Abstract
During social interactions, humans often unconsciously and unintentionally imitate the behaviors of others, which increases rapport, liking, and empathy between interaction partners. This effect is thought to be an evolutionary adaptation facilitating group living, and may therefore be shared with other primate species. Here we show that capuchin monkeys, a highly social primate species, prefer human imitators in a variety of ways: they look longer at imitators, spend more time in proximity to imitators, and choose to interact more frequently with imitators in a token exchange task. These results demonstrate that imitation can promote affiliation in nonhuman primates. Behavior matching that leads to prosocial behaviors towards others may have been one of the mechanisms at the basis of altruistic behavioral tendencies in capuchins and in other primates including humans.
In everyday life, we often imitate the body postures, gestures, and mannerisms of our social interaction partners without noticing (1), a phenomenon that has been termed the “chameleon effect” (2). This form of imitation, which occurs completely unconsciously and unintentionally, can have profound effects on subsequent social interactions: the imitated person reports increases in shared rapport, liking, and feelings of empathy with the interaction partner (2), and is also more likely to display prosocial behaviors such as helping others, leaving more generous tips, or donating money to charity (3, 4). Imitation therefore increases affiliation, empathy, and rapport between individuals and undoubtedly plays an important role in maintaining harmonious relationships with others (5).
Being able to cultivate successful social relationships is likely to carry significant adaptive value. Individuals with strong social bonds who receive support from others are thought to have an evolutionary advantage over those who are ostracized from a group (3, 5). Non-conscious imitation may therefore represent an evolutionary adaptation that facilitates group living, and may occur among other social primate species. While great apes and macaques share with us the capacity to recognize imitation (6, 7, 8), it is presently unclear whether imitation may also facilitate positive social interactions in nonhuman primates. That is, do other primates show increased levels of affiliation towards another individual who displays behaviors similar to their own? We addressed this question by studying the effects of imitation on the behavior of capuchin monkeys, a highly social and socially tolerant New World primate species. Observational and experimental evidence suggests that capuchins are easily influenced by others’ behavior (9, 10, 11), and are thus likely to recognize when others display behaviors matching their own actions. Moreover, since capuchins are strongly bonded into social groups, they may share with us this mechanism to facilitate social group living, namely increasing affiliation towards those who display matching behaviors. In the following series of experiments, we investigated whether capuchins recognize imitation, and whether imitation positively affects capuchins’ social interactions.
In Experiment 1, we investigated whether capuchins differentiate between an experimenter imitating them and an experimenter performing contingent actions but not imitating them. Following the established research designs with infants (12, 13), two experimenters, each holding a small plastic ball, faced each monkey (Fig. 1). During the baseline phase, both experimenters performed actions that are commonly performed by capuchins (poking the ball with their fingers, mouthing the ball, and pounding the ball on a surface; 14). Analysis of visual preferences showed that monkeys did not discriminate between experimenters (mean 36.23 sec and 40.05 sec, t(10)=-0.81, p=0.44). During the manipulation phase, monkeys were given an identical ball. One experimenter imitated the monkeys’ ball-directed actions whereas the other experimenter performed contingent but non-matching actions (14). Monkeys looked longer at the imitator while they were manipulating the ball and hence while they were being imitated by the imitator (t(10)=2.23, p=0.050; Fig. 2). Thus, capuchins are sensitive to the actions of others that match their own actions and they prefer to look at imitating individuals.
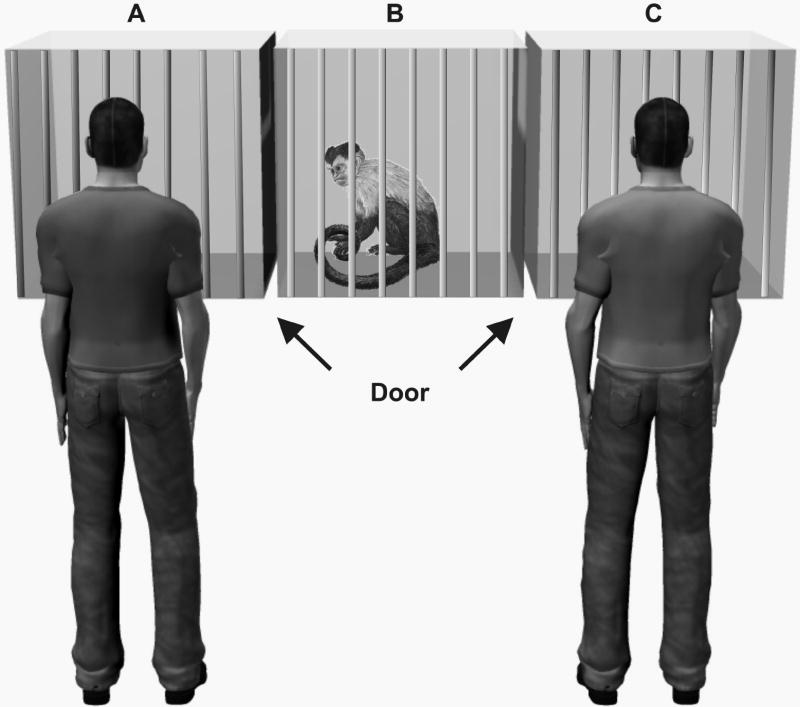
Figure 1.
