with eEF3-ribosome binding, promoting eEF3 ATPase activity and/or facilitating an eEF3 or ribosome conformational change that permits efficient dissociation of eEF3 from ribosomes following ATP hydrolysis. In the absence of Stm1p, eEF3 may not efficiently dissociate from ribosomes, potentially leading to an unproductive cycle of ATP binding, eEF3 conformational change and ATP hydrolysis. The failure of eEF3 to dissociate from the ribosome could prevent the release of deacyl-tRNA from the E-site and binding of eEF1A–GTP–aminoacyl-tRNA to the A-site, thereby retarding these final steps in the cycle of translation elongation. In addition, these effects are likely be exacerbated when concentrations of deacyl-tRNA are elevated and/or aminoacyl-tRNAs are depressed, exactly the circumstances that occur during nutrient deprivation (14). Similarly, given that the net effect of anisomycin on protein synthesis is to block aminoacyl-tRNA access to the ribosome peptidyltransferase center (32), this provides an explanation for the observed increased sensitivity of yeast lacking Stm1p or overexpressing eEF3 to this particular antibiotic. We believe that Stm1p, when bound to the ribosome, facilitates the release of the ATP-hydrolyzed conformation of eEF3, thereby permitting efficient translation elongation. However, high concentrations of Stm1p could also inhibit eEF3-80S ribosome binding and thereby reduce translation elongation, which we observed. The exact mechanism by which Stm1p binds to the ribosome and affects the function of eEF3 awaits further structural data and is currently under investigation.
Figure 5.
Role of Stm1p in translation elongation. Shown is a model of the yeast elongation cycle. Starting at the top left, Stm1p at high concentrations can inhibit the association of eEF3 (green ellipse) to post-translocation ribosomes. After ATP binding, which causes an elongated eEF3 conformation and unlocking of the E-site by moving the ribosome L1 stalk, bound Stm1p (red ellipse) can facilitate eEF3 dissociation from the ribosome and prevent reassociation. With eEF3 absent from the ribosome, the ternary complex eEF1A–GTP–aminoacyl-tRNA can then associate, delivering aminoacyl-tRNA (blue ellipse) to the ribosome A-site and releasing deacyl-tRNA (orange ellipse) from the E-site, steps inhibited by the antibiotic anisomycin (An). In the absence of Stm1p, eEF3 can inappropriately associate with ribosomes, thereby inhibiting subsequent ternary complex binding and deacyl-tRNA release and inhibiting efficient elongation, especially under conditions of translational stress (e.g. nutrient deprivation or antibiotic inhibition).
FUNDING
Grant G-1199 from the Robert A. Welch Foundation; and the University Cancer Foundation of The University of Texas M. D. Anderson Cancer Center. Funding for open access charge: Robert A. Welch Foundation G-1199.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank Jonathan Warner and Terri Goss Kinzy for their generous gifts of antibodies.