Schematic of experimental set up in Experiments 1-5. For proximity measures and token exchanges, monkeys had access to cages A-C. Monkeys were considered to be in proximity to an experimenter when they entered cages A or C. During the manipulation phases (imitation or gaze control), monkeys were restricted to cage B.
Figure 2.
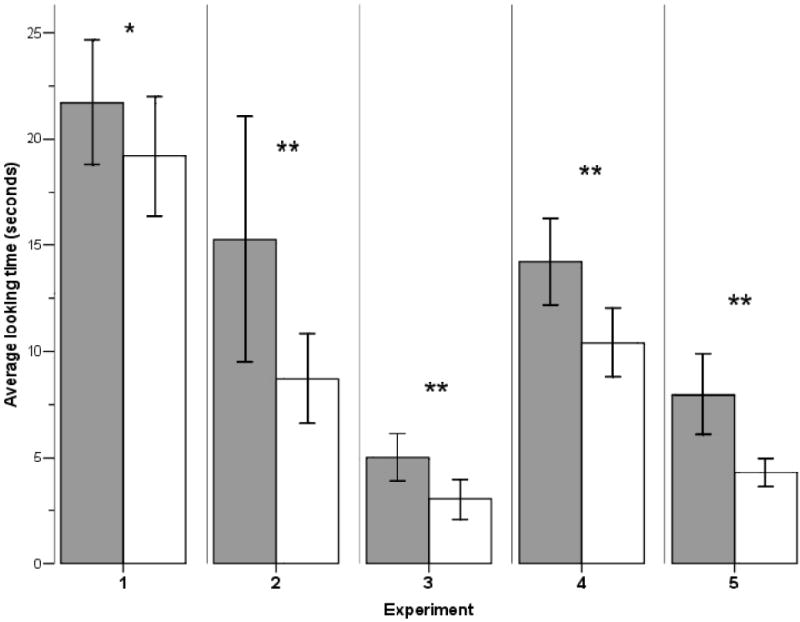
Average looking time at experimenters while monkeys were manipulating the ball during manipulation phases (imitation or gaze control) in Experiments 1-5. Dark bars represent imitator/experimenter facing monkey, white bars represent non-imitator/experimenter facing away from monkey. * = difference with p=0.05, ** = significant difference with p<0.05, two-tailed t-tests (Exp. 1&3: N=11, Exp. 2, 4 & 5: N=10, see (14)). Error bars represent SEM.
Proximity to others is a reliable indicator of underlying affiliative relationships in capuchins and other primates (15). We therefore tested whether capuchins show increased proximity to an imitator rather than a non-imitator. At the start of Experiment 2, two experimenters faced each monkey for a 1 minute period, one standing on the left side and one standing on the right side of a test cage. Monkeys could freely move within the three chambers of the test cage and choose whether to spend time in front of one of the experimenters or in a neutral middle position equidistant between experimenters (Fig. 1). During the first proximity measurement, monkeys spent similar amounts of time in front of the experimenters (41.2% and 36.0% of trial; t(9)=0.65, p=0.53; Fig. 3). Similar to Experiment 1, monkeys were then given a small plastic ball, and one experimenter imitated the monkey’s ball-directed actions while the other performed temporally contingent but structurally non-matching actions. Monkeys looked longer at the imitator while manipulating the ball and hence while being imitated (t(9)=2.95, p=0.016; Fig. 2).
Figure 3.
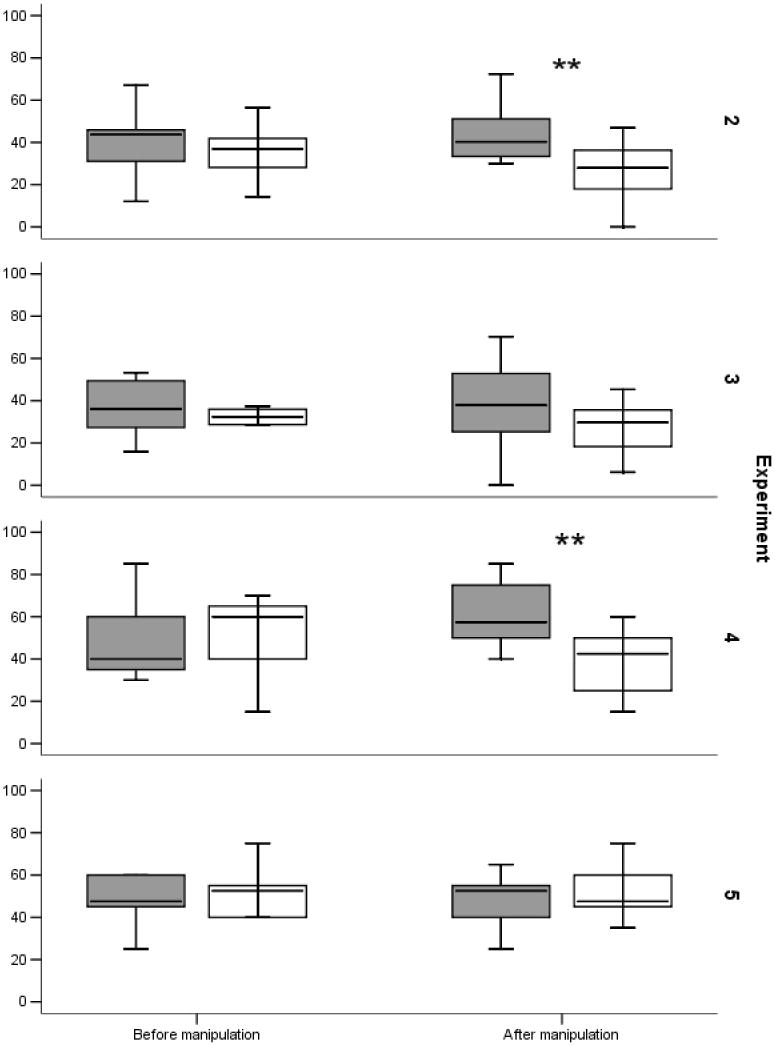
Average behavioral preferences (proximity and token exchanges) for experimenters in Experiments 2-5 before and after the manipulation phases (imitation and gaze control). Proximity measures in Experiments 2 and 3 were converted into a percentage of the total phase duration. Token exchanges in Experiments 4 and 5 were converted into a percentage of total exchanges (10 trials per phase = 100%). The top of the boxes represents the 75th percentile, the bottom of the boxes represents the 25th percentile, and the line in the middle represents the median. Dark boxes represent imitator/experimenter facing monkey, white boxes represent non-imitator/experimenter facing away from monkey. ** = significant difference with p<0.05, two-tailed t-tests.
Following the manipulation phase, imitator and non-imitator switched positions in front of the test cage, and for 1 minute monkeys could again choose whether to spend time in front of an experimenter or in the middle of the test cage equidistant between experimenters. Monkeys now spent significantly more time in front of the imitator rather than the non-imitator (mean imitator: 44.0%, mean non-imitator: 27.1% of trial, t(9)=2.29, p=0.048; Fig. 3).
One possible explanation for this effect might be that it is not imitation per se, but rather the difference in looking time during the manipulation phase that led to greater familiarity with the imitator and thereby might have caused the shift in experimenter preference. Alternatively, the imitator may have been perceived as being more attentive to the monkeys and therefore may have been preferred in the subsequent proximity test. To test whether attention alone may have caused the observed effect on social preference, in Experiment 3 we first conducted a baseline proximity preference as in Experiment 2.
Two experimenters stood in front of the monkeys, one to the left and one to the right of the test cage, and for 1 minute monkeys could choose to spend time in front of an experimenter or in the middle of the test cage equidistant between experimenters. Monkeys spent similar amounts of time in front of experimenters (36.9% and 32.0% of trial; t(10)=0.74, p=0.48; Fig. 3). Monkeys were then given a small plastic ball, and one experimenter faced and looked at the monkeys whereas the other experimenter turned around and faced away from the monkeys. Both experimenters remained passive throughout the test phase and did not move while the monkeys manipulated the ball.