REFERENCES
- 1.Sakai A, Chibazakura T, Shimizu Y, Hishinuma F. Molecular analysis of POP2 gene, a gene required for glucose-derepression of gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 1992;20:6227–6233. doi: 10.1093/nar/20.23.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kikuchi Y, Oka Y, Kobayashi M, Uesono Y, Toh-e A, Kikuchi A. A new yeast gene, HTR1, required for growth at high temperature, is needed for recovery from mating pheromone-induced G1 arrest. Mol. Gen. Genet. 1994;245:107–116. doi: 10.1007/BF00279756. [DOI] [PubMed] [Google Scholar]
- 3.Utsugi T, Toh-e A, Kikuchi Y. A high dose of the STM1 gene suppresses the temperature sensitivity of the tom1 and htr1 mutants in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1995;1263:285–288. doi: 10.1016/0167-4781(95)00123-x. [DOI] [PubMed] [Google Scholar]
- 4.Hata H, Mitsui H, Liu H, Bai Y, Denis CL, Shimizu Y, Sakai A. Dhh1p, a putative RNA helicase, associates with the general transcription factors Pop2p and Ccr4p from Saccharomyces cerevisiae. Genetics. 1998;148:571–579. doi: 10.1093/genetics/148.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligr M, Velten I, Fröhlich E, Madeo F, Ledig M, Fröhlich KU, Wolf DH, Hilt W. The proteasomal substrate Stm1 participates in apoptosis-like cell death in yeast. Mol. Biol. Cell. 2001;12:2422–2432. doi: 10.1091/mbc.12.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fröhlich KU, Madeo F. Apoptosis in yeast: a new model for aging research. Exp. Gerontol. 2001;37:27–31. doi: 10.1016/s0531-5565(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi N, Murakami S. STM1, a gene which encodes a guanine quadruplex binding protein, interacts with CDC13 in Saccharomyces cerevisiae. Mol. Genet. Genomics. 2002;267:806–813. doi: 10.1007/s00438-002-0712-3. [DOI] [PubMed] [Google Scholar]
- 8.Van Dyke MW, Nelson LD, Weilbaecher RG, Mehta DV. Stm1p, a G4 quadruplex and purine motif triplex nucleic acid-binding protein, interacts with ribosomes and subtelomeric Y' DNA in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:24323–24333. doi: 10.1074/jbc.M401981200. [DOI] [PubMed] [Google Scholar]
- 9.Van Dyke N, Baby J, Van Dyke MW. Stm1p, a ribosome-associated protein, is important for protein synthesis in Saccharomyces cerevisiae under nutritional stress conditions. J. Mol. Biol. 2006;358:1023–1031. doi: 10.1016/j.jmb.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Ohn T, Chiang YC, Lee DJ, Yao G, Zhang C, Denis CL. CAF1 plays an important role in mRNA deadenylation separate from its contact to CCR4. Nucleic Acids Res. 2007;35:3002–3015. doi: 10.1093/nar/gkm196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balagopal V, Parker R. Stm1 modulates mRNA decay and Dhh1 function in Saccharomyces cerevisiae. Genetics. 2009;181:93–103. doi: 10.1534/genetics.108.092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz JD, Gilbert W. A yeast gene product, G4p2, with a specific affinity for quadruplex nucleic acids. J. Biol. Chem. 1995;270:9413–9419. doi: 10.1074/jbc.270.16.9413. [DOI] [PubMed] [Google Scholar]
- 13.Nelson LD, Musso M, Van Dyke MW. The yeast STM1 gene encodes a purine-motif triple-helical DNA binding protein. J. Biol. Chem. 2000;275:5573–5581. doi: 10.1074/jbc.275.8.5573. [DOI] [PubMed] [Google Scholar]
- 14.Merrick WC, Nyborg J. In: Translational Control of Gene Expression. Sonenberg N, Hershey JWB, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 89–126. [Google Scholar]
- 15.Skogerson L, Engelhardt D. Dissimilarity in protein chain elongation factor requirements between yeast and rat liver ribosomes. J. Biol. Chem. 1977;252:1471–1475. [PubMed] [Google Scholar]