Being sensitive to gaze (16), monkeys looked significantly longer at the experimenter facing them during the manipulation phase (t(10)=6.41, p<0.001; Fig. 2). Following the manipulation phase, the two experimenters switched places in front of the test cage and monkeys could again choose for 1 minute whether to spend time in front of one of the experimenters or in the middle of the test cage equidistant between experimenters. Unlike Experiment 2, monkeys now spent similar amounts of time in front of experimenters (mean looking at monkey: 37.8%, mean looking away from monkey: 27.4% of trial; t(10)=1.56, p=0.15; Fig. 3). Thus, it appears that it is the process of being imitated rather than simple familiarity that caused monkeys to increase proximity to the imitator in Experiment 2.
To investigate whether imitation also affects social interactions, we tested the effects of imitation on monkeys’ interactions with an imitator in a token exchange task. A token exchange by its very nature is an interaction between two partners, one providing a token and the other providing a food item (17). Several factors may influence a monkey’s willingness to exchange tokens with an experimenter, for example a monkey might refuse to exchange tokens with a person whom they fear (supporting online text). Therefore, a monkey’s emotional reaction towards an experimenter may significantly affect a monkey’s willingness to exchange tokens. All of our monkeys had previously been trained to exchange small metal or plastic tokens with human experimenters for food rewards. In Experiment 4, monkeys could choose to exchange a token with one of two experimenters, both offering the same food reward (a small piece of marshmallow).
During the first token exchange session, monkeys did not prefer either experimenter for their token exchanges (mean 4.55 and 5.15 exchanges; t(9)=-0.25, p=0.81; Fig. 3). Immediately following this first token exchange session, we replicated Experiment 1. During the baseline imitation phase, monkeys did not visually discriminate between the experimenters (mean 37.25 sec and 40.19 sec, t(9)=-0.93, p=0.37). During the experimental imitation manipulation, monkeys showed a visual preference for the imitator during imitated actions (t(9)=3.12, p=0.012; Fig. 2). Finally, we conducted a second token exchange session where monkeys could choose to exchange a token with either the imitator or the non-imitator, again receiving the same food reward from both experimenters. Monkeys now exchanged significantly more frequently with the imitator (mean imitator: 6.05, mean non-imitator: 4.95 exchanges; t(9)=2.30, p=0.047; Fig. 3), indicating that being imitated increased the frequency of monkeys’ interactions with the imitator.
To confirm that, like proximity measurements, the token exchanges were not merely facilitated by increased familiarity or perceived attentiveness of the imitator, we ran Experiment 5 as a control study. At the start of Experiment 5, monkeys could exchange tokens with one of two experimenters, who offered identical food rewards. As in Experiment 4, monkeys initially did not prefer either experimenter (mean 4.55 and 5.45 exchanges; t(9)=-0.95, p=0.37; Fig. 3). We then replicated the gaze control manipulation from Experiment 3, i.e. one experimenter faced and looked directly at the monkeys whereas the other experimenter turned around and faced away from the monkeys, which led to monkeys looking significantly longer at the experimenter facing them (t(9)=3.51, p=0.007; Fig. 2). A second token exchange session immediately following the manipulation phase revealed, however, that monkeys did not prefer to exchange tokens with the experimenter who had faced them during the manipulation phase (mean looking at monkey: 4.8, mean looking away from monkey: 5.2 exchanges; t(9)=-0.49, p=0.64; Fig. 3). Experiment 5 therefore confirms that it is the process of being imitated that led to increased interactions with the imitator.
These experimental results demonstrate that imitation significantly affects the behavior of capuchin monkeys: they look longer at imitators, spend more time in proximity to imitators, and prefer to interact with imitators in a token exchange task. As control experiments 3 and 5 show, these behavioral preferences cannot solely be explained by familiarity or perceived attentiveness of the imitator. Thus, imitation positively affects subsequent social interactions not only in humans, but also in capuchin monkeys.
Increased affiliation in human studies is observed after the matching of subtle gestures (2, 5) or synchronized movement between individuals (19), not conspicuous imitation as in the present study. Moreover, it is generally accepted that capuchins do not explicitly match actions in the precise and timed manner as the human imitator in the present study (9, 15, 18). One might therefore wonder how well these effects observed under controlled laboratory conditions transfer to capuchins’ natural group environment. While precise data on this phenomenon in group settings are clearly needed, it is known that wild capuchin groups routinely synchronize their behavior for example for travel, feeding, and predator defense (15). It is possible that such group synchronization may provide a sufficient degree of behavioral matching to produce positive effects on subsequent social interactions. Moreover, monkeys are unlikely to understand others’ intentions to imitate them (8, supporting online text) which suggests that explicit and implicit matching of behaviors are likely to affect them in similar ways. Matching or synchronization of behaviors may therefore carry significant adaptive value not only as a mechanism of social learning (20) but also through its effects on subsequent social interactions.
It has been argued that the link between behavior matching and increases in affiliation might have played an important role in human evolution by helping to maintain harmonious relationships between individuals (21). We propose that the same principle also holds for other group-living primates. Matching or coordination of behaviors may lead to higher levels of tolerance and affiliation as well as decreases in aggressive behaviors, thereby increasing group cohesion. Behavior matching can therefore be regarded as a type of “social glue”, helping to bind individuals together (21). Importantly, the effects of behavior matching are not necessarily restricted to only two interaction partners, but may also lead to prosocial behaviors towards other individuals who were not directly involved in the social imitative exchange (3). An empathic connection resulting from behavior matching (2) may therefore extend to others in the social environment and promote altruistic behavioral tendencies in capuchins (22) and humans (3, 4).
Supplementary Material
Footnotes
Supporting Online Material
Materials and Methods
Supporting online text
Supporting references
References and Notes
- 1.La France M. Soc Psychol Quart. 1979;42:66. [Google Scholar]
- 2.Chartrand TL, Bargh JA. J Pers Soc Psychol. 1999;76:893. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- 3.van Baaren RB, Holland RW, Kawakami K, van Knippenberg A. Psychol Sci. 2004;15:71. doi: 10.1111/j.0963-7214.2004.01501012.x. [DOI] [PubMed] [Google Scholar]
- 4.van Baaren RB, Holland RW, Steenaert B, van Knippenberg A. J Exp Soc Psychol. 2003;39:393. [Google Scholar]
- 5.Lakin JL, Chartrand TL. Psychol Sci. 2003;14:334. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen M, Collier-Baker E, Davis JM, Suddendorf T. Anim Cogn. 2004;8:31. doi: 10.1007/s10071-004-0232-0. [DOI] [PubMed] [Google Scholar]
- 7.Haun DBM, Call J. Curr Biol. 2008;18:R288. doi: 10.1016/j.cub.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Paukner A, Anderson JR, Borelli E, Visalberghi E, Ferrari PF. Biol Lett. 2005;1:219. doi: 10.1098/rsbl.2004.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visalberghi E, Fragaszy DM. In: Imitation in animals and artifacts. Dautenhahn K, Nehanic K, editors. MIT Press; Cambridge, MA: 2002. pp. 247–273. [Google Scholar]
- 10.Perry S, et al. Curr Anthropol. 2003;44:241. [Google Scholar]
- 11.Agostini I, Visalberghi E. Am J Primatol. 2005;65:335. doi: 10.1002/ajp.20120. [DOI] [PubMed] [Google Scholar]
- 12.Meltzoff AN, Moore KM. In: Intersubjective Communication and emotion in early ontogeny. Braten S, editor. Cambridge University Press; Cambridge: 1998. pp. 47–62. [Google Scholar]
- 13.Agnetta B, Rochat P. Infancy. 2004;6:1. [Google Scholar]
- 14.Further details on materials and methods are available as supporting material on Science online.
- 15.Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- 16.Hattori Y, Kuroshima H, Fujita K. Anim Cogn. 2007;10:141. doi: 10.1007/s10071-006-0049-0. [DOI] [PubMed] [Google Scholar]
- 17.Addessi E, Crescimbene L, Visalberghi E. Proc R Soc B. 2007;274:2579. doi: 10.1098/rspb.2007.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilthermuth SS, Heath C. Psychol Sci. 2009;20:1. doi: 10.1111/j.1467-9280.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- 19.Herve N, Deputte BL. Primates. 1993;34:227. [Google Scholar]
- 20.de Waal FBM. The ape and the sushi master: cultural reflections of a primatologist. Basic Books; New York: 2001. [Google Scholar]
- 21.Lankin JL, Jefferis VE, Chang CM, Chartrand TL. J Nonverbal Behav. 2003;27:145. [Google Scholar]
- 22.de Waal FBM, Leimgruber K, Greenberg AR. Proc Natl Acad Sci U S A. 2008;105:13685. doi: 10.1073/pnas.0807060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.We gratefully acknowledge the help of Mary Huntsberry, Dan Hipp, Seth Bower, Franziska Dege, Steve Dinterman, Gino Coude, Giulia Sirianni, Eugenia Polizzi, and Barbara Gambetta. We thank Jim Anderson and two anonymous reviewers for valuable comments on earlier drafts of this paper. This research was supported by the Division of Intramural Research, NICHD, and the TECT-ESF Project SOCCOP.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.