- 16.Dasmahapatra B, Chakraburtty K. Protein synthesis in yeast. I. Purification and properties of elongation factor 3 from Saccharomyces cerevisiae. J. Biol. Chem. 1981;256:9999–10004. [PubMed] [Google Scholar]
- 17.Belfield GP, Tuite MF. Translation elongation factor 3: a fungus-specific translation factor? Mol. Microbiol. 1993;9:411–418. doi: 10.1111/j.1365-2958.1993.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 18.Qin SL, Moldave K, McLaughlin CS. Isolation of the yeast gene encoding elongation factor 3 for protein synthesis. J. Biol. Chem. 1987;262:7802–7807. [PubMed] [Google Scholar]
- 19.Sandbaken M, Lupisella JA, DiDomenico B, Chakraburtty K. Isolation and characterization of the structural gene encoding elongation factor 3. Biochim. Biophys. Acta. 1990;1050:230–234. doi: 10.1016/0167-4781(90)90172-x. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki M, Uritani M, Kagiyama H. Intrinsic ATPase activity of yeast peptide chain elongation factor 3 (EF-3) and its direct interaction with various nucleotides. Nucleic Acids Symp. Ser. 1986;17:171–174. [PubMed] [Google Scholar]
- 21.Miyazaki M, Uritani M, Kagiyama H. The yeast peptide elongation factor 3 (EF-3) carries an active site for ATP hydrolysis which can interact with various nucleoside triphosphates in the absence of ribosomes. J. Biochem. 1988;104:445–450. doi: 10.1093/oxfordjournals.jbchem.a122487. [DOI] [PubMed] [Google Scholar]
- 22.Kamath A, Chakraburtty K. Role of yeast elongation factor 3 in the elongation cycle. J. Biol. Chem. 1989;264:15423–15428. [PubMed] [Google Scholar]
- 23.Triana-Alonso FJ, Chakraburtty K, Nierhaus KH. The elongation factor 3 unique in higher fungi and essential for protein biosynthesis is an E site factor. J. Biol. Chem. 1995;270:20473–20478. doi: 10.1074/jbc.270.35.20473. [DOI] [PubMed] [Google Scholar]
- 24.Kambampati R, Chakraburtty K. Functional subdomains of yeast elongation factor 3. Localization of ribosome-binding domain. J. Biol. Chem. 1997;272:6377–6381. doi: 10.1074/jbc.272.10.6377. [DOI] [PubMed] [Google Scholar]
- 25.Anand M, Chakraburtty K, Marton MJ, Hinnebusch AG, Kinzy TG. Functional interactions between yeast translation elongation factor (eEF) 1A and eEF3. J. Biol. Chem. 2003;278:6985–6991. doi: 10.1074/jbc.M209224200. [DOI] [PubMed] [Google Scholar]
- 26.Anand M, Balar B, Ulloque R, Gross SR, Kinzy TG. Domain and nucleotide dependence of the interaction between Saccharomyces cerevisiae translation elongation factors 3 and 1A. J. Biol. Chem. 2006;281:32318–32326. doi: 10.1074/jbc.M601899200. [DOI] [PubMed] [Google Scholar]
- 27.Andersen CB, Becker T, Blau M, Anand M, Halic M, Balar B, Mielke T, Boesen T, Pedersen JS, Spahn CM, et al. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature. 2006;443:663–668. doi: 10.1038/nature05126. [DOI] [PubMed] [Google Scholar]
- 28.Lingner J, Cech TR, Hughes TR, Lundblad V. Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc. Natl Acad. Sci. USA. 1997;94:11190–11195. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amberg DC, Burke DJ, Strathern J. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2005. pp. 199–121. [Google Scholar]
- 30.Vilardell J, Warner J. Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol. Cell Biol. 1997;17:1959–1965. doi: 10.1128/mcb.17.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr-Schmid A, Durko N, Cavallius J, Merrick WC, Kinzy TG. Mutations in a GTP-binding motif of eukaryotic elongation factor 1A reduce both translational fidelity and the requirement for nucleotide exchange. J. Biol. Chem. 1999;274:30297–30302. doi: 10.1074/jbc.274.42.30297. [DOI] [PubMed] [Google Scholar]
- 32.Rakauskaite R, Dinman JD. rRNA mutants in the yeast peptidyltransferase center reveal allosteric information networks and mechanism of drug resistance. Nucleic Acids Res. 2008;36:1497–1507. doi: 10.1093/nar/gkm1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dresios J, Panopoulos P, Frantziou CP, Synetos D. Yeast ribosomal protein deletion mutants possess altered peptidyltransferase activity and different sensitivity to cycloheximide. Biochemistry. 2001;40:8101–8108. doi: 10.1021/bi0025722. [DOI] [PubMed] [Google Scholar]
- 34.Brodersen DE, Clemons WM, Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell. 2000;103:1143–1154. doi: 10.1016/s0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 35.Vicens Q, Westhof E. Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site. Structure. 2001;9:647–658. doi: 10.1016/s0969-2126(01)00629-3. [DOI] [PubMed] [Google Scholar]
- 36.Spirin AS, Ryazanov AG. Regulation of elongation rate. In: Traschel H, editor. Translation in Eukaryotes. Boca Raton, FL: CRC Press; 1991. pp. 325–350. [Google Scholar]
- 37.Gregio APB, Cano VPS, Avaca JS, Valentini SR, Zanelli CF. eIF5A has a function in the elongation step of translation in yeast. Biochem. Biophys. Res. Comm. 2009;380:785–790. doi: 10.1016/j.bbrc.2009.01.148. [DOI] [PubMed] [Google Scholar]
- 38.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galkin O, Bentley AA, Gupta S, Compton B, Mazumder B, Kinzy TG, Merrick WC, Hatzoglou M, Pestova TV, Hellen CUT, et al. Roles of the negatively charged N-terminal extension of Saccharomyces cerevisiae ribosomal protein S5 revealed by characterization of a yeast strain containing human ribosomal protein S5. RNA. 2007;13:2116–2128. doi: 10.1261/rna.688207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capa L, Mendoza A, Lavandera JL, Gomez de las Heras F, Garcia-Bustos JF. Translation elongation factor 2 is part of the target for a new family of antifungals. Antimicrob. Agents Chemother. 1998;42:2694–2699. doi: 10.1128/aac.42.10.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dominguez JM, Martin JJ. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob. Agents Chemother. 1998;42:2279–2283. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foley BT, Moehring JM, Moehring TJ. Mutations in the elongation factor 2 gene which confer resistance to diphtheria toxin and Pseudomonas exotoxin A. Genetic and biochemical analyses. J. Biol. Chem. 1995;270:23218–23225. doi: 10.1074/jbc.270.39.23218. [DOI] [PubMed] [Google Scholar]
- 43.Justice MC, Hsu MJ, Tse B, Ku T, Balkovec J, Schmatz D, Nielsen J. Elongation factor 2 as a novel target for selective inhibition of fungal protein synthesis. J. Biol. Chem. 1998;273:3148–3151. doi: 10.1074/jbc.273.6.3148. [DOI] [PubMed] [Google Scholar]
- 44.Savelsbergh A, Katunin VI, Mohr D, Peske F, Rodnina MV, Wintermeyer W. An elongation factor G-induced ribosome rearrangement precedes tRNA–mRNA translocation. Mol. Cell. 2003;11:1517–1523. doi: 10.1016/s1097-2765(03)00230-2. [DOI] [PubMed] [Google Scholar]
- 45.Zavialov AV, Hauryliuk VV, Ehrenberg M. Guanine-nucleotide exchange on ribosome-bound elongation factor G initiates the translocation of tRNAs. J. Biol. 2005;4:9–14. doi: 10.1186/jbiol24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connell SR, Takemoto C, Wilson DN, Wang H, Murayama K, Terada T, Shirouzu M, Rost M, Schüler M, Giesebrecht J, et al. Structural basis for interaction of the ribosome with the switch regions of GTP-bound elongation factors. Mol. Cell. 2007;25:751–764. doi: 10.1016/j.molcel.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Spahn CM, Gomez-Lorenzo MG, Grassucci RA, Jørgensen R, Andersen GR, Beckmann R, Penczek PA, Ballesta JP, Frank J. Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 2004;23:1008–1019. doi: 10.1038/sj.emboj.7600102. [DOI] [PMC free article] [PubMed] [Google Scholar]